Abstract
Coronary artery anomalies are a well recognized feature of many cardiac malformations and have been catalogued in a number of reviews. This overview concentrates on 1) the interplay between congenital heart defects and coronary morphogenesis, examining how some of the embryology fits with the experiments of nature encountered in clinical practice; and 2) the influence of coronary anatomy on patient management. This overview uses, as examples, pulmonary atresia with intact ventricular septum, complete and congenitally corrected transpositions of the great arteries, and tetralogy of Fallot. (Tex Heart Inst J 2002;29:279–89)
Key words: Coronary vessel anomalies; heart defects, congenital
Coronary artery anomalies are a well recognized feature of cardiac malformations and have been catalogued in a number of reviews. 1–3 After some comments about coronary imaging and reporting in patients with cardiac malformations, this overview will concentrate on 2 main areas: 1) the interplay between congenital heart defects and coronary morphogenesis, examining how the embryology fits with the experiments of nature encountered in clinical practice; and 2) the influence of coronary anatomy on patient management. This overview will use, as examples, pulmonary atresia with intact ventricular septum (PA-IVS), complete and congenitally corrected transpositions of the great arteries (d-TGA and CC-TGA, respectively), and tetralogy of Fallot.
Imaging and Reporting of Coronary Anatomy
While angiography has been the mainstay of detailed coronary imaging in patients with congenital heart disease, 2 sonography has played an important role, especially in children with d-TGA. 4,5 Increasingly, cross-sectional imaging with both gated computed tomography (CT) and magnetic resonance imaging (MRI) is being explored. 6–8 Whatever the imaging method chosen—a decision usually guided by the management issue at hand—imaging can supply such information as the relationship of the great arteries, the number of coronary ostia and their locations within the sinuses, the proximal courses of vessels, branching patterns, and regions of supply. Whether a physician uses 2 views at right angles during angiography 9,10 or cross-sectional imaging, he or she needs a good understanding of the normal in order to recognize some of the subtle variations from normal, such as whether the vessel runs an intramuscular course rather than the more typical epicardial course; this cannot be emphasized enough.
Once the coronaries have been imaged, the important issue becomes one of communication. Many systems for denoting coronary anatomy in patients with congenital heart defects have been proposed and are reviewed elsewhere, 2,11–14 but our surgeons prefer a short description supplemented by a simple diagram. A short description might be “rightward anterior aorta with usual coronaries from the midpoint of the facing sinuses and no significant commissural malalignment.” When communicating more detail of the coronary course and distribution is relevant, we use a diagram (Fig. 1), which enables the course and myocardial distribution of each vessel to be represented. 15,16
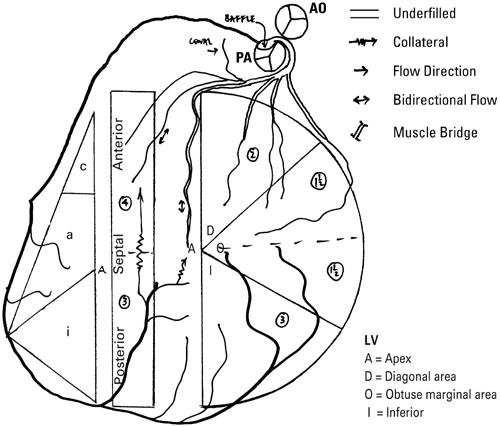
Fig. 1 Diagrammatic representation of coronary artery abnormalities. Complete transposition with single sinus origin of both coronary arteries, with the left coronary artery having an interarterial course, managed by the arterial switch and the Takeuchi tunnel. The displayed anatomy following the procedure shows the pulmonary artery (PA) in a rightward anterior position with underfilling of the left coronary artery. Collaterals from the right coronary artery fill septal perforators and the left anterior descending coronary artery retrograde. Numbers in circles denote the amount of myocardium supplied by each vessel (see refs. 15, 16 for detailed explanation).
Ao = aorta; LV = left ventricle
(Modified from: Brandt PW, Partridge JB, Wattie WJ. Coronary arteriography; method of presentation of the arteriogram report and a scoring system. Clin Radiol 1977;28:361–5. 15
© 1977. Reproduced by permission of Elsevier.)
As an aside, let me remark that in day-to-day clinical practice, widely used shorthand terminology is well understood, although it may not be descriptively accurate. For instance, a patient with d-TGA might be described as having “side-by-side great arteries with inverted coronaries.” In this case, the coronaries themselves are not “inverted” (they are designated as morphologically left or right depending on their distal course and distribution 14); rather, their origins and proximal courses are opposite from those usually seen in d-TGA, with the right coronary artery connected to the left-anterior-facing sinus and coursing over the right ventricular (RV) free wall to reach the right atrioventricular (AV) groove, and the left coronary artery connected to the right posterior facing sinus and traveling a retropulmonary course to reach the left AV groove. In this paper, shorthand terminology is denoted in quotation marks.
Influence of Congenital Heart Defects on Coronary Morphogenesis and Patient Management
Clinicians' interest in the coronary arteries of their patients with cardiac malformations has been driven primarily by issues of patient management. Yet information on the development of the coronary vessels has led to a new appreciation of the interplay between cardiac malformations and coronary morphogenesis.
Pulmonary Atresia with Intact Ventricular Septum.
The signal example of the relationship between a malformation and coronary morphogenesis is PA-IVS. This malformation is characterized by complete muscular or membranous obstruction of the RV outflow tract, an intact ventricular septum, an obligatory shunt at atrial level, and pulmonary blood flow mediated through a patent ductus arteriosus. However, as Freedom has articulated, 2 this characterization does no justice to the disorder and is akin to characterizing Da Vinci as “just a painter,” for there is tremendous morphological and functional heterogeneity, not least in the coronary arteries. These may be normal or grossly abnormal and are one of the major determinants of outcome, for myocardial perfusion can be partly or wholly dependent on communications between the RV and the coronary arteries (the “RV-dependent circulation”).
Abnormalities of coronary origin, course, and distribution—such as common, mixed trunk (terminology advocated in a recent review 3), and connections to a pulmonary artery—exhibit the same spectrum as that seen in normal hearts. 17,18 In addition, there is interplay between the cardiac malformation and coronary morphogenesis (or vice versa), in which the development of ventriculo–coronary arterial communications (VCAC) is followed by secondary changes in the vessels.
The development of VCAC in these patients has been discussed elsewhere in these presentations. The incidence of such communications is variable in reported autopsy and angiographic series 17,19,20 and was about 45% among patients for whom data were available in the Congenital Heart Surgeons Study. 20 These communications are usually to the right coronary artery (RCA) or left anterior descending coronary artery (LAD) (Fig. 2) and are often multiple, 17–19 although they may be to remote coronary branches, such as the circumflex, and may be single. Also, the vessels that are connected with VCAC may be dilated (Fig. 3).
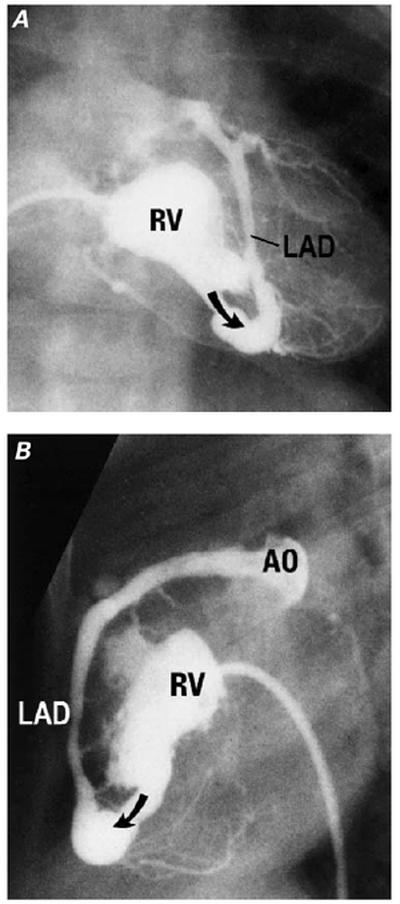
Fig. 2 Angiographic appearances of ventriculo-coronary arterial communications. A) Frontal and B) lateral projections of a right ventriculogram show a small right ventricle (RV) with only the inlet portion clearly defined. The left anterior descending coronary artery (LAD) opacifies retrograde (arrow) via the communication, as far as the aorta (AO).
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
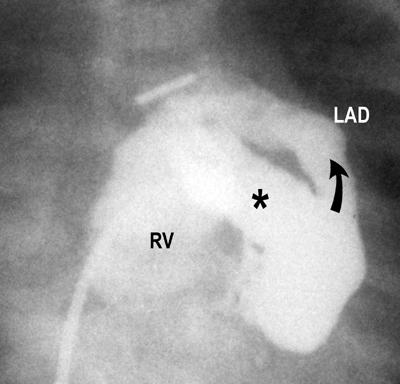
Fig. 3 Angiographic appearances of ventriculo-coronary arterial communications. Right ventricular injection filmed in a frontal projection shows only an inlet component to the right ventricle (RV). A huge communication (*) fills a markedly dilated left anterior descending coronary artery (LAD) retrograde (arrow).
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
Coronary arteries involved in VCAC show characteristic histopathologic changes of myointimal hyperplasia, 21 which is thought to be a consequence of repeated injury to the intima from the high-pressure, turbulent, RV systolic flow, mediated through the communications. Indeed, in the vast majority of patients, this process results in profound distortion of the angiographic appearance of the coronaries, 18,19 ranging from irregularity to luminal narrowing and frank interruption in one-half to two-thirds of those who have VCAC (Fig. 4). Interruptions can be seen anywhere along the length of an affected coronary artery. 17–19 Such proliferative lesions have been found even in the fetus 22 and neonate, as well as in older children, and are thought to progress as long as the coronaries are exposed to the systemic and suprasystemic pressures generated by the hypertensive RV. 18 It remains unclear whether lack of a proximal lumen is caused by defective ingrowth or lumenization of coronaries, occlusion due to myointimal hyperplasia at a later stage, or all of these.
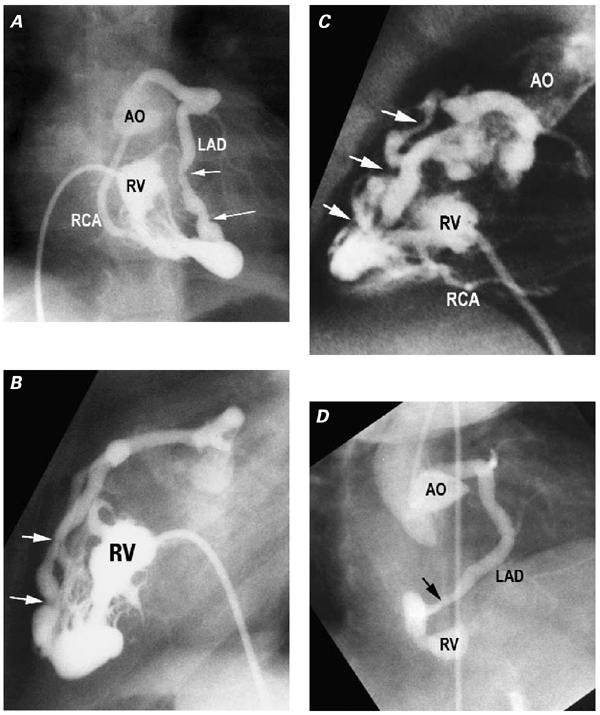
Fig. 4 Caliber changes in coronary arteries reflecting myointimal hyperplasia. A) Frontal and B) lateral projections of a right ventriculogram (RV) in which both the left anterior descending coronary artery (LAD) and the right coronary artery (RCA) fill retrograde via ventriculo–coronary arterial communications (VCAC). The irregularities of the LAD are particularly prominent (arrows). Note that both LAD and RCA are connected to the aorta (AO). C) Another patient. Right ventricular injection filmed in a lateral projection shows the LAD and distal RCA filling retrograde via VCAC. The LAD shows multiple levels of stenosis and interruption (arrows), promoting an RV-dependent coronary circulation. D) Another patient. A right anterior oblique projection of an aortogram shows a communication (arrow) between the LAD and the diminutive RV. Caliber changes are evident. Despite multiple injections in the aortic root (AO), the RCA was never opacified, which is good evidence that it lacked a normal aortic connection.
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
This coronary arterial anatomy has a profound influence on patient management and outcomes. 20 In those who lack a connection between a proximal coronary artery and the aorta, or in whom there is severe luminal stenosis or interruption, part or all of the coronary circulation is dependent upon perfusion from the RV cavity, the “RV-dependent circulation” (Fig. 4). In normal hearts, left coronary arterial flow is predominantly a diastolic event, whereas right coronary flow is both systolic and diastolic, due to the lower transmural pressure. In those with an RV-dependent circulation, the diastolic component is decreased, because retrograde flow has to be at sufficient pressure to overcome both stenoses and the prolonged isometric ventricular contraction that causes a relatively high wall tension. 23 Worse, desaturated blood from the RV cavity must supply the nutritional demands of a hypertrophied and disordered myocardium that generates systemic or suprasystemic pressures. Therefore, these patients have the substrate for myocardial ischemia, especially ischemia of the RV subendocardial region. Maneuvers that obliterate the cavity, such as thromboexclusion or tricuspid valve oversewing, or that decompress the cavity, such as RV-outflow-tract reconstruction or valve excision, decrease or eliminate the driving pressure for coronary flow and exacerbate ischemia.
In patients who have continuity between the aorta, coronary arteries, and RV cavity, bidirectional flow is evident in the coronaries. A small number of these patients exhibit diastolic runoff to the RV cavity, with ischemia resulting from a steal phenomenon. Maneuvers that drop the RV cavity pressure may worsen the shunt, while those that drop the aortic pressure, such as use of prostaglandins or creation of a systemic shunt to the pulmonary arteries, may decrease the driving pressure. Both exacerbate ischemia.
Complete Transposition of the Great Arteries.
Patients with d-TGA still pose significant management challenges for clinicians, despite the strides of the last several decades. Early attempts at switching the great arteries were essentially limited by the difficulties of reimplanting the coronary arteries. It was not until the concept of coronary “buttons” was established 24 that the arterial switch procedure became a viable alternative to the atrial switch procedures developed by Mustard 25 and by Senning, 26 and to the intracardiac repair developed by Rastelli. 27 While there had been a longstanding interest in the variations of coronary anatomy in patients with d-TGA, the development of the arterial switch repair provoked a great deal of publication in the 1970s and 1980s on the subjects of nomenclature, anatomy, risk stratification of the variants, imaging for preoperative recognition of variants, and surgical maneuvers for dealing with specific variants.
Most commonly, patients with d-TGA have a rightward anterior aorta relative to the pulmonary artery. This position, an aorta directly to the right (“side-by-side”) or directly in front of the pulmonary artery, accounts for the vast majority of patients; rarely, a right posterior or left anterior aorta is encountered. Origins are usually from the midpoints of facing sinuses, but can be ectopic within or above a sinus, and rarely may arise from the nonfacing sinus. Commissural malalignment or a bicuspid valve may contribute to a variant origin (for a detailed discussion of commissural alignment and malalignment, and its surgical implications, see reference 28). With ectopic or commissural origins, there is a risk of a slit-like origin and oblique proximal course, which may be in the wall of a vessel. These intramural segments are seen in about 3% of patients 28,29 and are usually (but not always) associated with interarterial courses (Fig. 5). 14 In cases of rightward anterior aorta, approximately two thirds of patients will have 2 coronaries from facing sinuses with typical branching patterns and distribution (so-called “usual” coronaries) (Fig. 6). In the next most common pattern, the RCA from the right-posterior facing sinus gives off a circumflex, which travels a retropulmonary course (Fig. 7). Beyond that, the variations are almost endless, although as pointed out, 12 9 types account for 95% of the cases (Fig. 8). Note should be made that patients with side-by-side great arteries have an increased incidence of unusual patterns, including “inverted” coronaries (Fig. 9).
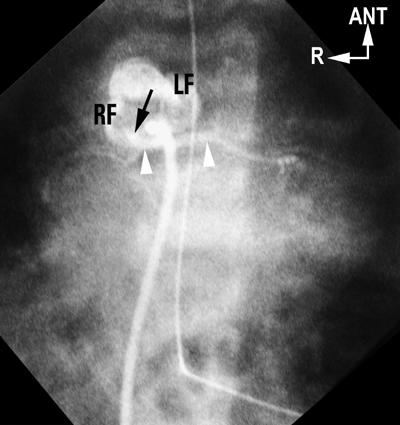
Fig. 5 Single-sinus origin of coronary arteries in d-TGA. Antegrade balloon-occlusion aortogram, in a caudally tilted, shallow, left anterior oblique (“laid-back”) projection, shows a rightward anterior aorta. No vessel arises from the left-anterior-facing sinus (LF) but early frames show a vessel (arrow) from the right-posterior-facing sinus (RF) having an interarterial course (arrow-heads). This eventually divided into left anterior descending and circumflex, and a separate origin for the right coronary artery was evident (later frames not shown). Findings were confirmed at surgery.
ANT = anterior; d-TGA = complete transposition of the great arteries; R = right
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
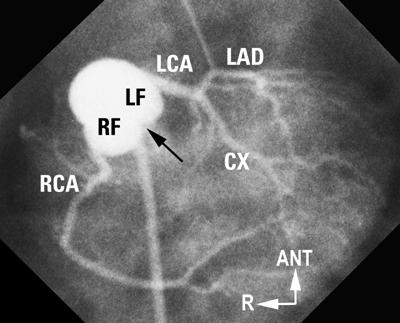
Fig. 6 “Usual” coronary artery anatomy in d-TGA. Laid-back aortogram shows a rightward anterior aorta. Both facing sinuses are well seen, as is the facing commissure (arrow). Both coronaries arise from the mid-points of their respective facing sinuses (RF, LF) and show a good length of proximal vessel before the 1st branch.
ANT = anterior; CX = circumflex; d-TGA = complete transposition of the great arteries; LAD = left anterior descending; LCA = left coronary artery; LF = left facing; R = right; RCA = right coronary artery; RF = right facing
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
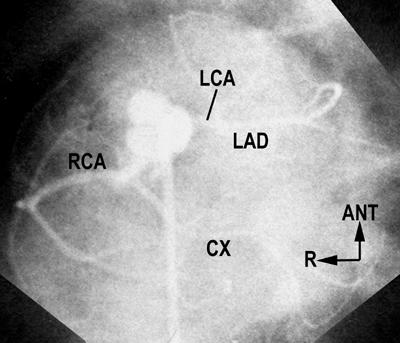
Fig. 7 Anomalous circumflex in d-TGA. Laid-back aortogram shows a rightward anterior aorta and 2 coronaries arising from the mid-points of their respective facing sinuses. The right coronary artery (RCA) gives off the circumflex (CX), which travels a retropulmonary course to reach the left atrioventricular groove.
ANT = anterior; d-TGA = complete transposition of the great arteries; LAD = left anterior descending; LCA = left coronary artery; R = right
(Courtesy of Dr. S-J Yoo, Hospital for Sick Children, Toronto, Canada.)
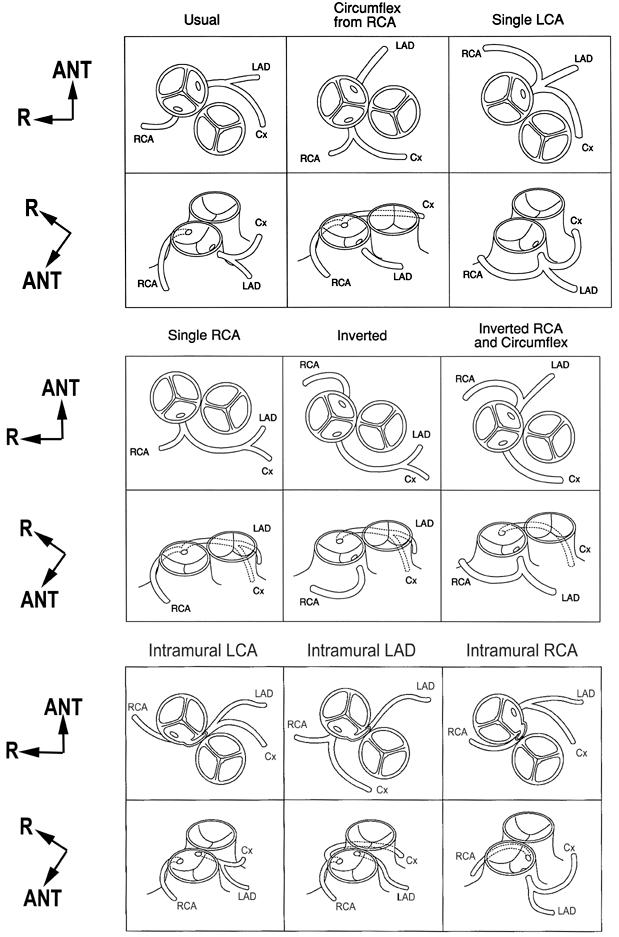
Fig. 8 The 9 most common variations of coronary artery anatomy in d-TGA.
ANT = anterior; Cx = circumflex; d-TGA = complete transposition of the great arteries; LAD = left anterior descending; LCA = left coronary artery; R = right; RCA = right coronary artery
(From: Wernovsky G, Sanders SP. Coronary artery anatomy and transposition of the great arteries. Coron Artery Dis 1993;4:148–57. 12 Reproduced, with modifications, by permission of Lippincott Williams & Wilkins, Baltimore.)
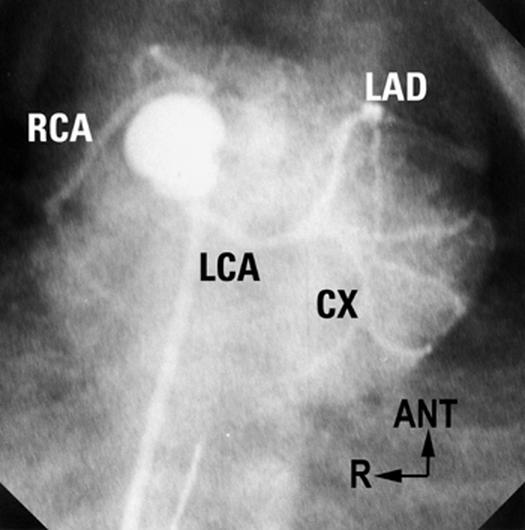
Fig. 9 “Inverted” coronary arteries in d-TGA (see text for discussion of terminology). Laid-back aortogram in a patient with almost side-by-side great arteries. Both facing sinuses are well seen, with 2 coronaries from their mid-points. Note the long proximal courses of the RCA and LCA.
ANT = anterior; CX = circumflex; d-TGA = complete transposition of the great arteries; LAD = left anterior descending; LCA = left coronary artery; R = right; RCA = right coronary artery
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
Identified as significant operative risk factors are “inverted” coronaries, single (or mixed common trunk) coronary artery, and an intramural coronary artery (oblique segment within the wall of the aorta). 14,30–33 Less-well-defined risk factors may include a course between the great arteries (the so-called “interarterial” course), a short distance to the 1st branch, ectopic origin within a sinus, origin from the nonfacing sinus, and malalignment of facing commissures. As experience builds and operations are concentrated in fewer centers, these risk factors may be diminishing. The recent recognition of the 10% to 15% incidence of late coronary complications after the arterial switch procedure has stimulated yet further interest in the coronary arteries. 34–36
The concepts of ingrowth of epicardial cell strands to an adjacent great artery 37 and their subsequent development of a lumen 38,39 have been discussed in earlier presentations. The postulate that epicardial vessels contact the aortic sinus to which they are closest would explain the usual coronary artery anatomy seen in patients with d-TGA who have a typical rightward anterior aorta: the morphologically right coronary artery is connected to the right posterior facing sinus, which is closest to the right AV groove, and the left coronary artery is connected to the left posterior facing sinus, which is closest to the anterior interventricular sulcus and left AV groove.
Alfred North Whitehead said “Seek simplicity and distrust it.” 40 Indeed, as attractive as such an explanation of the morphogenesis of the coronary connection and proximal course may be, it is an oversimplification that doesn't easily explain the almost endless variants of coronary anatomy that represent the experiments of nature seen in d-TGA, including those in which the connection and proximal course are as long as they could possibly be (Fig. 9), rather than as short. Presumably, a defect in ingrowth to a facing sinus, in lumenization, or in both, promotes lumenization of an alternative potential pathway in the peritruncal ring. Our understanding of these processes that influence the morphogenesis of the coronaries continues to advance.
Tetralogy of Fallot.
In angiographic, 41,42 surgical, 43 and autopsy 41 series, coronary artery abnormalities have been reported in 2% to 9% of patients with tetralogy of Fallot. Abnormalities cover a spectrum of variants of origin, course, and distribution, but 2 are of particular importance: those in which a vessel crosses the RV outflow tract, and those in which a coronary artery contributes to pulmonary blood flow, particularly in patients with tetralogy-type pulmonary atresia.
The great majority of tetralogy patients with coronary abnormalities have some component of their supply to the LAD territory coming from the RCA via a vessel crossing the RV free wall a variable distance below the pulmonary annulus, or they have a single right or left coronary artery (mixed common trunk) and therefore have the potential to have a crossing vessel (Fig. 10). All such crossing vessels (but especially those concealed in muscle or epicardial fat) are vulnerable during surgical outflow tract reconstruction, the location of which sometimes necessitates the placement of a conduit rather than a transannular patch. The postulate that epicardial vessels contact the aortic sinus to which they are closest provides a neat explanation of how these crossing vessels develop: clockwise rotation of the aortic root as viewed from the apex (Fig. 11), or a side-by-side arrangement of the great arteries, brings the right anterior-facing sinus into a higher, more leftward and anterior position, closer to the developing LAD. 44 This postulate may also explain the connection of the RCA to the right anterior facing sinus (with the proximal course crossing the RV outflow tract), in those rare tetralogy patients with a leftward anterior aorta relative to the pulmonary artery. However, as discussed in patients with d-TGA, the postulate does not easily explain the anatomy in those patients who, despite a rightward posterior aorta, still have a vessel crossing the RV outflow tract, nor does it easily explain single coronary arteries (mixed common trunk).
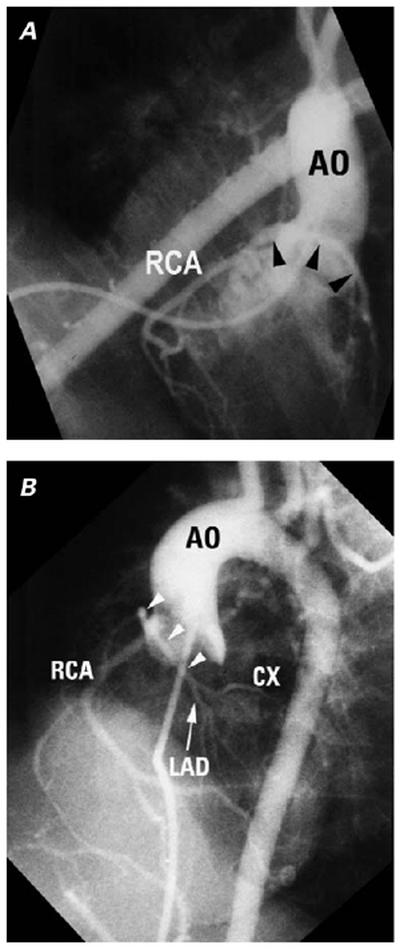
Fig. 10 Single coronary artery (mixed common trunk) in tetralogy of Fallot. A) Right and B) cranially tilted left anterior oblique projections of an antegrade aortogram show a single origin for the coronary arteries. The left main describes an anterior, cranial loop (arrowheads) consistent with a right ventricular free wall course close to the level of the pulmonary annulus. It divides into LAD and CX, which are of modest size.
AO = aorta; CX = circumflex; LAD = left anterior descending; RCA = right coronary artery
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
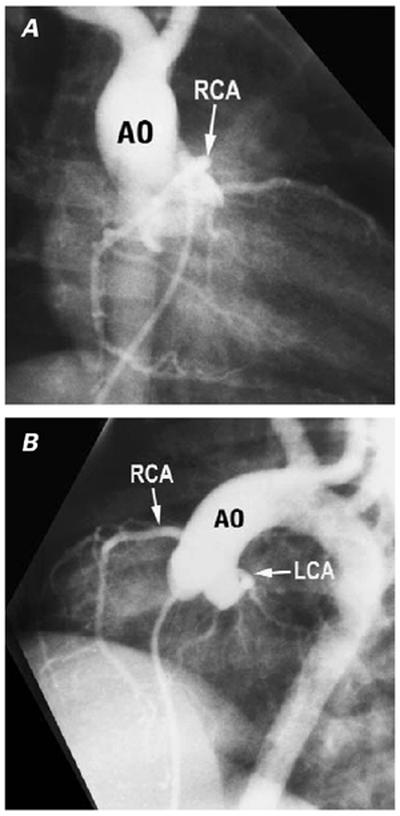
Fig. 11 Rotated aortic root in tetralogy of Fallot. A) Right anterior oblique (RAO) and B) cranially tilted left anterior oblique projections (LAO) of an antegrade aortogram show clockwise rotation of the root as viewed from the apex, bringing the right coronary artery (RCA) origin into a higher, more anterior position than usual, so that its origin is profiled in the RAO, rather than the LAO, projection.
Ao = aorta; LCA = left coronary artery
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
In patients with tetralogy-type pulmonary atresia, a variety of systemic sources contribute to pulmonary blood flow, most often the patent ductus arteriosus. In those who receive some or all of their pulmonary blood supply via vessels from the aorta or other splanchnic arteries, the coronary arteries contribute flow in about 10% of cases. 45 Very rarely, the coronary artery may be connected to the pulmonary artery, thereby serving as a major or sole 46 source of flow.
Congenitally Corrected Transposition of the Great Arteries.
In clinical practice, patients with CC-TGA (atrioventricular and ventriculoarterial discordance) are a rather heterogeneous group, often having ventricular septal defects, AV valve abnormalities, semilunar valve abnormalities, tetralogy-type malformations including pulmonary atresia, and twisted or hypoplastic ventricles. At 1st glance, these patients may appear to have bizarre coronary artery anatomy, not only because the ventricular and great artery relationships (left anterior aorta) are different from what most angiographers are accustomed to, but because patients with atrial situs solitus often have dextrocardia, and a modest number of patients have atrial situs inversus. Coronary artery anatomy has been clinically relevant in the numerically significant subgroup of patients with tetralogy-type malformations who require RV outflow tract reconstruction. More recently, interest in the double switch procedure has given even more relevance to the coronary anatomy. 47,48
Typically, 2 vessels arise from facing sinuses, 49,50 and the postulate that epicardial vessels contact the aortic sinus to which they are closest also accounts for these. Abnormalities of coronary origin and proximal course are seen, such as common, mixed trunk (single coronary artery), and vessels crossing the RV outflow tract. Beyond its origin and proximal course, the morphology of the coronary artery has for many years been observed to “follow” that of the ventricle. 49 Thus, when there is a leftward-anterior aorta, the coronary connecting to the right anterior facing sinus has the epicardial distribution of a morphologically left coronary artery, while the coronary connecting to the left posterior facing sinus runs in the left AV groove and supplies the RV and posterior interventricular septum as the RCA would do (Fig. 12). This principle has been useful not only in identifying ventricular morphology, but also in working out the size of the ventricle or the plane of the interventricular septum in patients with hypoplastic or twisted ventricles. 51
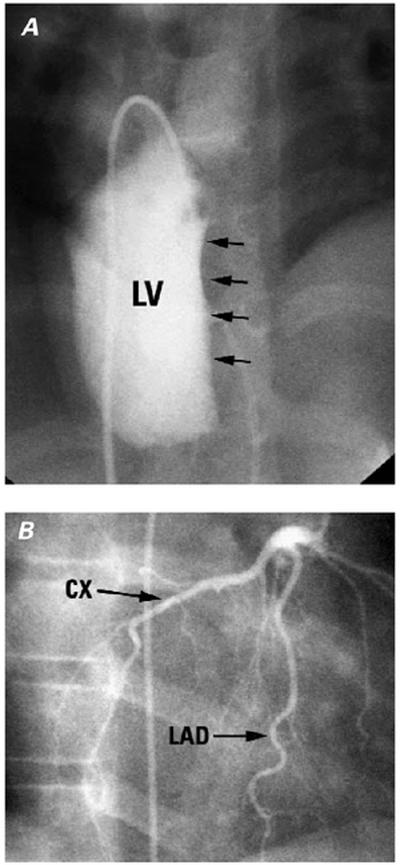
Fig. 12 Patient with congenitally corrected transposition of the great arteries (CC-TGA). A) Left ventricular (LV) injection filmed in a cranially tilted frontal projection shows the plane of the interventricular septum (arrows). B) Selective left coronary artery injection filmed in a projection similar to that of (A) shows the left anterior descending (LAD) in the anterior interventricular groove and the circumflex (CX) coursing around the right-sided (mitral) atrioventricular groove.
(Modified from: Freedom RM, et al. 2 With permission of Futura Publishing Company, Inc.)
Development of the epicardial and myocardial coronary arteries involves the predictable migration of developing vessels along the planes of the atrioventricular and interventricular grooves 39,52 (Fig. 13). If this predictable migration holds no matter what the orientation of these planes or the sizes of the ventricles, then this migration accounts in large measure for the anatomical observation that coronary artery morphology “follows” the ventricle and provides yet another example of the interplay between congenital heart disease and coronary artery morphology.
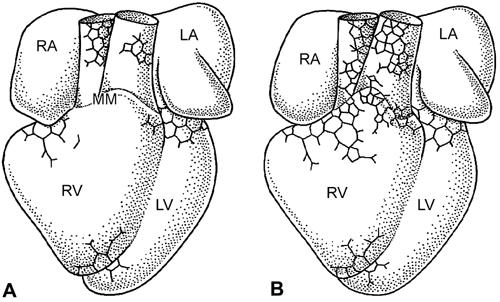
Fig. 13 Ventral views of A) HH29 and B) HH30 quail embryo hearts. The coronary vessel plexus expands from the base of the heart around the atrioventricular groove and over the myocardium and great arteries at the level of the myocardium-mesenchyme (MM) boundary.
LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle
(From: Vrancken-Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn 1997;208:338–48. 39 Copyright © 1997 Wiley-Liss. Reprinted by permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons.)
Summary
As our collective experience in handling patients with congenital heart disease continues to expand, no doubt interest in the relationship between specific lesions and coronary morphogenesis, and between coronary anatomy and management, will expand, too. Many of the postulates that have been alluded to in this paper will be further refined, explaining more and more of the experiments of nature that are encountered in clinical practice.
Acknowledgments
I acknowledge the helpful comments of Drs. L. Calder, J.A.G. Culham, A.C. Gittenberger-de Groot, and S. Sett during the preparation of this manuscript.
Footnotes
Address for reprints: Dr. John Mawson, Department of Radiology, Children's and Women's Health Centre of British Columbia, 4480 Oak Street, Vancouver, BC, Canada V6H 3V4
This paper has its basis in a presentation made at the symposium Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart® Institute, Houston, Texas.
References
- 1.Neufeld HN, Schneeweiss A. Coronary artery disease in infants and children. Philadelphia: Lea and Febiger; 1983.
- 2.Freedom RM, Mawson JB, Yoo S-J, Benson LN. Congenital heart disease: textbook of angiocardiography. New York: Futura Publishing Company Inc.; 1997.
- 3.Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999.
- 4.Pasquini L, Parness IA, Colan SD, Wernovsky G, Mayer JE, Sanders SP. Diagnosis of intramural coronary artery in transposition of the great arteries using two-dimensional echocardiography. Circulation 1993;88:1136–41. [DOI] [PubMed]
- 5.Pasquini L, Sanders SP, Parness IA, Wernovsky G, Mayer JE Jr, Van der Velde ME, et al. Coronary echocardiography in 406 patients with d-loop transposition of the great arteries. J Am Coll Cardiol 1994;24:763–8. [DOI] [PubMed]
- 6.Post JC, van Rossum AC, Bronzwaer JG, de Cock CC, Hofman MB, Valk J, Visser CA. Magnetic resonance angiography of anomalous coronary arteries. A new gold standard for delineating the proximal course? Circulation 1995;92:3163–71. [DOI] [PubMed]
- 7.Mousseaux E, Hernigou A, Sapoval M, Darmon O, Beyssen B, Gaux JC. Coronary arteries arising from the contralateral aortic sinus: electron beam computed tomographic demonstration of the initial course of the artery with respect to the aorta and the right ventricular outflow tract. J Thorac Cardiovasc Surg 1996;112:836–40. [DOI] [PubMed]
- 8.Taylor AM, Thorne SA, Rubens MB, Jhooti P, Keegan J, Gatehouse PD, et al. Coronary artery imaging in grown up congenital heart disease: complementary role of magnetic resonance and x-ray coronary angiography. Circulation 2000;101:1670–8. [DOI] [PubMed]
- 9.Ishikawa T, Brandt PW. Anomalous origin of the left main coronary artery from the right anterior aortic sinus: angiographic definition of anomalous course. Am J Cardiol 1985;55:770–6. [DOI] [PubMed]
- 10.Serota H, Barth CW 3rd, Seuc CA, Vandormael M, Aguirre F, Kern MJ. Rapid identification of the course of anomalous coronary arteries in adults: the “dot and eye” method. Am J Cardiol 1990;65:891–8. [DOI] [PubMed]
- 11.Anderson RH. Description of the origins and epicardial course of the coronary arteries in complete transposition [editorial]. Cardiol Young 1991;1:11–2. [DOI] [PubMed]
- 12.Wernovsky G, Sanders SP. Coronary artery anatomy and transposition of the great arteries. Coron Artery Dis 1993;4:148–57. [DOI] [PubMed]
- 13.Amato JJ, Zelen J, Bushong J. Coronary arterial patterns in complete transposition—classification in relation to the arterial switch procedure. Cardiol Young 1994;4:329–39.
- 14.Angelini P, de la Cruz MV, Valencia AM, Sanchez-Gomez C, Kearney DL, Sadowinski S, Real GR. Coronary arteries in transposition of the great arteries. Am J Cardiol 1994;74:1037–41. [DOI] [PubMed]
- 15.Brandt PW, Partridge JB, Wattie WJ. Coronary arteriography; method of presentation of the arteriogram report and a scoring system. Clin Radiol 1977;28:361–5. [DOI] [PubMed]
- 16.Kirklin JW, Barratt-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications. 2nd ed. New York: Churchill Livingstone; 1993.
- 17.Calder AL, Co EE, Sage MD. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum. Am J Cardiol 1987;59:436–42. [DOI] [PubMed]
- 18.Burrows PE, Freedom RM, Benson LN, Moes CA, Wilson G, Koike K, Williams WG. Coronary angiography of pulmonary atresia, hypoplastic right ventricle, and ventriculocoronary communications. Am J Roentgenol 1990;154:789–95. [DOI] [PubMed]
- 19.Sauer U, Bindl L, Pilossoff V, Hultzsch W, Buhlmeyer K, Gittenberger-de Groot AC, et al. Pulmonary atresia with intact ventricular septum and right ventricle–coronary artery fistulae: selection of patients for surgery. In: Doyle EF, Engle MA, Gersony WM, Rashkind WJ, Talner NS, editors. Pediatric cardiology. Proceedings of the Second World Congress. New York: Springer-Verlag; 1986. p. 566–78.
- 20.Hanley FL, Sade RM, Blackstone EH, Kirklin JW, Freedom RM, Nanda NC. Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg 1993;105:406–27. [PubMed]
- 21.Wilson GJ, Freedom RM, Koike K, Perrin D. The coronary arteries: anatomy and histopathology. In: Freedom RM, editor. Pulmonary atresia with intact ventricular septum. Mount Kisco (NY): Futura Publishing Company Inc.; 1989. p. 75.
- 22.Sandor GG, Cook AC, Sharland GK, Ho SY, Potts JE, Anderson RH. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum diagnosed during fetal life. Cardiol Young 2002;12:436–44. [DOI] [PubMed]
- 23.Freedom RM, Wilson GJ. Endomyocardial abnormalities. In: Freedom RM, editor. Pulmonary atresia with intact ventricular septum. Mount Kisco (NY): Futura Publishing Company Inc.; 1989. p. 89.
- 24.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976;72:364–70. [PubMed]
- 25.Mustard WT. Successful two-stage correction of transposition of the great vessels. Surgery 1964;55:469–72. [PubMed]
- 26.Senning A. Surgical correction of transposition of the great vessels. Surgery 1959;45:966–80. [PubMed]
- 27.Rastelli GC, Wallace RB, Ongley PA. Complete repair of transposition of the great arteries with pulmonary stenosis. A review and report of a case corrected by using a new surgical technique. Circulation 1969;83–95. [DOI] [PubMed]
- 28.Gittenberger-de Groot AC, Sauer U, Oppenheimer-Decker A, Quaegebeur J. Coronary arterial anatomy in transposition of the great arteries: a morphologic study. Pediatr Cardiol 1983;4(Suppl 1):15–24.
- 29.Gittenberger-de Groot AC, Sauer U, Quaegebeur J. Aortic intramural coronary artery in three hearts with transposition of the great arteries. J Thorac Cardiovasc Surg 1986;91:566–71. [PubMed]
- 30.Mayer JE Jr, Sanders SP, Jonas RA, Castenada AR, Wernovsky G. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries. Circulation 1990;82(5 Suppl):IV139–45. [PubMed]
- 31.Day RW, Laks H, Drinkwater DC. The influence of the coronary anatomy on the arterial switch operation in neonates. J Thorac Cardiovasc Surg 1992;104:706–12. [PubMed]
- 32.Kirklin JW, Blackstone EH, Tchervenkov CI, Castenada AR. Clinical outcomes after the arterial switch operation for transposition. Patient, support, procedural, and institutional risk factors. Circulation 1992;86:1501–15. [DOI] [PubMed]
- 33.Wernovsky G, Mayer JE, Jonas RA, Hanley FL, Blackstone EH, Kirklin JW, Castaneda AR. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 1995;109:289–302. [DOI] [PubMed]
- 34.Tsuda E, Imakita M, Yagihara T, Ono Y, Echigo S, Takahashi O, Kamiya T. Late death after arterial switch operation for transposition of the great arteries. Am Heart J 1992;124:1551–7. [DOI] [PubMed]
- 35.Tanel RE, Wernovsky G, Landzberg MJ, Perry SB, Burke RP. Coronary artery abnormalities detected at cardiac catheterization following the arterial switch operation for transposition of the great arteries. Am J Cardiol 1995;76:153–7. [DOI] [PubMed]
- 36.Bonhoeffer P, Bonnet D, Piechaud JF, Stumper O, Aggoun Y, Villain E, et al. Coronary artery obstruction after the arterial switch operation for transposition of the great arteries in newborns. J Am Coll Cardiol 1997;29:202–6. [DOI] [PubMed]
- 37.Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, Peault BM, Huysmans HA. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol 1989;180:437–41. [DOI] [PubMed]
- 38.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 1993;73:559–68. [DOI] [PubMed]
- 39.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn 1997;208:338–48. [DOI] [PubMed]
- 40.Whitehead AN. The concept of nature. Tarner lectures delivered in Trinity College, November 1918. Cambridge: Cambridge University Press; 1920.
- 41.Fellows KE, Freed MD, Keane JF, Van Praagh R, Bernhard WF, Castaneda AC. Results of routine preoperative coronary angiography in tetralogy of Fallot. Circulation 1975;51:561–6. [DOI] [PubMed]
- 42.Dabizzi RP, Teodori G, Barletta GA, Caprioli G, Baldrighi G, Baldrighi V. Associated coronary and cardiac anomalies in the tetralogy of fallot. An angiographic study. Eur Heart J 1990;11:692–704. [DOI] [PubMed]
- 43.Berry BE, McGoon DC. Total correction for tetralogy of Fallot with anomalous coronary artery. Surgery 1973;74:894–8. [PubMed]
- 44.Van Praagh R, Van Praagh S, Nebesar RA, Muster AJ, Sinha SN, Paul MH. Tetralogy of Fallot: underdevelopment of the pulmonary infundibulum and its sequelae. Am J Cardiol 1970;26:25–33. [DOI] [PubMed]
- 45.Amin Z, McElhinney DB, Reddy VM, Moore P, Hanley FL, Teitel DF. Coronary to pulmonary artery collaterals in patients with pulmonary atresia and ventricular septal defect. Ann Thorac Surg 2000;70:119–23. [DOI] [PubMed]
- 46.Moss RL, Backer CL, Zales VR, Florentine MS, Mavroudis C. Tetralogy of Fallot with anomalous origin of the right coronary artery. Ann Thorac Surg 1995;59:229–31. [DOI] [PubMed]
- 47.Yeh T Jr, Connelly MS, Coles JG, Webb GD, McLaughlin PR, Freedom RM, et al. Atrioventricular discordance: results of repair in 127 patients. J Thorac Cardiovasc Surg 1999;117:1190–203. [DOI] [PubMed]
- 48.Van Praagh R, Papagiannis J, Grunenfelder J, Bartram U, Martanovic P. Pathologic anatomy of corrected transposition of the great arteries: medical and surgical implications. Am Heart J 1998;135(5 Pt 1):772–85. [DOI] [PubMed]
- 49.Shea PM, Lutz JF, Vieweg WV, Corcoran FH, Van Praagh R, Hougen TJ. Selective coronary arteriography in congenitally corrected transposition of the great arteries. Am J Cardiol 1979;44:1201–6. [DOI] [PubMed]
- 50.McKay R, Anderson RH, Smith A. The coronary arteries in hearts with discordant atrioventricular connections. J Thorac Cardiovasc Surg 1996;111:988–97. [DOI] [PubMed]
- 51.Anderson RH, Becker AE. Coronary arterial patterns: a guide to identification of congenital heart disease. In: Becker AE, Losekoot G, Marcelletti C, Anderson RH, editors. Paediatr cardiol. Edinburgh: Churchill Livingstone: 1981;251–62.
- 52.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199:367–78. [DOI] [PubMed]


