Abstract
We have developed “vertical” magnetic tweezers capable of exerting controlled pico and subpico Newton forces. Using this apparatus, we apply a point-like force to a vesicle that is adhered by means of specific bonds between the vesicle-carrying oligosaccharide sialyl LewisX and the surface-grafted E-selectin. An exponential decrease of the bound vesicle area with the decay rate that is insensitive to the force and the composition of the system is observed. We measure an equilibrium under force that is associated with an increased binding in the center of the contact zone. We also show that the determination of the shape is potentially sufficient to determine the number of formed specific bonds.
Adhesion and deadhesion are vital processes for normal cell functioning. However, particularly from the physical point of view, deadhesion is the much less studied, and therefore less well understood. The standard means of inducing deadhesion is the application of a force to ligand-receptor bonds. The crucial step in grasping the consequences of such forces was the explanation of the unbinding process of a single bond on the basis of the Arrhenius law of chemical kinetics, with the caveat that bonds appear more resistant to higher force-load rates (1). When multiple bonds are considered, they can respond as if in series, where only molecules in the outer rim feel the force, or as if in parallel, where the force is shared by all the bonds and cooperative effects arise due to the statistical nature of the bond lifetime (2). The unbinding of large membrane-confined aggregates of bonds is even more complex and, although the subject of several studies (3,4), is still an open and challenging issue.
Motivated by recent theoretical advances in modeling specific vesicle adhesion (5) and the shapes of vesicles under force (6), we studied a reversible, force-induced deadhesion process of vesicles specifically bound to the substrate. We identify a new type of bond cooperativity that is a result of the response of the entire thermodynamically driven system where lateral reorganization of bonds plays a critical role.
In our experiments, the vesicle adhesion is mediated by the formation of bonds between sialyl LewisX (sLeX) glycosphingolipids (15 mol% of sLeX are incorporated into GUVs (DMPC/ cholesterol/PEG 2000 1:1:0.01–0.03); see Supplemental Materials for details on materials and methods) (8) and surface-immobilized E-selectin at coverages of the order of 102 and 103 receptors/μm2. Once the vesicle-substrate system reaches an adhesion equilibrium, forces of 0–50 pN are exerted with vertical magnetic tweezers mounted either on a confocal or a reflection interference contrast microscope. Due to the high concentration of sLeX in the vesicle, a uniform contact zone is formed upon adhesion (the bound area is proportional to the number of formed bonds). Furthermore, the low surface coverage by E-selectin and the relatively low binding constant of the pair ensures that the vesicle remains in the weak-adhesion regime (the vesicle does not assume the shape of a spherical cap).
As can be seen in Fig. 1 (top), the application of a constant force induces an exponential decay of the bound area A(t) from the initial A0 + A1 size into the final equilibrium size A1, which is finite, but smaller:
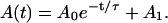 |
(1) |
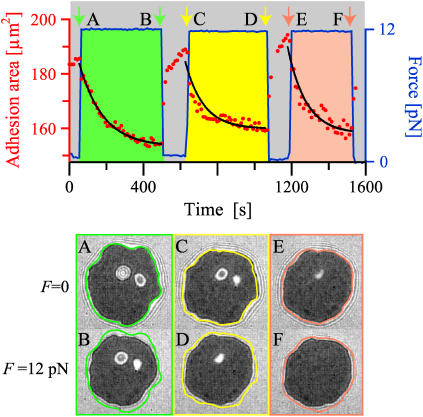
FIGURE 1 .
Time evolution of the bound area in the contact zone (data and exponential fits as symbols and lines, respectively) is shown for three force pulses. Each pulse is associated with a color. The contact zones in the equilibrium at both zero and 12 pN force (second and third row) are shown for every pulse, as indicated by the capital letters. The black area in the contact is a signature of the specific binding. The edges of the bound areas are outlined for zero force and shown for comparison with zones under force.
We studied the properties of this decay function by changing system parameters such as the applied force, the surface coverage, and the concentration of PEG 2000 lipids (7) (which provide a repulsive contribution). Surprisingly, we find the decay constant τ to be very insensitive to all these parameters. However, consistent with expectations, at very low surface coverages we measure increased relative loss of the bound area with i), increased force acting on the vesicle, and ii), increased PEG 2000 content in the vesicle, because the repulsive contribution competes with the ligand-receptor binding. At high surface coverages, the final state is insensitive to the PEG 2000 content (with typical loss of 20% of bound area), as the adhesive contribution now outweighs the repulsion. Lastly, at high surface coverage and high forces, the membrane experiences instabilities due to the built-up tension, and the reduced volume is no longer constant.
Inspection of the equilibrium contact zones (second and third row in Fig. 1) reveals that numerous binding events take place under force resulting in an increased density of bonds in the contact zone. For this vesicle, this can be inferred from the spontaneous disappearance of the blisters in the middle of the zone. Releasing the vesicle at the end of the pulse results in the restoration of the initial strongly bound area. However, there is no re-establishment of the blister, showing that the newly formed bonds do not dissociate upon release.
The above behavior can be explained in light of the recently developed model for adhesion of vesicles (5). There it was shown that the equilibrium density of formed bonds increases upon decreasing the size of the contact zone until all receptors are bound and the bond density reaches the saturation level. Furthermore, the formation of bonds was found to produce an effective adhesion strength that acts as a spreading pressure of the vesicle. This quantity always increases with the decrease of the contact zone (5). In the context of the results shown in Fig. 1, such spreading pressure counteracts the applied force. The thermodynamic equilibrium, achieved upon the reorganization of bonds, can then be understood as a balance between the applied force and the spreading pressure that characterizes the resulting size of the contact zone.
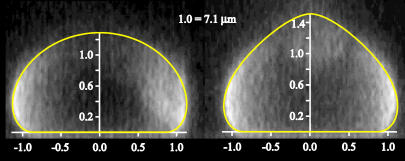
The second important prediction emerging from the adhesion model (5) is the decoupling of the vesicle shape from the binding in the contact zone that arises due to the different energy scales of these two contributions. As the force provides a contribution to the free energy that is of the same magnitude as the bending energy (6), such decoupling must also be applicable in the presence of force. Consequently, because the adhesion equilibrium of our system corresponds to a weak adhesion regime, the shapes calculated in the continuous model (6) should be observable. We have tested this hypothesis by performing confocal measurements of the vesicle shape (Fig. 2) and fitting the results with shapes obtained theoretically.
FIGURE 2 .
Overlay of equilibrium shapes of the same vesicle at zero force (left) and pulled with a constant force (right) originating from the experiments and the continuous model.
The theoretical shape is first determined in the absence of force. Using a bending stiffness of the membrane of κ = 100 kBT, the fit provides a shape corresponding to a reduced volume of 0.89 ± 0.01 and an effective adhesion strength of 0.12 ± 0.01 μNm−1 (Fig. 2, left). These values are in good agreement with the measured reduced volume of 0.8 ± 0.1 and the average adhesion strength of 0.15 ± 0.05 μNm−1. The latter was evaluated independently using the Young-Dupré equation for wetting droplets as described in Guttenberg et al. (9). Keeping these parameters constant, shapes under force are then calculated (Fig. 2, right), and the matching shape is found for F = 0.73 ± 0.05 pN. This corresponds well to the experimentally applied subpico-Newton force.
The agreement between the theoretically predicted and experimentally observed shapes is an indication that the macroscopic vesicle shape can indeed be decoupled from the details of the specific binding by the construction of an effective potential. In other words, when the effective adhesion strength is known, the shape can be obtained merely by considering the free energy of curvature, subject to constraints concerning the force and the size of the contact zone. When, on the other hand, the shape is experimentally available, the effective adhesion can be, in principle, determined by matching the theoretical shapes. Because a unique mapping is possible, the obtained effective adhesion strength can be associated with a given number of bonds. However, in this case, the surface coverage and the binding strength of the ligand-receptor pair must be well known.
In conclusion, we show that the mechanical force applied vertically serves as a control mechanism for maintaining a particular size of a contact zone, which is an integral part of a macroscopic shape. The force is opposed by the overall effective adhesion strength in what can be understood as a thermodynamic response of a system that exhibits cooperative bond effects. If only the bonds at the rim of the contact zone were to experience the force (bonds in series), the force per bond would be inversely proportional to the circumference of the zone. Hence, once the outermost bonds were broken, there should be no further bonds capable of withstanding the force, simply because the force per bond increases at smaller circumferences. If force was to be transmitted to the bonds in parallel, so that the cooperativity results from the statistics of bond association and dissociation, then the growth of the cluster under force should not be observed (2). Instead, the calculations and the experiments presented herein show that the bonds in the contact zone respond as a subsystem capable of organizing to oppose the detachment by means of a “collective” spreading pressure.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
We thank U. Seifert for insightful discussions.
This work has been sponsored by the Sonderforschungsbereich 563/C3.
References
- 1.Evans, E., and K. Ritchie. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 72:1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seifert, U. 2000. Rupture of multiple parallel molecular bonds under dynamic loading. Phys. Rev. Lett. 84:2750–2753. [DOI] [PubMed] [Google Scholar]
- 3.Dembo, M., D. C. Torney, K. Saxman, and D. Hammer. 1988. The reaction limited kinetics of membrane to surface adhesion and detachment. Proc. Roy. Soc. London. 234:55–83. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz, U. S., N. Q. Balaban, D. Riveline, A. Bershadsky, B. Geiger, and S. A. Safran. 2002. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys. J. 83:1380–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith, A.-S., and U. Seifert. 2005. Effective adhesion strength of specifically bound vesicles. Phys. Rev. E. 71:061902. [DOI] [PubMed] [Google Scholar]
- 6.Smith, A.-S., E. Sackmann, and U. Seifert. 2003. Effects of a pulling force on the shape of a bound vesicle. Europhys. Lett. 64:281–287. [Google Scholar]
- 7.Reference deleted in proof.
- 8.Gege, C., S. Oscarson, and R. R. Schmidt. 2001. Synthesis of fluorescence labeled sialyl LewisX-glycosphingo-lipids. Tetrahedron Lett. 105:5178–5185. [Google Scholar]
- 9.Guttenberg, Z., A. Bausch, B. Hu, R. Bruinsma, L. Moroder, and E. Sackmann. 2000. Measuring ligand-receptor unbinding forces with magnetic beads: molecular leverage. Langmuir. 16:8984–8993. [Google Scholar]