Abstract
When intervention is indicated for anomalous origination of a coronary artery from the opposite sinus, stent-angioplasty may seem more attractive than coronary artery bypass grafting. However, in the case of anomalous origination of a coronary artery from the opposite sinus, the anatomy is quite different from that encountered in atherosclerotic disease, and stent-angioplasty would involve unusual challenges, both in technique and prognostic outcomes. We illustrate these points by presenting the 2 first cases in which intervention was indicated because of severe symptoms. We conclude from this preliminary study that coronary artery bypass grafting should still be considered the preferred (although unproven) method of revascularization in patients who have symptomatic anomalous origination of a coronary artery from the opposite sinus. Until adequate data have been gathered to evaluate the late results of stent-angioplasty in these patients (in comparison with the results of surgical and medical treatment), the procedure should be performed only in selected patients, enrolled in prospective, controlled studies. (Tex Heart Inst J 2002;29:308–13)
Key words: Angioplasty, transluminal, percutaneous coronary; coronary artery bypass; coronary disease; coronary vessel anomalies; heart defects, congenital; myocardial revascularization/methods; stents; ultrasonography, interventional
Some congenital coronary artery anomalies commonly cause ischemic manifestations. Until recently, coronary artery bypass grafting (CABG) was the only alternative to medical treatment in these cases. However, stenting has become an increasingly attractive alternative for managing congenital anomalies that result in vascular stenosis, because of its success in treating atherosclerotic stenoses. For some congenital anomalies, it seems apparent that stent-angioplasty is not advisable. For instance, the occasional use of stents to treat muscular bridges has recently been reported. Nevertheless, our preliminary experience and that of others indicates that this practice should generally be avoided because of the prohibitive rate of restenosis, possibly due to crushing of the stent (as a consequence of phasic compression) or mechanical stimulation.
Anomalous origination of a coronary artery from the opposite sinus (ACAOS) is a congenital anomaly that might be presumed to respond favorably to stent-angioplasty. Recently, intravascular ultrasonography (IVUS) imaging at baseline and after testing by pharmacologic provocation has suggested that, in ACAOS, the main mechanism of ischemia originates with the proximal ectopic vessel, which runs intramurally at the aortic root while crossing the aortopulmonary septum (Figs. 1 and 2). This type of coronary stenosis is usually relatively mild at baseline; only in exceptional cases does it become clinically significant (typically during or after extreme exertion). In such cases, arterial conduits such as a left internal mammary implant tend to develop poorly, and soon occlude in the absence of a favorable hemodynamic regimen (a significant pressure drop beyond the stenotic area in the recipient vessel).
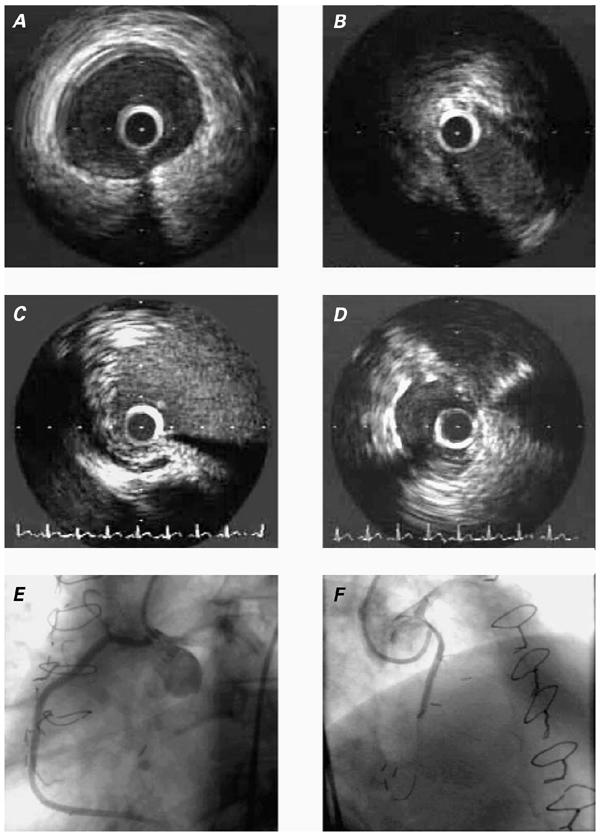
Fig. 1 Patient 1. A) Intravascular ultrasonographic (IVUS) image of the ectopic right coronary artery (RCA), at a segment just distal to the intramural segment. B) IVUS image of the intramural segment during maximal narrowing (end-systole). C) Ectopic RCA ostium, as revealed by IVUS. D) IVUS image of the stented intramural RCA. E) Angiographic appearance of the RCA in the left anterior oblique projection. No stenosis is recognizable in this projection. F) Angiographic appearance of the RCA in the cranial, right anterior oblique projection. A significant proximal stenosis is clearly visible.
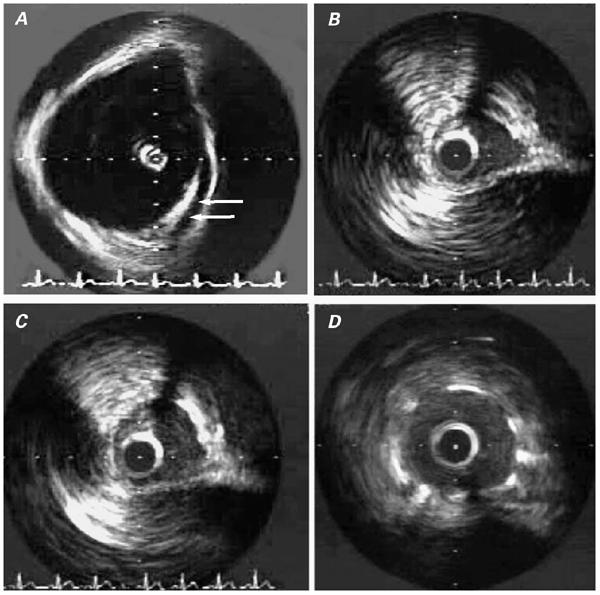
Fig. 2 Patient 2. Intravascular ultrasonographic (IVUS) images: A) Image obtained within the aortic root, illustrating the tangential, intramural course of the proximal right coronary artery (arrows). B) Intramural segment of the right coronary artery (RCA) during systole. C) Same segment as in B, during late diastole (maximal lumen). D) Intramural RCA after stent implantation.
We present 2 cases in which coronary stenting was used to treat ACAOS—cases which possibly are the first ever to test the hypothesis that this treatment is preferable to surgery. We also discuss potentially favorable and unfavorable features of this procedure, which still should be considered unproved and insufficiently tested in this setting.
Case Reports
Patient 1
In 2001, a 51-year-old man was admitted to our hospital because of resting and exercise-induced chest pain, accompanied by dyspnea and lightheadedness. In 1988, he had had a myocardial infarction and undergone heart catheterization, which showed anomalous origination of the right coronary artery (RCA) from the left anterior sinus of Valsalva. The patient underwent surgical implantation of the right internal mammary artery (RIMA) into the RCA. He was totally asymptomatic until the end of the 1st postoperative year, when his original symptoms began to recur. In 1989, a new coronary angiogram revealed occlusion of the RIMA implant. Numerous episodes of unstable angina or myocardial infarction necessitated repeated hospitalization, culminating in the admission to our institution. By that time, the patient's heart condition was sufficiently disabling that he had been declared unable to work. His risk factors included a one-pack-per-day smoking habit.
Upon his hospital admission, the patient's physical findings were not remarkable. A pharmacologic sestamibi stress test showed reversible ischemia of the inferior wall, and a resting electrocardiogram revealed nonspecific ST-T changes in the precordial leads. The patient underwent heart catheterization, including intravascular ultrasonography (IVUS) (Fig. 1) at baseline and during pharmacologic provocation with a dobutamine, atropine, and saline bolus. Because the results suggested substantial, phasic obliteration of the proximal, ectopic artery, he agreed to undergo implantation of a stent to protect the proximal RCA from compression. Initial, precarious catheter positioning was achieved with a 3.5-cm left Judkins-curve, 6-F guiding catheter. A 0.014-inch PT Graphics® guidewire (Boston Scientific; Maple Grove, Minn) was then selectively advanced into the ectopic RCA, followed by a balloon catheter and then a 40-MHz IVUS catheter (Atlantis Catheter Company; Galway, Ireland). After selective, coaxial guiding-catheter positioning had been attained, excellent back-up support was acquired in order to advance the Penta 3.0/15-mm stent (Guidant; Temecula, Calif) into the proximal RCA. Preoperatively, the lumen had a minimal area of 5.1 mm2 at baseline and 4.7 mm2 during pharmacologic stress testing, with an elliptical shape (Fig. 1B). Dilation at 12 atm resulted in adequate stent expansion (final diameter, 3.15 mm; luminal area, 9.0 mm2 with a circular shape), as documented by IVUS. In spite of the fact that the more distal RCA had a larger luminal area (13.2 mm2), we stopped the dilation at this stage because of the perceived risk of aortic dissection by overdilation. The patient was treated with clopidogrel, 75 mg/day for 3 months, during which time he was mostly asymptomatic.
At 4 months, while undergoing routine re-evaluation, he reported mild, atypical chest pain (without dyspnea or lightheadedness) but had a normal sestamibi pharmacologic stress-test result. On coronary angiography, his RCA was widely patent, but the IVUS catheter could not be advanced subselectively; aggressive attempts were deemed inadvisable due to the risk of crushing the ostial stent. Since that time, the patient has continued to report mild, atypical chest pain that is unrelated to exertion. Compared with his preoperative state, his condition is clearly improved.
Patient 2
In 2001, a 45-year-old man reported chest pain that was both aching and sharp in character and that had progressed during the year before admission. These episodes had been particularly noticeable during sexual intercourse. He had been treated for hypertension for 15 years. On admission, physical findings were not especially noteworthy, except for mild obesity. A resting electrocardiogram showed nonspecific ST-T changes in the inferior leads. An exercise stress test revealed a reversible, mild, inferolateral perfusion defect. Coronary angiography disclosed a normal left coronary artery with a codominant circumflex pattern. The RCA originated anomalously from the left sinus of Valsalva and had no obstructive disease. The patient was initially treated with verapamil, which did not relieve his chest pain. After having an episode of nausea and near syncope, he was referred to our hospital for a 2nd opinion.
Cardiac catheterization was performed specifically to delineate the origin, course, and functional significance of the anomalous RCA; IVUS (Fig. 2) was performed at baseline and during pharmacologic stress testing with an intravenous dobutamine, atropine, and saline bolus. Initial attempts at selective engagement of the ostium with a 4.0-cm, left Judkins-curve catheter were unsuccessful, but we were able to place a 3.5-cm, left Judkins-curve, 6-F guiding catheter adjacent to the ostium of the anomalous RCA. The ostium was then selectively cannulated with a 0.014-inch, PT Graphics guidewire (Boston Scientific), which was advanced into the distal RCA. An IVUS catheter (40-MHz; Atlantis) was advanced over the wire into the RCA, which was found to be elliptical in cross-section and which coursed inside the aortic media. Systolic lateral compression of the intramural segment worsened during pharmacologic stress testing. A 12-MHz IVUS catheter, positioned in the aortic root, confirmed that the anomalous RCA traversed the aortic root, running tangentially from the left toward the right sinus of Valsalva (Fig. 2A). Because of the patient's persistent symptoms and the evidence that the proximal segment of the anomalous RCA was the likely culprit, he consented to undergo stenting of the anomalous RCA.
Accordingly, a 2.5 × 13.0-mm stent (Tetra, Guidant Corporation; Indianapolis, Ind) was advanced and positioned so as to cover the segment of artery that traversed the aortic root and to reach the ostium. The stent was deployed at 14 atm and was postdilated with an NC Raptor balloon (3.0- × 15-mm at 16 atm) (Cordis Corporation; Miami, Fla). Intravascular ultrasonography showed that the luminal area had improved from 3.7 to 7.5 mm2. More importantly, the configuration of the lumen changed from an ellipse to a circle, without undergoing phasic changes.
After treatment, the patient's symptoms were relieved, but they returned approximately 4 weeks later. An exercise stress test was negative for reversible ischemia. Three months postoperatively, the patient underwent elective repeat angiography and IVUS, which revealed mild restenosis of the proximal anomalous segment, inside a persistently well-expanded stent (minimal luminal diameter, 2.8 mm), and no systolodiastolic variation in luminal diameter. The arterial segment was redilated with a 3.25-mm balloon at 12 atm, achieving a final luminal area of 8 mm2. The atypical chest-pain syndrome was improved but not totally eliminated, even 1 week after this procedure, when the nuclear stress test remained normal.
Discussion
The 2 cases described here were part of a pilot study that our group recently initiated regarding the use of stents for treating ACAOS. All together, 10 ACAOS patients have undergone IVUS studies (under an internal review board protocol) in our catheterization laboratory for the evaluation of symptoms, abnormal test results, or both. In addition to the 2 patients whose cases are described above, there was 1 patient with a left coronary artery originating from the right anterior sinus, who underwent surgical bypass. The other 7 patients were treated medically because their findings, at least initially, were considered mild or benign. Their information was entered into a database designed to study, prospectively, the prognoses of patients with ACAOS.
The mechanism of ischemia in ACAOS, as well as the prognosis for individual forms, is not yet well understood; but extensive clinical experience has been accumulated in the surgical revascularization in these patients. 3 Because the long-term results of CABG are less than ideal 4 (probably due to competitive flow in the presence of only mild proximal stenosis at baseline), alternative surgical procedures have been attempted. 3–7 In ACAOS patients, surgical reimplantation of the ectopic vessel is quite involved and has unpredictable results, chiefly because of difficulty in creating the proximal anastomosis and the possibility of impairing aortic valve function. 3,4 The recently suggested method that involves surgical translocation of the pulmonary artery 8 is an attempt to solve the unlikely problem of an aortopulmonary scissors mechanism. When the intramural segment does not involve the implantation site of the anterior aortic commissure, surgical enlargement of the ostium by opening the intussuscepted segment into the aorta yields promising results. 4–6
In cases of atherosclerotic stenosis, the introduction of coronary stents has accelerated the tendency to substitute catheter angioplasty for bypass surgery. 9 Therefore, it is natural to explore the possibility of using this approach for ACAOS, as well. Several considerations are relevant in judging the value of coronary stents in patients with ACAOS:
1. Anatomy and Pathophysiology.
The anatomy of ACAOS (especially in the most severe cases, which require intervention to prevent sudden death) is quite different from that usually seen in atherosclerotic disease. The anomalous artery's ostium is ectopic, and it is often hard to reach with a guiding catheter designed for the normal coronary anatomy. Even if the ectopic ostium is reachable with the guiding catheter, cannulation and institution of coaxiality, with adequate back-up support, is frequently difficult. Indeed, the ectopic ostium is usually juxtacommissural, and the proximal course of the ectopic artery is tangential to the aortic wall; in contrast, the normal artery is oriented in an orthogonal plane and situated in the middle of the proper sinus of Valsalva. Diagnostic and guiding catheters designed specifically for these coronary anomalies are urgently needed, and our group is evaluating different catheter designs for this purpose.
2. Hypoplasia of the Intramural Segment in ACAOS.
Both the circumference and the area (not only the minimal diameter) of the anomalous vessel's proximal, intramural segment are smaller than those of the more distal “normal” (epicardial) artery. Developmentally, this finding probably signifies not poststenotic dilatation but, rather, the fact that the intramural artery cannot grow normally as the patient ages and as the dependent myocardial territory grows, because the artery is situated within the confines of the aortic media. This feature of ACAOS leads to uncertainty about the ideal degree of stent dilation. Should one aim at simply eliminating the lateral compression (by fully opening the stent, so that its diameter is midway between the minimal and maximal diameters of the ellipsoid segment)? Should one match the maximal diameter of the ellipsoid transverse section (as established by IVUS) or, alternatively, match the distal “reference” diameter? On the one hand, the largest possible initial diameter would seem to offer the best chance of preventing restenosis; yet the simple presence of a stent prevents collapse of the vessel, which seems to be the main pathophysiologic mechanism of restenosis in these patients. The risk of aortic root dissection as a consequence of aggressive coronary dilation needs to be studied.
3. No Intimal Thickening of the Intramural Segment in ACAOS.
At the intramural coronary segment, no adventitia is present, but only the aortic media and the inner elastic lamina. In this unusual environment, studying the effects of a stent (especially on the incidence of restenosis) could be an interesting pathophysiologic experiment. Indeed, some authors suggest that the remodeled, stretched adventitia plays a prominent role in initiating restenosis after angioplasty for atherosclerotic disease. 10 Our initial observations are inadequate to answer this question, but the development of moderate restenosis in patient 2 suggests that the disease process may start at the endothelial level and be activated by platelet deposition. In patient 2, as documented by follow-up IVUS, lateral compression did not crush the stent. With respect to the threat of aortic-wall dissection, overdilation during the 2nd procedure (as in our patient 2) may be safer than overdilation during the initial procedure.
4. Immediate Results.
Stenting of the intramural coronary artery in ACAOS initially eliminated chest pain and dyspnea in both of our patients. The later recurrence of milder, atypical chest pain in both patients was not correlated with a positive nuclear stress test result and may have been a spurious association, as is frequently the case in patients with similar coronary anomalies. More clinical experience with ACAOS is necessary before final conclusions can be reached. In our 2 patients, the restenosis, although mild, was a confounding factor, which made it harder for us to clarify the relationship between the symptoms and stress test abnormality upon presentation, the changes noted on IVUS imaging, and the effects of stenting. Undoubtedly, in many instances ACAOS is identified angiographically when the patient is being studied for atypical (and possibly unrelated) chest pain. 11
5. Late Results.
In routine stent (and balloon) angioplasty, restenosis is a problem mainly during the first 6 to 12 postoperative months. Before stent indications are liberalized, cardiologists should perform close IVUS follow-up in a reasonable number of cases during this vulnerable period, especially when treating a very dominant RCA or the left coronary artery in young individuals with ACAOS. The imminent introduction of drug-eluting stents, which promise to lower the restenosis rate to near zero, 12 may encourage the use of stent-angioplasty in ACAOS patients.
As IVUS shows, in neither muscular bridging nor ACAOS is an atherosclerotic process present; in this environment, a patient would usually be considered at low risk for in-stent restenosis. Nevertheless, the poor results obtained after stenting of muscular bridges suggest that restenosis may be more frequent in the presence of phasic compressive forces, which surely persist also in ACAOS patients, even after stent implantation.
For the time being, we advise that CABG be considered the preferred (although unproven) method of revascularization in ACAOS patients and that stent-angioplasty be done only in selected cases, at selected institutions, within the constraints of a prospective study approved by an institutional review board. The greater current challenge is to decide which cases require interventional treatment at all. This ability depends on increased understanding of the pathophysiologic mechanisms involved and the specific prognosis of each form of ACAOS.
Footnotes
Address for reprints: Paolo Angelini, MD, P.O. Box 20206, Houston, TX 77225-0206
This paper has its basis in a presentation made at the symposium Coronary Artery Anomalies: Morphogenesis, Morphology, Pathophysiology, and Clinical Correlations, held on 28 Feb.–1 March 2002, at the Texas Heart® Institute, Houston, Texas.
References
- 1.Akilli A, Kultursay H, Akin M, Payzin S, Can L, Altintig A, Turkoglu C. Stenting of myocardial bridging. J Invasive Cardiol 1997;9:529–33. [PubMed]
- 2.Klues HG, Schwartz ER, vom Dahl J, Reffelmann T, Minartz J, Hanrath P. Intracoronary stent implantation—a new therapeutical approach in highly symptomatic patients with myocardial bridging [abstract]. J Am Coll Cardiol 1997;29(Suppl):220A.
- 3.Fernandes ED, Kadivar H, Hallman GL, Reul GJ, Ott DA, Cooley DA. Congenital malformations of the coronary arteries: the Texas Heart Institute experience. Ann Thorac Surg 1992;54:732–40. [DOI] [PubMed]
- 4.Rinaldi RG, Carballido J, Giles R, Del Toro E, Porro R. Right coronary artery with anomalous origin and slit ostium. Ann Thorac Surg 1994;58:829–32. [DOI] [PubMed]
- 5.Mustafa I, Gula G, Radley-Smith R, Durrer S, Yacoub M. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg 1981;82:297–300. [PubMed]
- 6.Nelson-Piercy C, Rickards AF, Yacoub MH. Aberrant origin of the right coronary artery as a potential cause of sudden death: successful anatomical correction. Br Heart J 1990;64:208–10. [DOI] [PMC free article] [PubMed]
- 7.Di Lello F, Mnuk JF, Flemma RJ, Mullen DC. Successful coronary reimplantation for anomalous origin of the right coronary artery from the left sinus of Valsalva. J Thorac Cardiovasc Surg 1991;102:455–6. [PubMed]
- 8.Rodefeld MD, Culbertson CB, Rosenfeld HM, Hanley FL, Thompson LD. Pulmonary artery translocation: a surgical option for complex anomalous coronary artery anatomy. Ann Thorac Surg 2001;72:2150–2. [DOI] [PubMed]
- 9.Angelini P, Vaughn WK, Zaqqa M, Wilson JM, Fish RD. Impact of the “stent-when-feasible” policy on in-hospital and 6-month success and complication rates after coronary angioplasty: single-center experience with 17,956 revascularization procedures (1993–1997). Tex Heart Inst J 2000;27:337–45. [PMC free article] [PubMed]
- 10.Moreno PR, Purushothaman KR, Fuster V, O'Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation 2002;105:2504–11. [DOI] [PubMed]
- 11.Angelini P, Villason S, Chan AV Jr, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 27–150.
- 12.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773–80. [DOI] [PubMed]


