Abstract
Background
The possibility for commercial mining of deep-sea manganese nodules is currently under exploration in the abyssal Clarion-Clipperton Fracture Zone. Nematodes have potential for biomonitoring of the impact of commercial activity but the natural biodiversity is unknown. We investigate the feasibility of nematodes as biomonitoring organisms and give information about their natural biodiversity.
Results
The taxonomic composition (at family to genus level) of the nematode fauna in the abyssal Pacific is similar, but not identical to, the North Atlantic. Given the immature state of marine nematode taxonomy, it is not possible to comment on the commonality or otherwise of species between oceans. The between basin differences do not appear to be directly linked to current ecological factors. The abyssal Pacific region (including the Fracture Zone) could be divided into two biodiversity subregions that conform to variations in the linked factors of flux to the benthos and of sedimentary characteristics. Richer biodiversity is associated with areas of known phytodetritus input and higher organic-carbon flux. Despite high reported sample diversity, estimated regional diversity is less than 400 species.
Conclusion
The estimated regional diversity of the CCFZ is a tractable figure for biomonitoring of commercial activities in this region using marine nematodes, despite the immature taxonomy (i.e. most marine species have not been described) of the group. However, nematode ecology is in dire need of further study.
Background
The area where there is the most interest in commercial deep-sea mining lies within the Clarion-Clipperton Fracture Zone (CCFZ) in the abyssal Pacific west of Central America. Three zones, in particular, are of especial commercial importance, 8° 27'N, 150° 47' W; 11° 42' N, 138° 24' W; and 15° 00' N, 126° 00' W) Fig. 1. The International Sea Authority is required to develop regulations for mining to take place, including regulations for environmental monitoring. Such monitoring will involve surveys of the benthic fauna.
Figure 1.
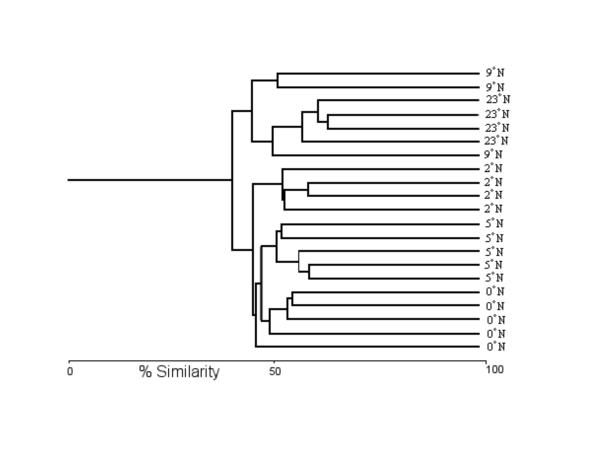
Cluster analysis of samples on fourth root transformed data using the Bray-Curtis index of similarity and single linkage clustering. The samples are labelled according to the station from which they were collected.
The most abundant metazoan group in deep-sea sediments is the marine Nematoda [1]. Deep-sea nematode assemblages are also highly diverse and species rich at local (point diversity and alpha diversity sensu Gray [2]) scales [3] but estimates of regional and global species richness remain speculative [4,5]. The utility of nematodes as monitoring organisms has been demonstrated in shallow water studies where they are now used routinely in this role [6,7].
There are, however, difficulties in utilising deep-sea nematodes as monitoring organisms. The taxonomy of the group is immature; few species have been described and geographic coverage is uneven [5]. There are few nematode taxonomic studies of the deep Pacific. An as yet unpublished taxonomic study of Pacific abyssal nematodes of the Peru-Beckens region has been undertaken as part of the DISCOL project [8]. 358 nematode specimens taken from the ECHO I site within the CCFZ have been taxonomically studied and 148 putative species discovered [9]. It should be noted that although most deep-sea nematode species have never been described, they can nevertheless be sorted into 'morphotypes' (morphological operational taxonomic units) which are often considered to be equivalent to species for the purpose of biodiversity studies.
The study of deep-sea nematode biodiversity is in an early stage. There have been no published biodiversity studies at the species level on Pacific abyssal nematode fauna, and only one on Pacific bathyal fauna [10]. Most studies have taken place in the North Atlantic and it is not clear how similar nematode assemblages in the Atlantic are to those in the mining regions in the Indo-Pacific [11]. Furthermore, studies have usually been made at individual stations rather than over larger areas, so regional diversity is unknown but is assumed to be high because local diversity is high [4,5].
Deep-sea mining is likely to cause physical disturbance and smothering [12]. Natural physical and smothering disturbance, such as that resulting from turbidites and benthic storms, has been associated with a small but statistically significant reduction in North Atlantic deep-sea nematode diversity [13]. However, it was noteworthy that the effect of disturbance could be prolonged (e.g., lasting for decades to centuries), possibly through changes in sediment composition. The robustness of deep-sea nematode biodiversity to physical disturbance mirrors the conclusions of studies on dredging impacts in shallow water [14]. Such properties offer support for nematodes as monitoring organisms in that nematode assemblages are present for analysis at all levels of impact but display detectable changes in composition. These properties, coupled with high abundance and high sample/local diversity are ideal characteristics of a monitoring taxon but if regional diversity is high, then this, coupled with immature taxonomy, could present significant difficulties for routine monitoring. This is the first data set that covers a sufficient area to allow a credible estimation of regional nematode species richness in the abyss, or indeed anywhere apart from the north-west coast of Europe.
This paper has two objectives. The first is to augment previous papers on nematode abundance, biomass and species richness gradients [15,16] by making a preliminary investigation of the nematode biodiversity from the region of the CCFZ. In addition, the taxonomic composition of the region will be compared to abyssal sites previously investigated from the North Atlantic in order to evaluate between basin patterns in nematode biodiversity. The second objective of the study is to estimate deep-sea nematode regional diversity for the first time so as to establish whether the total number of species likely to be encountered is tractable for routine biomonitoring of commercial activities.
Results
In the central Pacific, twenty-five families were recorded from the equatorial station and all were found at least at one other station. The highest number of families was recorded from 2°N because of a family unique to that station, the Tripyloididae. Subsequently, the number of families decreased with increasing latitude to a minimum of 21 families at 9 and 23°N. Dominant families (>10% of total population in sample) recorded along the stations were divided into two communities (Table 1). At stations from 0–5°N, the dominant families were the Monhysteridae, the Chromadoridae and the Microlaimidae. At stations from 9–23°N, the Monhysteridae and the Chromadoridae were also dominant families, but the Xyalidae replaced the Microlaimidae. The Oxystominidae were a subdominant family (>5%) at all stations and the Microlaimidae were subdominant at stations at 9 and 23°N. At the 0–5°N stations, other subdominant families varied from station to station (Table 1).
Table 1.
Mean percentage of dominant (>10%) and subdominant (>5%) nematode families present in 0–1 cm sediment horizon from the stations of the Clarion Clipperton Fracture Zone.
| Equator | 2°N | 5°N | 9°N | 23°N |
| Monhysteridae 32% | Monhysteridae 28% | Monhysteridae 30% | Monhysteridae 36% | Monhysteridae 33% |
| Chromadoridae 10% | Microlaimidae 21% | Microlaimidae 24% | Xyalidae 13% | Xyalidae 18% |
| Microlaimidae 8% | Chromadoridae 10% | Chromadoridae 11% | Chromadoridae 9% | Chromadoridae 10% |
| Leptolaimidae 7% | Xyalidae 7% | Oxystominidae 5% | Oxystominidae 6% | Microlaimidae 9% |
| Xyalidae 6% | Desmoscolecidae 6% | Microlaimidae 6% | Oxystominidae 6% | |
| Aegialoalaimidae 6% | ||||
| Meyliidae 5% |
The families are listed in order of dominance per station.
At the genus level, Thalassomonhystera dominated at all Pacific stations, varying in abundance from 18% at the equatorial station, to 33% at 9 and 23°N (Table 2). Acantholaimus also occurred with a high frequency at all stations. Other dominant to subdominant genera (>5% and >1% respectively) included Halalaimus, Microlaimus, Molgolaimus, Leptolaimus and Aponema. The patterns of dominant and subdominant genera varied from station to station, but followed the general replacement of the Microlaimidae by the Xyalidae, between 5 and 9°N.
Table 2.
Mean percentage of dominant (>5%) and subdominant (>1%) nematode genera present in the 0–1 cm sediment horizon from the stations from the Clarion-Clipperton Fracture Zone.
| Equator | 2°N | 5°N | 9°N | 23°N |
| Thalassomonhystera 18.3% | Thalassomonhystera 22.9% | Thalassomonhystera 22.8% | Thalassomonhystera 32.5% | Thalassomonhystera 32.1% |
| Acantholaimus 9.6% | Microlaimus 7.1% | Molgolaimus 8.5% | Acantholaimus 5.9% | Acantholaimus 7.3% |
| Quadricoma 5.7% | Molgolaimus 6.8% | Acantholaimus 7.6% | Halalaimus 5.5% | Linhystera 6.5% |
| Leptolaimus 5.5% | Acantholaimus 5.7% | Microlaimus 5.4% | Microlaimus 4.1% | Halalaimus 5.3% |
| Microlaimus 5.2% | Desmoscolex 5.1% | Aponema 4.6% | Leptolaimus 4.1% | Theristus 4.0% |
| Diplopeltoides 5.2% | Aponema 4.8% | Halalaimus 3.7% | Linhystera 2.6% | Aponema 3.5% |
| Halalaimus 3.5% | Diplopeltoides 3.1% | Quadricoma 2.8% | Manganonema 2.6% | Microlaimus 3.5% |
| Desmodora 3.2% | Halalaimus 2.8% | Desmodora 2.6% | Diplopeltula 2.6% | Leptolaimus 3.5% |
| Diplopeltula 3.0% | Quadricoma 2.5% | Leptolaimus 2.4% | Aegialoalaimus 2.2% | Aegialoalaimus 2.5% |
| Manganonema 2.7% | Ascolaimus 2.3% | Diplopeltoides 2.4% | Quadricoma 2.2% | Daptonema 2.3% |
| Thalassomonhystera 2.5% | Syringolaimus 2.0% | Ascolaimus 2.2% | Prochromadorella 1.9% | Manganonema 2.0% |
| Desmoscolex 2.2% | Nox 2.0% | Manganonema 2.0% | Desmodora 1.9% | Diplopeltoides 1.8% |
| Actinonema 2.0% | Campylaimus 2.0% | Syringolaimus 1.7% | Diplopeltoides 1.9% | Desmoscolex 1.8% |
| Camacolaimus 1.7% | Actinonema 1.4% | Tubolaimoides 1.7% | Cobbia 1.9% | Neochromadora 1.3% |
| Aponema 1.7% | Desmodora 1.4% | Cervonema 1.3% | Syringolaimus 1.5% | Pselionema 1.3% |
| Linhystera 1.2% | Linhystera 1.4% | Acantholaimus 1.1% | Molgolaimus 1.5% | Quadricoma 1.3% |
| Molgolaimus 1.0% | Manganonema 1.4% | Desmoscolex 1.1% | Nox 1.1% | Campylaimus 1.3% |
| Eudraconema 1.0% | Rhips 1.1% | Linhystera 1.1% | Desmoscolex 1.1% | Actinonema 1.0% |
| Campylaimus 1.0% | Eudraconema 1.1% | Amphimonhystera 1.1% | Desmodora 1.0% | |
| Pselionema 1.1% | Molgolaimus 1.0% | |||
| Metadesmolaimus 1.0% | ||||
| Southerniella 1.0% |
The genera are listed in order of dominance at each station.
A total of 3,321 individuals were sorted into 200 species, All but seven species could be assigned to known genera. The domination of Thalassomonhystera was caused by more than one species (Table 3). Three species of this genus were dominant across the stations (with one exception) and an additional species was dominant in the phytodetritus-influenced stations at 0°N, 2°N, and 5°N. Many genera were represented by more than one species (Table 4). Five genera included 22% of the species, and ten genera contained 33% of the species.
Table 3.
Mean percentage of dominant (>1%) nematode species present in 0–1 cm sediment horizon at the stations from the Clarion Clipperton Fracture Zone.
| Equator | 2°N | 5°N | 9°N | 23°N | |
| Thalassomonhystera sp. .A | 5.4% | 5.3% | 10.0% | 7.3% | 13.3% |
| Thalassomonhystera sp. .B | 6.0% | 2.3% | 3.8% | 7.0% | 5.0% |
| Thalassomonhystera sp. G | 0% | 3.3% | 1.3% | 8.3% | 9.5% |
| Acantholaimus sp. .A | 2.6% | 3.0% | 2.8% | 4.7% | 2.8% |
| Molgolaimus sp. B | 1.0% | 4.8% | 6.6% | 2.0% | 2.0% |
| Aponema sp. A | 1.4% | 4.25% | 2.4% | 0% | 4.7% |
| Microlaimus sp. .B | 1.8% | 3.8% | 1.0% | 5.0% | 3.5% |
| Thalassomonhystera sp. D | 2.5% | 4.8% | 2.8% | 0% | 0% |
| Diplopeltoides sp B | 3.0% | 2.5% | 2.7% | 0.7% | 1.7% |
| Acantholaimus sp. B | 2.6% | 0% | 3.8% | 0% | 0% |
| Microlaimus sp. A | 4.0% | 5.0% | 2.0% | 0% | 0% |
| Linhystera sp. A | 1.25% | 0% | 1.0% | 3.5% | 6.5% |
| Quadricoma sp. A | 2.3% | 2.3% | 2.5% | 2.5% | 1.0% |
| Quadricoma sp. C | 2.4% | 0% | 1.5% | 1.0% | 2.0% |
| Halalaimus sp. D | 1.2% | 2.0% | 4.0% | 1.5% | 2.0% |
| Desmoscolex sp. .A | 1.0% | 2.8% | 1.7% | 1.0% | 2.0% |
| Manganonema sp. A | 2.8% | 2.5% | 1.8% | 1.0% | 2.0% |
| Leptolaimus sp. B | 3.7% | 1.0% | 2.5% | 1.5% | 3.3% |
| Acantholaimus sp. C | 1.0% | 2.3% | 0% | 0% | 4.3% |
The species are listed in order of dominance from the whole data set.
Table 4.
Genera with three or more species (in brackets) recorded from various locations; (i) Clarion-Clipperton Fracture Zone (CCFZ, this study), (ii) Porcupine Abyssal Plain 1989 study (PAP) [45], (iii) San Diego Trough (SDT) [10], (iv) Rockall Trough 535 metre station (RT 535) [10], (v) Irish Sea (IS, Ferrero unpublished data) [3], and (vi) Clyde Inland Sea low water spring tide (CIS) [6].
| CCFZ | PAP 1989 | SDT | RT535 | IS | CIS |
| Thalassomonhystera 12 | Acantholaimus 8 | Daptonema 8 | Thalassomonhystera 7 | Daptonema 8 | Daptonema 8 |
| Halalaimus, 11 | Halalaimus 8 | Halalaimus 7 | Halalaimus 5 | Microlaimus 7 | Theristus 8 |
| Acantholaimus 7 | Daptonema 4 | Microlaimus 6 | Daptonema 3 | Halalaimus 5 | Microlaimus 5 |
| Desmodora 7 | Diplopeltula 4 | Ceramonema 4 | Microlaimus 3 | Sabatieria 5 | Neochromadora 4 |
| Leptolaimus 7 | Thalassomonhystera 4 | Desmodora 4 | Metadesmolaimus 3 | Paracanthonchus 4 | Cyartonema 3 |
| Diplopeltoides 5 | Microlaimus 3 | Leptolaimus 3 | Tricoma 3 | Pomponema 4 | Dichromadora 3 |
| Syringolaimus 5 | Oxystomina 3 | Molgolaimus 3 | Prochromadorella 4 | Metadesmolaimus 3 | |
| Cervonema 4 | Quadricoma 3 | Prochromadorella 3 | Theristus 4 | Pomponema 3 | |
| Desmoscolex 4 | Ceramonema 3 | ||||
| Diplopeltula 4 | Desmodora 3 | ||||
| Linhystera 4 | Richtersia 3 | ||||
| Oxystomina 4 | Tricoma 3 | ||||
| Ascolaimus 3 | |||||
| Campylaimus 3 | |||||
| Campylaimus 3 | |||||
| Cobbia 3 | |||||
| Litinium 3 | |||||
| Marylynnia 3 | |||||
| Microlaimus 3 | |||||
| Molgolaimus 3 |
Note that the Rockall data are based on only 3 samples so may underestimate numbers of species per genus. All other data are based on at least six samples.
Similar dendrograms were obtained from both the untransformed and transformed data. This is not unexpected because the samples showed a high equitability and the percentage dominance of the most abundant species was low. Therefore, only the dendrogram for the transformed data is depicted here to save space, transformed data being commonly employed in this type of analysis (Fig. 1).
The first split in the dendrogram at approximately 40% divided the samples into two regions, the southern phytodetritus-influenced region from 0°N to 5°N and the northerly region from 9°N to 23°N. The northerly two stations did not separate, although the 23°N HOT station formed a cluster at the 55% level. The southern samples split into the three stations, with one exception, but did not cluster according to geography. Instead, the 0°N station was most similar to the 5°N station. The topography of the dendrogram was 'flat', with samples being only slightly more similar to each other than either station or region splits, indicating high variability between the samples.
Neither the species accumulation per number of individual curves for the whole region nor either of the two subregions came to asymptote indicating that the sampling had been insufficient to discover regional diversity (Fig. 2). The curves do indicate beta diversity, the differentiation diversity, between samples (i.e. the rate of change of species between samples), across the CCFZ and the two subregions. The northern non-phytodetritus subregion is noticeably less beta-diverse.
Figure 2.
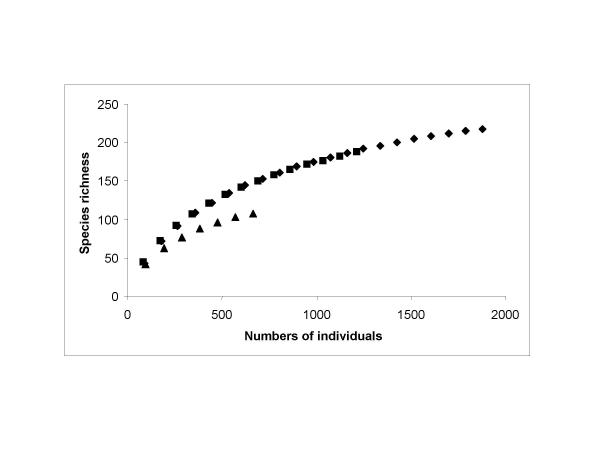
Plots of the species accumulation curves against numbers of individuals for the whole data set (diamonds), the southern phytodetritus-influenced samples (squares) and the northern samples (triangles).
A sigmoidal growth model best fitted the species accumulation curves for the whole data set and the southern subregion, stations 0°N, 2°N, & 5°N. A Hoerl model best fitted the northern subregion data (r = 0.99998) but the sigmoidal model was almost as good a fit so was also used for estimating species richness in this subregion also for consistency. The estimated maximum number of species for each data set is shown in Table 5.
Table 5.
Estimations of regional species richness using an extrapolation of an MMF model (y = (ab + cxd)/(b + xd) of a plot of species accumulation per number of individuals at near asymptote at 108 individuals. The r is a measure of the 'fit' of the model to the data.
| Data | Sigmoidal Growth Model Parameters | r | Estimated Species Richness |
| All Samples | a = -12.1378 b = 154.4177 c = 360.9705 d = 0.7323 | 0.99999 | 361 |
| Southern Samples (O°N, 2°N & 5°N) | a = -16.7132 b = 109.1754 c = 385.8644 d = 0.6642 | 0.99997 | 386 |
| Northern Samples (9°N & 23°N) | a = 12.6610 b = 485.3579 c = 158.5034 d = 1.0503 | 0.99996 | 159 |
The results for non-parametric statistical estimators are shown in Table 6. They tend to give slightly lower estimates for species richness than the sigmoidal growth model suggesting that the curve extrapolation is not underestimating regional species richness across the transect.
Table 6.
Non-parametric statistical estimators of species richness from Colwell's EstimateS program [47].
| Data | ICE | Chao 2 | Jackknife 2 |
| All Samples | 281 | 280 | 313 |
| Southern Samples (O°N, 2°N & 5°N) | 264 | 260 | 287 |
| Northern Samples (9°N & 23°N) | 139 | 129 | 147 |
Discussion
The presence of a number of closely related and apparently morphologically similar species in the same local area has been noted as a characteristic of marine nematode fauna in shallow water studies [17]. However, this phenomenon is particularly exaggerated in the CCFZ (Table 4).
At the family level, there was a high degree of similarity in nematode community composition in these samples with only a small variation between the southern three phytodetritus-influenced and the northern two non-phytodetritus influenced regions. The non-phytodetritus influenced northern areas showed an increase in the percentage of Xyalidae compared to the southern three stations.
The dominant families recorded here are broadly similar to those from other abyssal data. Where there are differences, they seem to be associated more with biogeography than with the environment, see Table 7. For example, in the Pacific Monhysteridae dominate the stations, irrespective of the apparent influence of phytodetritus. In the North Atlantic, Chromadoridae dominate despite each station being influenced differently by a number of factors including phytodetritus flux [11], turbidites and benthic storms [13] all of which have been shown to be associated with variation in deep-sea nematode diversity.
Table 7.
Dominant nematode families present in a number of abyssal stations. CCFZ = Clarion-Clipperton Fracture Zone (this study), PAP = Porcupine Abyssal Plain [28], MAP = Madeira Abyssal Plain [28], HEBBLE = Scotian Rise [48], HAP = Hatteras Abyssal Plain [27].
| Station | Dominant Family | Subdominant Families | Ecological Notes | Geographic Location |
| CCFZ 0°N | Monhysteridae | Chromadoridae Microlaimidae | Phytodetritus influenced | Central Pacific |
| CCFZ 2°N | Monhysteridae | Microlaimidae Chromadoridae | Phytodetritus influenced | Central Pacific |
| CCFZ 5°N | Monhysteridae | Microlaimidae Chromadoridae | Phytodetritus influenced | Central Pacific |
| CCFZ 9°N | Monhysteridae | Xyalidae Chromadoridae | Central Pacific | |
| CCFZ 23°N | Monhysteridae | Xyalidae Chromadoridae | Central Pacific | |
| HEBBLE | Chromadoridae | Xyalidae Oxystominidae | Benthic Storms | Northwest North Atlantic |
| PAP 1989 | Chromadoridae | Xyalidae Monhysteridae | Phytodetritus influenced | Northeast North Atlantic |
| PAP 1991 | Chromadoridae | Monhysteridae Xyalidae | Phytodetritus influenced | Northeast North Atlantic |
| MAP | Chromadoridae | Xyalidae Monhysteridae | Turbidite | Northeast North Atlantic |
| HAP | Chromadoridae | Xyalidae Oxystominidae | Southwest North Atlantic |
Ecological factors that might influence nematode communities are listed, where they are known.
The association with biogeography rather than with local ecological factors implies that the differences in the proportions of the communities taken up by the dominant families are the product of historical processes rather than of current ecology. This is unexpected because the dominant families from each ocean basin are usually considered to be in different trophic groups. Deep-sea Monhysteridae tend to be small mouthed organisms, such as Thalassomonhystera, that presumably feed on bacteria or other small particles. In contrast, deep-sea Chromadoridae, such as Acantholaimus or Prochromadorella, tend to have buccal armature that is capable of piercing or scraping. The feeding preference of deep-sea members of this family is unknown but at least some of their shallow water relatives are associated with diatom feeding, the teeth being used to pierce or break the frustrule [18-23]. It is worth emphasising that observations on marine nematode feeding have been limited to a handful of opportunistic coastal species that can be cultured, so their feeding ecology may not be typical of offshore family members. Also, deep-sea Chromadoridae are dominated by the genus Acantholaimus (see below), which is rare in shallow water, while shallow-water Chromadoridae are typically dominated by Dichromadora and Neochromadora which are less common in the deep sea. Nevertheless, this apparent trophic variation between the ocean basins suggests that either there are errors in our comprehension of nematode trophic groups or that there have been powerful historical forces affecting the distribution of these groups that has left an imprint that is still detectable.
The genera discovered at the CCFZ were mostly familiar to science and the common genera have also been found commonly in North Atlantic abyssal fauna, notably Thalassomonhystera, Halalaimus, and Acantholaimus (Table 8) and we may conjecture that these genera are characteristic of abyssal nematode communities, although Thalassomonhystera is more important in the Pacific data and Acantholaimus in the Atlantic. Although phytodetritus and physical disturbance have been shown to influence nematode species richness [11,13,15], there is little evidence of characteristic dominant genera associated with the presence or absence of these factors.
Table 8.
Mean percentage of dominant (>5%) and subdominant (>1%) nematode genera present in 0–1 cm sediment horizon from the stations from the northeast North Atlantic [28].
| PAP 1989 | PAP 1991 | MAP |
| Acantholaimus 15.2% | Thalassomonhystera 17.3% | Acantholaimus 24.0% |
| Thalassomonhystera 15.0% | Acantholaimus 12.0% | Thalassomonhystera 11.7% |
| Halalaimus 9.0% | Halalaimus 7.0% | Daptonema 11.5 |
| Elzalia 6.4% | Prochromadorella 6.4% | Halalaimus 11.1 |
| Chromadorina 5.0% | Enchonema 6.3% | Metadesmolaimus 3.5 |
| Quadricoma 4.7% | Daptonema 5.2% | Campylaimus 3.5 |
| Daptonema 4.4% | Pareudesmoscolex 4.3% | Amphimonhystrella 3.0 |
| Campylaimus 4.1% | Desmoscolex 3.9% | Pomponema 1.6 |
| Microlaimus 3.9% | Molgolaimus 3.8% | Quadricoma 1.6 |
| Desmoscolex 2.7% | Campylaimus 3.8% | Chromadora 1.4 |
| Actinonema 2.0% | Quadricoma 3.0% | Cyartonema 1.4 |
| Aegialoalaimus 1.9% | Elzalia 2.6% | Desmoscolex 1.4 |
| Prochromadora 1.8% | Diplopeltua/ Diplopeltoides 2.6% | Actinonema 1.2 |
| Linhystera 1.6% | Diplolaimella/ Diplolaimelloides 1.7% | Innocuonema 1.2 |
| Metadesmolaimus 1.5% | Pareudesmoscolex 1.6% | Dolichoalaimus 1.1 |
| Diplopeltula 1.4% | Manganonema 1.6% | Syringolaimus 1.1 |
| Scaptrella 1.3% | Karkinochromadora 1.3% | Diplopeltoides 1.1 |
| Pierrickia 1.3% | Aegialoalaimus 1.1% | |
| Amphimonhystrella 1.0% | Amphimonhystrella 1.1% |
The genera are listed in order of dominance per station. PAP = Porcupine Abyssal plain, MAP = Madeira Abyssal Plain.
There was no evidence that the type of core sampler used had influenced the analysis, clusters did not group according to core sampler employed. The low similarity between samples from the same station demonstrates a high degree of variability between samples. The stations split weakly into two regions, divided between the southern three stations and the northern two. This was not a simple question of geographical distance as the distance is greater between 9°N and 23°N than between 5°N to 9°N, where the putative ecotone occurred. This division is associated both with the presence or absence of phytodetritus and the concomitant change in sediment structure. Both factors have been previously associated with deep-sea nematode species distributions [24-29].
It is noteworthy that the high 'point' (i.e. sample) diversity recorded for deep-sea systems is not reflected in the regional species-richness estimates. The estimated regional species diversity in the CCFZ is not especially high and is tractable for biodiversity analysis both for monitoring and scientific research. The CCFZ is an open system so the rarer species in the assemblage are likely to be somewhat different with each programme of work, changing with space and time. It should also be pointed out that this estimate is based on identifications using only light microscopy and does not incorporate the additional taxonomic resolution that might be determined using ultrastructure or molecular analysis. It is quite conceivable that sibling species remain undetected by light microscopy identification [30].
A higher estimated species richness was found in the southern phytodetritus-influenced stations than in the northern stations, reflecting the point (sample) species richness and diversity analysis [16]. However the fit of the model to the northern data was less good so there may be increased error in the calculation for this region. It is suspicious that a Hoerl model (which lacks a maxima) best fitted this data suggesting that the region may have been woefully under-sampled for estimating regional diversity.
Conclusions
The higher taxa in the region of the CCFZ are typical of the better known North Atlantic abyss but the relative abundance of any particular family or genus is different between the oceans, possibly because of historical rather than ecological processes. So extant nematode biodiversity data from North Atlantic abyssal depths may not be entirely representative of baseline conditions in the Pacific even where the local environmental factors are similar. The region spanning from the equator to 23°N can be further sub-divided into subregions based on nematode biodiversity differences that are associated with differences in the sediment and in the organic flux. The low estimated regional diversity is a tractable figure for the biomonitoring of commercial activities in this region using marine nematodes despite the immature taxonomy of the group. However, a lack of natural history data will limit the ecological inferences that could be drawn from changes to nematode community structure.
Methods
The study area is shown in Fig. 3. Sediment samples were collected at four "EqPac" sites along a latitudinal gradient of phytodetrital deposition and organic-carbon flux from 0 to 9°N at 140°W in the central equatorial Pacific, as part of the US Joint Global Ocean Flux Study (JGOFS). A visible input of phytodetritus has been reported at the stations at 0, 2 and 5°N on the 140°W transect [31]. The presence of measurable quantities of chlorophyll a and excess 234Thorium (tracers with degradation time-scales of less than one hundred days, phytoplankton with intact chloroplasts, and high respiration rates of associated microbial populations, implied that this material was recently settled and undegraded [31-33]. In contrast, at 9°N, the surface detritus appeared to be much more refractory in nature. Overall, the annual abyssal, particulate organic carbon (POC) flux exhibits a four-fold increase between 9°N and the equator [34,35] giving 'large latitudinal gradients in biogenic particle flux to the abyssal seafloor' [36].
Figure 3.
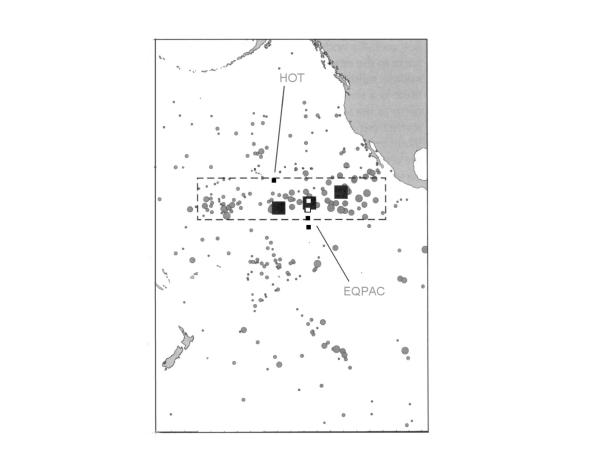
The Clipperton Clarion Fracture Zone in the central eastern Pacific showing the area where there is interest in deep-sea mining. The small squares show the stations from which samples were taken for this study.
A site at 23°N, 158°W, beneath the Hawaii Ocean Time Series (HOT) site, was also considered a non-phytodetritus site, as only a thin veneer of phytodetrital material was recorded from the seafloor at this site and on only one occasion. The deep carbon flux at 23°N was similar to the 9°N site [37].
All five stations were located on predominately flat, sediment-covered abyssal plain. Earlier studies indicated that they were positioned along a gradient of overlying primary productivity, POC flux and sediment accumulation rate [38]. As far as is known, all other environmental variables were approximately constant. Sampling locations, collection dates and water depths are listed in Table 9, Fig. 3[15]. The maximum distance between the stations is 3,195 km, or 2,523 km of latitude, which should be adequate to estimate regional diversity.
Table 9.
Sample locations, water depths, collecting date and device.
| Sample | Location | Water Depth (m) | Collecting Date | Collecting Device |
| BC4 | 00°06.00'N 139°43.90'W | 4328 | 15/Nov/92 | Box Corer |
| BC6 | 00°06.62'N 139°43.96'W | 4305 | 16/Nov/92 | Box Corer |
| BC7 | 00°06.40'N 139°44.10'W | 4307 | 18/Nov/92 | Box Corer |
| BC8 | 00°06.98'N 139°43.94'W | 4301 | 19/Nov/92 | Box Corer |
| MC15 | 00°06.57'N 139°43.42'W | 4304 | 19/Nov/92 | Multiple Corer |
| BC9 | 02°03.94'N 140°08.94'W | 4409 | 20/Nov/92 | Box Corer |
| BC10 | 02°04.00'N 140°07.90'W | 4414 | 21/Nov/92 | Box Corer |
| BC11 | 02°03.96'N 140°08.06'W | 4409 | 22/Nov/92 | Box Corer |
| BC12 | 02°03.80'N 140°07.90'W | 4410 | 23/Nov/92 | Box Corer |
| BC15 | 05°05.00'N 139°39.00'W | 4447 | 27/Nov/92 | Box Corer |
| BC16 | 05°04.42'N 139°38.90'W | 4446 | 28/Nov/92 | Box Corer |
| BC17 | 05°04.80'N 139°38.50'W | 4424 | 29/Nov/92 | Box Corer |
| BC18 | 05°04.20'N 139°38.40'W | 4320 | 30/Nov/92 | Box Corer |
| MC26 | 05°04.30'N 139°38.30'W | 4418 | 30/Nov/92 | Multiple Corer |
| BC19 | 08°55.08'N 139°52.20'W | 4986 | 3/Dec/92 | Box Corer |
| BC20 | 08°56.04'N 139°51.55'W | 4994 | 4/Dec/92 | Box Corer |
| BC22 | 08°55.80'N 139°52.30'W | 4991 | 6/Dec/92 | Box Corer |
| MC1 | 22°54.69'N 157°49.74'W | 4880 | 29/Jul/92 | Multiple Corer |
| MC2 | 22°54.95'N 157°49.93'W | 4871 | 29/Jul/92 | Multiple Corer |
| MC4 | 22°54.74'N 157°50.21'W | 4880 | 31/Jul/92 | Multiple Corer |
| MC6 | 22°54.64'N 157°49.86'W | 4884 | 1/8/92 | Multiple Corer |
At the southern stations, 0°N, 2°N and 5°N, the sediment consisted of predominantly calcareous foraminiferal muds At the two northern stations, 9°N and 23°N, the sediment consisted of fine-grained clays which more uniform in size [32,38]. Manganese nodules were present only at the 9°N station. The sediment at the three southern stations had 234Thorium bioturbation rates that were greater than at 9°N (mean Db values of 9.07 and <0.63) indicating a greater rate of sediment mixing [32].
Previous work has suggested that the stations could be analysed either as a transect of declining productivity or as two regions, a southern region that experienced noticeable phytodetritus input and a northern region that did not [13].
Samples were obtained using either a multiple-corer with 10-cm diameter tubes or a USNEL-type box-corer divided in situ into 10 × 10 cm subcores [39-41]. Box-cores can be inefficient collectors of meiofauna [42]. Analysis of these samples in earlier studies did not suggest sampler bias due to the two collecting methods was a problem for abundance[15] or species richness [16].
Following sampler recovery, cores were sliced at one centimetre vertical intervals and transferred to buffered formaldehyde, diluted to 4% v/v with seawater. A single multiple-core tube or box-core subcore was used per deployment. Overlying top water was combined with the 0–1 cm sediment layer. Nematodes were extracted using a modified Ludox-TM§ flotation method on a 45 μm sieve. All nematodes extracted from the sediments were mounted in anhydrous glycerine and are deposited in The Natural History Museum, London. Approximately 100 individuals were selected at random from the 0–1 cm sediment horizon for identification from each sample as there were too many nematodes recovered to identify them all [15]. This approach should help offset possible bias caused by inefficient collection in box cores, assuming such bias to be random with respect to species identity. Individuals were identified to genus level and sorted into morphotypes, which were considered to be identical to species, using the pictorial key to world genera developed by Platt and Warwick and also the wider taxonomic literature [43].
The nematode community composition was compared at the species level, using cluster analysis based upon the Bray-Curtis index of similarity and single linkage [44]. This is a hierarchical agglomerative clustering method [45]. The analysis was repeated after fourth root transformation of the data, reducing the influence of the dominant species. The BDPro program was used for cluster analysis [46].
Regional species richness was estimated by species per sample accumulation curves plotted against abundance, with order of sample input randomised 50 times to remove distortions due to the stations lying along a productivity gradient; productivity is known to affect deep-sea nematode diversity [5,16]. Colwell's EstimateS program was employed for this purpose.
Models were fitted to the resulting curves using non-linear regression in the shareware CurveExpert program, Table 5. The sigmoidal growth model (y = (ab + cxd)/(b + xd), gave the best fit to the total data and the southern region. The Hoerl model best fitted the northern region, this is a convex (or concave) power model that lacks inflection points or maxima (or minima).
There are a variety of non-parametric statistical approaches for estimating species richness from incomplete sampling using presence absence data [47]. These have been tested and reviewed for forest biodiversity. Estimator accuracy is reduced by patchy spatial distributions and deep-sea marine nematode data is known to be patchy [28,29] so the results of this analysis must be treated with caution. Some estimators are more robust than other to patchiness, notably ICE and Chao2. ICE, Chao2 and Jackknife 2 are also relatively insensitive to sample size [47] so these three estimators were deemed most suitable for this data. Second order Jacknife and Chao 2 estimators are based on the numbers of duplicates and singletons and the number of samples. ICE is an incidence-based coverage estimator where the sample coverage estimate is the proportion of all individuals in infrequent species that are not uniques. Chao2 and ICE tend to overestimate when patchiness is high whereas Jackknife 2 tends to be more stable for patchy than random distributions where it tends to overestimate. In general ICE gives the best estimate but it is useful to check the result against the other two estimators [47]. Colwell's EstimateS program was used for the calculations.
Authors' contributions
This paper was produced from the PhD thesis of CJB, supervised by LEH. PJDL conceived and supervised the project, carried out the species richness part of the analysis and wrote the paper. TJF supervised the taxonomy and NJM the laboratory analysis. CRS helped conceive and supervise the project, and conducted the field sampling.
Acknowledgments
Acknowledgements
The authors are grateful to Adam Cook for his advice and help in carrying out this study and for reading the manuscript. The work was funded in part by a Fulbright Commission scholarship and by US NSF grant no. OCE 90-22116 to CRS. The authors acknowledge the assistance of the International Seabed Authority for providing a venue and framework for discussion. This is contribution no. 6078 form SOEST, and no. 848 from the US JGOFS program.
Contributor Information
P John D Lambshead, Email: pjdl@nhm.ac.uk.
Caroline J Brown, Email: c1brown@uk.ibm.com.
Timothy J Ferrero, Email: tjf@nhm.ac.uk.
Lawrence E Hawkins, Email: lawrence.e.hawkins@soc.soton.ac.uk.
Craig R Smith, Email: csmith@soest.hawaii.edu.
Nicola J Mitchell, Email: n.mitchell@nhm.ac.uk.
References
- Lambshead PJD, Schalk P. Overview of Marine Invertebrate Biodiversity. In: Levin S, editor. Encyclopedia of Biodiversity. Vol. 3. Academic Press; 2001. pp. 543–549. [Google Scholar]
- Gray JS. The measurement of marine species diversity, with an application to the benthic fauna of the Norwegian continental shelf. J Exp Biol Ecol. 2000;250:23–49. doi: 10.1016/s0022-0981(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Boucher G, Lambshead PJD. Marine nematode ecological biodiversity in samples from temperate, tropical and deep-sea regions. Conservation Biology. 1995;9:1594–1604. [Google Scholar]
- Lambshead PJD. Recent developments in marine benthic biodiversity research. Oceanis. 1993;19:5–24. [Google Scholar]
- Lambshead PJD. Marine nematode biodiversity. In: Chen ZX, Chen SY, Dickson DW, editor. Nematology, advances and perspectives. ACSE-TUP Book Series; [Google Scholar]
- Lambshead PJD. Sub-catastrophic sewage and industrial waste contamination as revealed by marine nematode faunal analysis. Mar Ecol Prog Ser. 1986;29:247–260. [Google Scholar]
- Coull BC, Chandler GT. Pollution and meiofauna – field, laboratory, and mesocosm studies. Oceanogr Mar Biol. 1992;30:191–271. [Google Scholar]
- Bussau C. Taxonomische und Okologische Untersuchungen an Nematoden des Peru-Beckens. PhD Dissertation Christian-Albrechts-Universitat Kiel. 1993.
- Spiess FN, Hessler R, Wilson G, Weydert M. Environmental effects of deep-sea dredging. SIO reference:87-5, San Diego. 1987.
- Lambshead PJD, Elce BJ, Thistle D, Eckman JE, Barnett PRO. A comparison of the biodiversity of deep-sea marine nematodes from three stations in the Rockall Trough, Northeast Atlantic, and one station in the San Diego Trough, Northeast Pacific. Biodiversity Letters. 1994;2:95–107. [Google Scholar]
- Lambshead PJD, Tietjen J, Ferrero T, Jensen P. Latitudinal diversity gradients in the deep-sea with special reference to North Atlantic nematodes. Mar Eco Prog Ser. 2000;194:159–167. [Google Scholar]
- International Sea Authority Deep-seabed polymetallic nodule exploration: development of environmental guidelines. Kingston Jamaica. 1999.
- Lambshead PJD, Tietjen J, Glover A, Ferrero T, Thistle D, Gooday A. The impact of large-scale natural physical disturbance on the diversity of deep-sea North Atlantic nematodes. Mar Ecol Prog Ser. 2001;214:121–126. [Google Scholar]
- Schratzberger M, Rees HL, Boyd SE. Effects of simulated deposition on structure of nematode assemblages – the role of burial. Mar Biol. 2000;136:519–530. [Google Scholar]
- Brown CJ, Lambshead PJD, Smith CR, Hawkins LE, Farley R. Phytodetritus and the abundance and biomass of abyssal nematodes in the central, equatorial Pacific. Deep-Sea Res. 2001;48:555–565. [Google Scholar]
- Lambshead PJD, Brown CJ, Ferrero TJ, Nicola Mitchell J, Smith CR, Hawkins LE, Tietjen J. Latitudinal diversity patterns for deep-sea marine nematodes and organic fluxes – a test from the central equatorial Pacific. Mar Ecol Prog Ser. 2002;236:129–135. [Google Scholar]
- Castillo-Fernandez D, Lambshead PJD. Revision of the genus Elzalia Gerlach, 1957 (Nematoda: Xyalidae) including three new species from an oil producing zone in the Gulf of Mexico, with a discussion of the sibling species problem. Bull Br Mus nat Hist. 1990;56:63–71. [Google Scholar]
- Tietjen JH, Lee JJ. Feeding behaviour of marine nematodes. In: Coull BC, editor. In Ecology of Marine Benthos. University of South Carolina Press Columbia; 1977. pp. 21–35. [Google Scholar]
- Jensen P. Diatom-feeding behaviour of the free-living marine nematode Chromadorita tenuis. Nematologica. 1982;28:71–76. [Google Scholar]
- Romeyn K, Bouwman LA. Food selection and consumption by estuarine nematodes. Hydrobiological Bulletin. 1983;17:103–109. [Google Scholar]
- Jensen P. Food ingestion and growth of the diatom-feeding nematode Chromadorita tenuis. Marine Biology. 1984;81:307–310. [Google Scholar]
- Jensen P. Feeding ecology of free-living aquatic nematodes. Mar Ecol Prog Ser. 1987;35:187–196. [Google Scholar]
- Moens T, Vincx M. Observations on the feeding ecology of estuarine nematodes. J mar biol Ass UK. 1997;77:211–227. [Google Scholar]
- Tietjen JH. Distribution and species diversity of deep-sea nematodes off North Carolina. Deep Sea Research. 1976;23:755–768. [Google Scholar]
- Tietjen JH. Distribution and species diversity of deep-sea nematodes in the Venezuela Basin. Deep Sea Research. 1984;31:119–132. [Google Scholar]
- Jensen P. Nematode assemblages in the deep-sea benthos of the Norwegian Sea. Deep Sea Research. 1988;35:1173–1184. [Google Scholar]
- Tietjen JH. Ecology of deep-sea nematodes from the Puerto Rico Trench area and Hatteras Abyssal Plain. Deep-Sea Res. 1989;36:1579–1594. [Google Scholar]
- Rice AL, Lambshead PJD. Patch dynamics in the deep-sea benthos: the role of a heterogeneous supply of organic matter. In: Giller PS, Hildrew AG, Raffaelli D, editor. Aquatic Ecology: scale, pattern and process. 34th Symposium of The British Ecological Society. Oxford, Blackwell Scientific Publications; 1994. pp. 469–499. [Google Scholar]
- Lambshead PJD, Hodda M. The impact of disturbance on measurements of variability in marine nematode populations. Vie et Milieu. 1994;44:21–27. [Google Scholar]
- Knowlton N. Molecular genetic analysis of species boundaries in the sea. Hydrobiologia. 2000;420:73–90. [Google Scholar]
- Smith CR, Hoover DJ, Doan SE, Pope RH, Demaster DJ, Dobbs FC, Altabet MC. Phytodetritus at the abyssal seafloor across 10° of latitude at the central equatorial Pacific. Deep Sea Res. 1996;43:1309–1338. [Google Scholar]
- Stephens MP, Kadko DC, Smith CR, Latasa M. Chlorophylla and phaeopigments as tracers of labile organic carbon at the central equatorial Pacific seafloor. Geochim Cosmochim Acta. 1997;61:4605–4619. [Google Scholar]
- Aller RC, DeMaster DJ. Estimates of particle flux and reworking at the deep-sea floor using Th-234/U-238 disequilibrium. Earth planet Sci Lett. 1984;67:308–318. [Google Scholar]
- Dymond J, Collier R. Biogenic particle fluxes in the equatorial Pacific: evidence for both high and low productivity during the 1982–1983 El Niño. Global Biogeochemical Cycles. 1988;2:129–137. [Google Scholar]
- Honjo S, Dymond J, Collier R, Manganini SJ. Export production of particles to the interior of the equatorial Pacific Ocean during the 1992 EqPac experiment. Deep Sea Res. 1995;42:831–870. [Google Scholar]
- Smith CR, Berelson W, Demaster DJ, Dobbs FC, Hammond D, Hoover DJ, Pope RH, Stephens M. Latitudinal variations in benthic processes in the abyssal equatorial Pacific: Control by biogenic particle flux. Deep Sea Res. 1997;44:2295–2317. [Google Scholar]
- Karl D, Christian J, Dore J, Hebel D, Letelier R, Tupas L, Winn C. Seasonal and interannual variability in primary production and particle flux at Station Aloha. Deep-Sea Res. 1996;43:539–568. [Google Scholar]
- Berelson WM, Hammond DE, McManus J, Kilgore TE. Dissolution kinetics of calcium carbonate in equatorial Pacific sediments. Global Biogeochemical Cycles. 1994;8:219–235. [Google Scholar]
- Barnett PRO, Watson J, Connolly D. A multiple corer for taking virtually undisturbed samples from shelf, bathyal and abyssal sediments. Oceanol Acta. 1984;7:399–408. [Google Scholar]
- Hessler RR, Jumars PA. Abyssal community analysis from replicate box cores in the central north Pacific. Deep Sea Res. 1974;21:185–209. [Google Scholar]
- Jumars PA. Environmental grain and polychaete species diversity in a bathyal benthic community. Mar Biol. 1975;30:253–266. [Google Scholar]
- Bett BJ, Vanreusel A, Vincx M, Soltwedel T, Pfannkuche O, Lambshead PJD, Gooday AJ, Ferrero TJ, Dinet A. Sampler bias in the quantitative study of deep sea meiobenthos. Mar Ecol Progr Ser. 1994;104:197–203. [Google Scholar]
- Platt HM, Warwick RM. Free Living Marine Nematodes, Part I British Enoplids. Cambridge University Press Cambridge. 1983.
- Clarke KR, Warwick RM. Changes in marine communities: an approach to statistical analysis and interpretation. NERC Publication Swindon. 1994.
- Cormack RM. A review of classification. Journal of the Royal Statistical Society Series A. 1971;134:321–367. [Google Scholar]
- Lambshead PJD. Numerical biodiversity analysis with the Natural History Museum and Scottish Association for Marine Sciences BioDiversity Professional PC package. Oceanis. 2001;24:375–391. [Google Scholar]
- Chazdon RS, Colwell RK, Denslow JS, Guariguata MR. Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of northeastern Costa Rica. In: Dallmeier F, Comiskey JA, editor. In Forest biodiversity research, monitoring and modelling. Paris, UNESCO and the Parthenon Publishing Group; 1998. pp. 285–309. [Google Scholar]


