Abstract
We investigated the in vitro activity of nystatin and liposomal nystatin against 103 Candida isolates to determine the effect of both time and medium on MICs. We also compared the nystatin MICs with those of amphotericin B and fluconazole. Testing was performed in accordance with the National Committee for Clinical Laboratory Standards M27-A microdilution methodology with RPMI 1640, RPMI 1640 supplemented with glucose to 2% (RPMI-2), and antibiotic medium 3 supplemented with glucose to 2% (AM3). While nystatin MICs were similar to or slightly lower than liposomal nystatin MICs in RPMI 1640 and RPMI-2, they were markedly higher than liposomal nystatin MICs in AM3. Use of AM3 and determination of the MIC after 24 h of incubation provided a slightly wider range of liposomal nystatin MICs (0.06 to >16 μg/ml). Under these conditions, the MICs at which 90% of isolates were inhibited of nystatin and liposomal nystatin were 2 and 1 μg/ml, respectively. Nystatin and liposomal nystatin in general showed good activity against all Candida spp. tested. Although the MICs of nystatin and liposomal nystatin tended to rise in parallel with the amphotericin B MICs, nystatin and liposomal nystatin MICs of 1 to 2 and 0.5 to 1 μg/ml, respectively, were obtained for seven and six, respectively, of nine isolates for which amphotericin B MICs were ≥0.25 μg/ml. No correlation between fluconazole and nystatin or liposomal nystatin MICs was observed. As amphotericin B MICs of ≥0.25 μg/ml correlate with in vitro resistance, these results suggest that liposomal nystatin might have activity against some amphotericin B-resistant isolates. In vivo testing in animal models is required for clarification of this issue.
As acquired resistance has been described for both amphotericin B and the current azoles (4, 10, 30, 31, 34), there is interest in developing new antifungal agents. Nystatin, a polyene antibiotic derived from Streptomyces noursei, is known to be effective against a variety of fungal infections in humans. Although the drug has been widely used as oral and topical therapy for superficial mycoses (16), intravenous use has been limited by its toxic side effects. To overcome these problems, nystatin has been reformulated in a lipid complex that has demonstrated reduced side effects while maintaining antifungal activity in vitro and in vivo 6, 9, 11, 17, 18, 32; C. J. Jessup, T. J. Wallace, and M. A. Ghannoum, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-88, 1997).
The relevance, if any, of in vitro testing with lipid-based preparations of polyenes such as amphotericin B and nystatin (rather than the parent drug) is unclear. Some feel that the role of a lipid formulation is solely to alter in vivo drug delivery and that testing the in vitro activity of lipid formulations is of little relevance. On the other hand, use of a lipid-based polyene preparation could be viewed as simply being an alternative way to dissolve the polyene, as opposed to the use of the dimethyl sulfoxide (DMSO) specified by the National Committee for Clinical Laboratory Standards (NCCLS) methodology for these hydrophobic compounds. As the choice of solubilizing system has been shown to influence azole MICs (8), the possible relevance of use of a lipid polyene as the drug source for in vitro testing merits investigation. In studies to date with lipid formulations of amphotericin B, the MICs of some lipid formulations were higher than those of the parent compound in some studies (11, 23, 24), whereas similar MICs for lipid and conventional formulations were obtained by other workers (1).
Complicating these issues is the fact that in vitro susceptibility testing of amphotericin B is technically difficult. The NCCLS M27-A methodology fails to reliably detect amphotericin B-resistant isolates (20). Thus we (28; M. Lozano-Chiu, S. Arikan, F. M. Martin-Diez, V. Paetznick, J. L. Rodriguez-Tudela, and J. H. Rex, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-18, p. 455, and J-19b, p. 456, 1998) and others (14, 21) explored variants of M27-A that might resolve this problem. In our hands, use of antibiotic medium 3 supplemented with glucose to 2% as the test medium and an incubation period of 24 h improved detection of in vitro resistance to amphotericin B (28; Lozano-Chiu et al., 38th ICAAC, abstr. J-18 and J-19b). However, Nguyen et al. (21) reported that MICs in antibiotic medium 3 were insensitive in predicting outcome and that the best predictor for microbiological failure was amphotericin B minimum lethal (fungicidal) concentration (MLC) at 48 h. In these workers' hands, supplementation of the medium to 2% glucose also provided no advantage over plain antibiotic medium 3 in detection of resistant isolates. These differences highlight the importance of technical factors in the measurement of amphotericin B MICs.
Based on the structural similarity between nystatin and amphotericin B, we speculated that in vitro susceptibility testing of nystatin and liposomal nystatin might present difficulties similar to those observed for amphotericin B. We now report investigations of the comparative in vitro activity of nystatin and those of liposomal nystatin, amphotericin B, and fluconazole against several Candida spp. We tried to (i) determine the in vitro activity of nystatin against Candida isolates and the influence of species, time of reading, reading method, and the test medium on nystatin and liposomal nystatin MICs; (ii) compare the in vitro activity of nystatin with those of liposomal nystatin, amphotericin B, and fluconazole; and finally (iii) determine the possibility of cross-resistance between nystatin and amphotericin B or fluconazole.
(This work was presented in part previously [S. Arikan, M. Lozano-Chiu, V. Paetznick, D. Gordon, T. Wallace, and J. H. Rex, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. C-280, 1998]).
MATERIALS AND METHODS
Isolates.
Three groups of Candida isolates were tested. The first group consisted of four previously defined clinical isolates for which amphotericin B MICs were relatively high (≥0.25 μg/ml) (2, 28): 5W31 (C. lusitaniae), Y537 (C. albicans), CL2887 (C. lusitaniae), and MY1012 (C. tropicalis). The second group was composed of 95 randomly selected clinical isolates and included 38 isolates of C. albicans, 17 isolates of C. glabrata, 11 isolates of C. tropicalis, 10 isolates of C. parapsilosis, 9 isolates of C. krusei, 9 isolates of C. lusitaniae, and one isolate of C. lipolytica. The third group consisted of four quality control strains (25, 26): C. parapsilosis ATCC 22019, C. parapsilosis ATCC 90018, C. krusei ATCC 6258, and C. tropicalis ATCC 750. Identification of the strains was accomplished by standard methods (13).
Antifungal agents.
Liposomal nystatin (NYOTRAN) was obtained from Aronex Pharmaceuticals, Inc. (The Woodlands, Tex.) as a lyophilized product containing nystatin mixed with dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol (lot numbers 503-33-0010 and 503-33-0011). The product was reconstituted in sterile saline, yielding a concentration of 1 mg/ml. Once prepared, the fresh liposomal nystatin suspension was used to prepare the microdilution trays. Standard powder of nystatin was obtained from Gist-Brocades (Capua, Italy) through Aronex Pharmaceuticals and was dissolved in DMSO; the stock solution was kept at −70°C until use. Amphotericin B (Bristol-Myers Squibb, Plainsboro, N.J.) and fluconazole (Pfizer Pharmaceuticals, New York, N.Y.) were obtained from their respective manufacturers as standard powders. Stock solutions for amphotericin B were prepared by dissolving the drug in DMSO. Fluconazole was dissolved in sterile distilled water. Both solutions were stored at −70°C until use.
Drug preparation and antifungal susceptibility testing.
Except as noted, the testing was performed according to the procedures for the microdilution variant of the NCCLS M27-A standard (20). Liposomal nystatin, nystatin, amphotericin B, and fluconazole were tested in twofold dilutions on microtiter plates. The concentrations ranged from 0.03 to 16 μg/ml for liposomal nystatin and nystatin, 0.078 to 4 μg/ml for amphotericin B, and 0.125 to 64 μg/ml for fluconazole. Dilution series were prepared in DMSO (amphotericin B and nystatin) or water (fluconazole and liposomal nystatin). Amphotericin B, nystatin, and fluconazole microtiter plates were prepared in advance and kept at −70°C until use. These are conditions under which both prior work in our laboratory and concurrent control testing demonstrated the stability of the materials. Liposomal nystatin plates were used on the day of preparation. Susceptibility to amphotericin B was determined in antibiotic medium 3 (BBL, Becton Dickinson, lot JD4ZSG) supplemented to 20 g of glucose/liter and buffered to pH 7.0 with 0.01 M phosphate (AM3) 28; Lozano-Chiu et al., 38th ICAAC, abstr. J-18). Susceptibility to nystatin and liposomal nystatin was determined in the standard NCCLS M27-A medium (RPMI 1640 with glutamine and buffered with 0.165 M MOPS [morpholinepropanesulfonic acid] [RPMI]), RPMI 1640 supplemented to 2% glucose (RPMI-2), and AM3. Fluconazole susceptibility testing was performed in RPMI. Each isolate was tested in duplicate, and four reference strains were included for quality control. MICs for reference strains were determined four times in duplicate for liposomal nystatin and once in duplicate for nystatin. Twenty-eight isolates were tested four times to establish the reproducibility of the methods.
Interpretation of the results.
MICs (in micrograms per milliliter) were read both visually and by spectrophotometer. As we have previously found that determining the MIC after 24 h rather than the NCCLS M27-A-specified 48 h improves the correlation between in vitro and in vivo results for both amphotericin B (28) and fluconazole (29), MICs were recorded after both 24 and 48 h. Visual readings were done after shaking (Minishaker; Dynatech Laboratories) the plates for 1 to 2 min (3). Spectrophotometric readings were based on the reduction of growth compared to that in a growth control well for each isolate. For this, microtiter plates were agitated and the optical densities of the wells were determined at 530 nm (model EL310 EIA Autoreader; Biotek Instruments). The background optical density of the sterility check control well was subtracted from the optical densities of all of the other wells. The resulting optical density values were divided by the optical density of the drug-free growth control well to calculate the percentage of growth compared to the growth in the growth control well (29).
For visual readings of the nystatin, liposomal nystatin, and amphotericin B MICs, the least concentration of the drug which produced an optically clear well was recorded as the MIC. The spectrophotometric MICs of these agents were the least drug concentrations which resulted in 95% reduction in growth by spectrophotometric measurements. Reduction by 95% was found to correspond to an optically clear well and was used instead of 100% in the spectrophotometric MIC calculation to compensate for minor imperfections in the well. For fluconazole, the MIC was determined according to NCCLS M27-A procedures. As we have also observed that the MIC obtained by reading after 24 h and by using a spectrophotometric end point of 50% reduction in growth may correlate more reliably with the clinical outcome (29), this MIC was also recorded.
Based on the previous in vitro and in vivo data (28), isolates for which the amphotericin B MICs were relatively high, defined as ≥0.25 μg/ml, were considered putatively resistant to amphotericin B.
Determination of MLC values of amphotericin B and nystatin.
MLC values for amphotericin B and nystatin were obtained by the method described by Nguyen et al. (21). Briefly, after visual determination of the 48-h MIC and after shaking the microtiter plate, 25 μl from each clear well was plated on Sabouraud dextrose agar and incubated at 35°C for 48 h. The MLC was taken as the concentration of the first clear well that showed no CFU growth, corresponding to the least concentration of drug which produced a >98% reduction of the initial inoculum.
RESULTS
Influence of species, time of reading, and reading method on nystatin and liposomal nystatin MICs.
Nystatin and liposomal nystatin MICs for clinical and American Type Culture Collection isolates are shown in Tables 1 and 2, respectively. There was no species-related variation of nystatin and liposomal nystatin MICs. MICs for most isolates at 48 h were twofold greater than those at 24 h. Excellent agreement between visual assessments of no growth and spectrophotometric 95%-reduction end point values was observed. At 24 h, visual and spectrophotometric readings produced identical MICs for 66 and 80 of 99 isolates tested against nystatin and liposomal nystatin, respectively. Similarly, these two reading parameters were within one twofold dilution for 94 (95%) and 98 (99%) of 99 isolates tested against nystatin and liposomal nystatin, respectively. Visual and spectrophotometric MICs were thus essentially the same, and spectrophotometric readings are reported throughout for both drugs. Finally, repetitive (n = 4) testing of 28 isolates against liposomal nystatin showed that MICs varied by one dilution or less in all three media. Similar results were obtained in duplicate testing of 10 strains against nystatin.
TABLE 1.
Nystatin and liposomal nystatin MICs for the clinical isolates after 24 and 48 h in the three test media
| Isolate type (n) | Antifungal agent | MIC parameter | MIC (μ/ml) at indicated time (h) in:
|
|||||
|---|---|---|---|---|---|---|---|---|
| RPMI
|
RPMI-2
|
AM3
|
||||||
| 24 | 48 | 24 | 48 | 24 | 48 | |||
| AMB-Ra (4) | Nystatin | Range | 0.5-8 | 1-16 | 0.5->16 | 1->16 | 2->16 | 4->16 |
| Liposomal nystatin | Range | 1->16 | 2->16 | 1->16 | 2->16 | 1->16 | 2->16 | |
| Other clinicalb (95) | ||||||||
| C. albicans (38) | Nystatin | 50 | 0.5 | 1 | 0.5 | 1 | 2 | 4 |
| 90 | 0.5 | 1 | 0.5 | 1 | 2 | 8 | ||
| Range | 0.125-0.5 | 0.5-1 | 0.125-1 | 0.5-2 | 1-2 | 1-8 | ||
| Liposomal nystatin | 50 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | |
| 90 | 1 | 2 | 0.5 | 1 | 0.5 | 1 | ||
| Range | 0.25-1 | 1-2 | 0.25-1 | 0.5-2 | 0.25-0.5 | 0.5-2 | ||
| C. glabrata (17) | Nystatin | 50 | 0.25 | 0.5 | 0.25 | 0.5 | 1 | 2 |
| 90 | 0.5 | 1 | 0.5 | 1 | 2 | 4 | ||
| Range | 0.125-0.5 | 0.25-1 | 0.03-1 | 0.25-1 | 0.5-2 | 1-4 | ||
| Liposomal nystatin | 50 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | |
| 90 | 1 | 2 | 0.5 | 1 | 1 | 1 | ||
| Range | 0.25-1 | 1-2 | 0.25-1 | 1-2 | 0.25-1 | 0.5-2 | ||
| C. tropicalis (11) | Nystatin | 50 | 0.5 | 1 | 0.5 | 1 | 2 | 4 |
| 90 | 0.5 | 1 | 0.5 | 1 | 4 | 8 | ||
| Range | 0.25-0.5 | 1 | 0.5 | 1 | 1-4 | 2-8 | ||
| Liposomal nystatin | 50 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | |
| 90 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | ||
| Range | 0.25-1 | 0.5-1 | 0.25-0.5 | 0.5-1 | 0.06-1 | 0.25-2 | ||
| C. parapsilosis (10) | Nystatin | 50 | 0.5 | 1 | 0.5 | 1 | 2 | 4 |
| 90 | 1 | 1 | 1 | 1 | 2 | 8 | ||
| Range | 0.125-2 | 0.5-4 | 0.25-2 | 1-2 | 0.25-2 | 2-16 | ||
| Liposomal nystatin | 50 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | |
| 90 | 1 | 2 | 0.5 | 2 | 1 | 1 | ||
| Range | 0.25-2 | 1-2 | 0.25-1 | 0.5-2 | 0.125-1 | 0.25-2 | ||
| C. krusei (9) | Nystatin | 50 | 0.5 | 1 | 1 | 1 | 1 | 4 |
| 90 | 1 | 1 | 1 | 1 | 2 | 8 | ||
| Range | 0.5-1 | 1 | 0.5-2 | 1-2 | 1-4 | 2-8 | ||
| Liposomal nystatin | 50 | 1 | 2 | 1 | 1 | 0.5 | 1 | |
| 90 | 2 | 2 | 1 | 1 | 0.5 | 1 | ||
| Range | 1-2 | 2 | 0.5-1 | 1-2 | 0.5 | 0.5-1 | ||
| C. lusitaniae (9) | Nystatin | Range | 0.25-2 | 0.5-2 | 0.25-0.5 | 0.5-1 | 0.5-2 | 2-4 |
| Liposomal nystatin | Range | 0.25-1 | 0.5-2 | 0.25-1 | 0.5-2 | 0.25-1 | 0.25-2 | |
| C. lipolytica (1) | Nystatin | Range | 0.5 | 1 | 1 | 1 | 2 | 8 |
| Liposomal nystatin | Range | 2 | 2 | 1 | 2 | 0.5 | 1 | |
5W31, CL2887, Y537, and MY1012. AMB-R, amphotericin B resistant.
Isolates randomly selected from a collection of clinical Candida strains.
50, MIC50; 90, MIC90.
TABLE 2.
Nystatin and liposomal nystatin MICs for American Type Culture Collection isolates after 24 and 48 h in the three test media
| Reference strain and antifungal agent | MICa (μg/ml) at indicated time (h) in:
|
|||||
|---|---|---|---|---|---|---|
| RPMI
|
RPMI-2
|
AM3
|
||||
| 24 | 48 | 24 | 48 | 24 | 48 | |
| C. parapsilosis ATCC 22019 | ||||||
| Nystatin | 0.25 | 1 | 0.5 | 1 | 2 | 2 |
| Liposomal nystatin | 0.5 | 1 | 0.5 | 1 | 0.25-1 | 0.5-1 |
| C. parapsilosis ATCC 90018 | ||||||
| Nystatin | 0.5 | 1 | 0.5 | 1 | 2 | 2 |
| Liposomal nystatin | 0.25-1 | 1-2 | 0.5-1 | 1-2 | 0.25-0.5 | 0.25-1 |
| C. krusei ATCC 6258 | ||||||
| Nystatin | 1 | 1 | 0.5 | 1 | 1 | 4 |
| Liposomal nystatin | 0.5-1 | 2-4 | 0.5-1 | 1-2 | 0.5-1 | 0.5-2 |
| C. tropicalis ATCC 750 | ||||||
| Nystatin | 0.5 | 1 | 0.5 | 0.5 | 1 | 2 |
| Liposomal nystatin | 0.5-1 | 1 | 0.5-1 | 0.5-1 | 0.125-0.25 | 0.5-1 |
Liposomal nystatin MICs were determined four times in duplicate. Nystatin MICs are results of single testing.
Comparison of nystatin and liposomal nystatin MICs.
As shown in Tables 1 and 2, nystatin and liposomal nystatin had favorable activity against all Candida spp., with the exception of some amphotericin B-resistant strains. Comparison of nystatin MICs with those of liposomal nystatin showed that the differences were medium dependent. Nystatin in general generated similar or slightly lower MICs than liposomal nystatin in RPMI and RPMI-2, but nystatin MICs were consistently and sometimes markedly higher than liposomal nystatin MICs in AM3. This effect was observed for all Candida spp. tested as well as the putatively amphotericin B-resistant isolates included in the study.
Influence of test media on nystatin MICs.
Nystatin MICs in RPMI-2 were in general similar to or slightly higher than those in RPMI at both 24 and 48 h. Nystatin MIC ranges in RPMI and RPMI-2 were similarly wide for all species other than C. glabrata. AM3 consistently generated the highest nystatin MICs at both 24 and 48 h. This effect resulted in a wide nystatin MIC range in AM3, with a shift toward higher MICs.
Influence of test media on liposomal nystatin MICs.
A wider range of liposomal nystatin MICs in AM3 than in RPMI and RPMI-2 was obtained for some strains, and especially for the C. tropicalis isolates. The wider range in AM3 in general was due to generation of MICs for some isolates that were one to three twofold dilutions lower than those generated in the other two media. This observation for AM3 was less pronounced at 48 h. The numbers of isolates for which the MICs in AM3 were lower than those in RPMI were 73 and 56 at 24 and 48 h, respectively. The MIC range for AM3 at 24 h was identical with the range for RPMI for the putatively amphotericin B-resistant isolates.
Nystatin and liposomal nystatin MICs for isolates for which amphotericin B MICs are high.
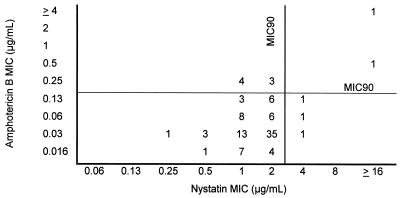
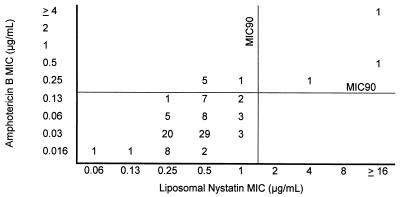
Based on our previous demonstrations that amphotericin B MICs determined at 24 h were most likely to be of clinical relevance and provided good discrimination of resistant isolates (28), we focused our attention on these MICs of nystatin and liposomal nystatin as well. The comparison of amphotericin B MICs with those of nystatin and liposomal nystatin in AM3 at 24 h of incubation is shown in Fig. 1 and 2, respectively. Under these conditions, the MICs at which 90% of isolates were inhibited (MIC90s) of nystatin and liposomal nystatin were 2 and 1 μg/ml, respectively. Amphotericin B MICs for five isolates in the group of randomly selected clinical isolates were also found to be ≥0.25 μg/ml. This made a total of nine isolates which were putatively amphotericin B resistant. Relatively higher nystatin and liposomal nystatin MICs were associated with increased amphotericin B MICs. However, MICs of nystatin and/or liposomal nystatin for the isolates that were putatively resistant to amphotericin B (MIC, ≥0.25 μg/ml) (2, 28) were not always elevated (Table 3). While nystatin and liposomal nystatin MICs for two (Y537 and MY1012) and three (CL2887, Y537, and MY1012) of the nine amphotericin B-resistant isolates, respectively, were high (4 to >16 μg/ml), the remaining seven and six isolates, respectively, yielded nystatin and liposomal nystatin MICs that were lower than the MIC90 for the overall population of the corresponding drug.
FIG. 1.
Nystatin MICs relative to amphotericin B MICs for the clinical isolates included in the study (n = 99). The MICs were determined in AM3 after 24 h of incubation. The numbers are numbers of isolates for which the MICs of nystatin and amphotericin B were as shown.
FIG. 2.
Liposomal nystatin MICs relative to amphotericin B MICs for the clinical isolates included in the study (n = 99). The MICs were determined in AM3 after 24 h of incubation. The numbers are numbers of isolates for which MICs of liposomal nystatin and amphotericin B were as shown.
TABLE 3.
Nystatin and liposomal nystatin MICs for isolates with amphotericin B MICs of ≥0.25 μg/ml
| Isolate | Species | AMBb MIC | Drugc | MICa (μg/ml) at indicated time (h) in:
|
Susceptibility to AMB (reference or source) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RPMI
|
RPMI-2
|
AM3
|
||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||||
| CL2887 | C. lusitaniae | 0.25 | N | 0.5 | 1 | 1 | 1 | 2 | 4 | Resistant in animal model (2) |
| LN | 2-4 | 4-16 | 1-4 | 4 | 2-4 | 4-8 | ||||
| 5W31 | C. lusitaniae | 0.25 | N | 0.5 | 1 | 0.5 | 1 | 2 | 4 | Resistant in animal model (2) |
| LN | 0.5-1 | 1-2 | 0.25-1 | 1-2 | 0.25-1 | 0.5-2 | ||||
| 34-016-027.01 | C. krusei | 0.25 | N | 0.5 | 1 | 0.5 | 1 | 2 | 4 | Relatively high MIC (present study) |
| LN | 1 | 2 | 0.5 | 1 | 0.5 | 1 | ||||
| 34-501-035 | C. krusei | 0.25 | N | 1 | 1 | 1 | 1 | 1 | 4 | Relatively high MIC (present study) |
| LN | 1 | 2 | 1 | 1 | 0.5 | 1 | ||||
| 34-013-031.01 | C. krusei | 0.25 | N | 0.5 | 1 | 0.5 | 1 | 1 | 2 | Relatively high MIC (present study) |
| LN | 2 | 2 | 1 | 1 | 0.5 | 1 | ||||
| 34-504-050 | C. krusei | 0.25 | N | 0.5 | 1 | 1 | 1 | 1 | 4 | Relatively high MIC (present study) |
| LN | 2 | 2 | 1 | 1 | 0.5 | 1 | ||||
| 34-044-040 | C. krusei | 0.25 | N | 0.5 | 1 | 0.5 | 1 | 1 | 4 | Relatively high MIC (present study) |
| LN | 1 | 2 | 0.5 | 1 | 0.5 | 0.5 | ||||
| Y537 | C. albicans | 0.5 | N | 4 | 8 | 4 | 8 | 16 | 16 | Relatively high MIC (28) |
| LN | 8->16 | 16->16 | 4->16 | 8->16 | 8->16 | 16 | ||||
| MY1012 | C. tropicalis | 4 | N | 8 | 16 | >16 | >16 | >16 | >16 | Relatively high MIC (28) |
| LN | >16 | >16 | 16->16 | 16->16 | >16 | >16 | ||||
The isolates for which there are available MIC ranges were tested four times in duplicate, whereas those for which there are single MIC values were tested once in duplicate. In case of variable MICs obtained in different runs for a single isolate, the highest value was used.
AMB, amphotericin B.
N, nystatin; LN, liposomal nystatin.
Nystatin and liposomal nystatin MICs for isolates with low amphotericin B MICs.
Nystatin had low MICs for 87 of 90 strains for which the amphotericin B MICs were low. Interestingly, the nystatin MICs for the remaining three isolates were higher than the MIC90 of the drug (Fig. 1). Unlike what was found for nystatin, low amphotericin B MICs were associated with low liposomal nystatin MICs for all isolates (Fig. 2).
Of interest, the amphotericin B MICs for all C. krusei isolates (n = 9) tested were relatively high (0.125 to 0.25 μg/ml). The nystatin and liposomal nystatin MIC ranges for these isolates were 1 to 4 and 0.5 μg/ml, respectively. These results indicated that, for eight of nine C. krusei isolates, nystatin MICs were lower than the nystatin MIC90, while for all of them the liposomal nystatin MICs were lower than the liposomal nystatin MIC90.
Comparison of MICs with MLCs for nystatin and amphotericin B.
Comparison of MICs and MLCs for each individual isolate showed that, for 92 (92.9%) and 81 (81.8%) of the clinical isolates, the MLCs of nystatin and amphotericin B, respectively, were identical to or one twofold dilution higher than the corresponding MICs. For the remaining isolates the MLCs of nystatin and amphotericin B were at most three and four twofold dilutions higher, respectively, than the corresponding MICs. For all of these isolates the MICs were in the higher end, and for some the MLCs were >16 μg/ml.
Comparison of fluconazole MICs with those of nystatin and liposomal nystatin.
Comparison of the nystatin and liposomal nystatin MICs with that of fluconazole for each individual isolate showed that there was no relationship between the in vitro efficacy and MIC trend of nystatin or liposomal nystatin with fluconazole (data not shown).
DISCUSSION
With the exception of some of the putatively amphotericin B-resistant isolates, nystatin and liposomal nystatin showed good activity against our test isolates. Data reported by others on in vitro activity of liposomal nystatin against Candida isolates are similar to our results. Jessup et al. (37th ICAAC), Carillo-Munoz et al. (5), and Quindos et al. (27) also demonstrated that liposomal nystatin had good in vitro activity against several Candida spp. Similarly, and as reported by Johnson et al. (11), liposomal nystatin was shown to be as active as free nystatin and to have MICs lower than or similar to those of nystatin against Candida spp. Data on in vivo efficacy of liposomal nystatin in candidiasis (9; D. Gordon, I. Baird, R. Darouiche, V. Fainstein, L. Juaregui, C. Levy, and P. Lewis, Program Abstr. 35th Annu. Meet. Infect. Dis. Soc. Am., abstr. 144, 1997; G. H. Reyes, L. A. Long, F. Florentino, and M. A. Ghannoum, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1676, p. 385, 2000) also suggest a favorable in vivo activity of liposomal nystatin in this clinical setting.
No set of MICs for any of the Candida spp. tested were consistently high, and nystatin and liposomal nystatin MICs for each of the species tested were comparable. In terms of nystatin and liposomal nystatin MIC end points, we initially determined the concentrations which resulted in complete visual inhibition of growth (MIC-0) and 95% inhibition as detected by spectrophotometer. These end points were chosen based on the fungicidal activity of the parent drug, which we have confirmed by MLC determinations, the ability of the drug to give clear end points without significant trailing and its being similar to the end point used for determining amphotericin B MICs. As expected, the visual MIC-0 values were in excellent agreement with MICs from the spectrophotometric 95% reduction for both drugs, and we reported our spectrophotometric readings.
The major modifications we made in the standard NCCLS method were related to the test media and the incubation period. The current NCCLS recommendations for antifungal susceptibility testing of yeasts involve the use of RPMI and a 48-h incubation period (20). RPMI-2 and AM3 were also tested in the present study, and the MICs were evaluated at 24 h of incubation as well as 48 h. Our data show that addition of 2% glucose to standard RPMI 1640 did not alter the nystatin and liposomal nystatin MICs significantly. The only remarkable influence of RPMI-2 on nystatin MICs was the wider range obtained for C. glabrata. Consistent with our previous work with amphotericin B, AM3 gave the widest liposomal nystatin MIC range of all media at 24 h of incubation. The widening of the range was particularly due to the lower MICs for a few isolates with AM3 than with other media. Although this might have indicated that AM3 simplified the detection of isolates for which MICs were relatively high, more data are required for definitive conclusions on this issue. AM3, also known as Penassay broth, was previously shown to lower amphotericin B MICs (33) and to provide discrimination between amphotericin B-susceptible and -resistant isolates (28; Lozano-Chiu et al., 38th ICAAC, abstr. J-18 and J-19b).
On the other hand, comparison of the in vitro activity of the parent compound, nystatin, with that of its lipid formulation, liposomal nystatin, also showed that the test medium influenced the results. The influence of AM3 on nystatin MICs and that on liposomal nystatin MICs were completely opposite. Nystatin-AM3 MICs were higher than those of nystatin-RPMI and nystatin-RPMI-2 in general. In contrast, liposomal nystatin-AM3 MICs were lower than those of liposomal nystatin-RPMI and liposomal nystatin-RPMI-2. These results suggest that the incorporation of nystatin into liposomes somehow resulted in generation of lower AM3 MICs. However, whether the use of AM3 falsely elevated the nystatin MICs or falsely lowered the liposomal nystatin MICs is not clear. The clinical significance of this finding is also unknown.
Determination of the in vitro activity of nystatin and liposomal nystatin against amphotericin B-resistant isolates was the other major objective of our study. Of nine isolates for which amphotericin B MICs were ≥0.25 μg/ml, the MICs of nystatin and liposomal nystatin for seven and six, respectively, in AM3 at 24 h were low. Despite the structural similarity between amphotericin B and nystatin, cross-resistance between the two drugs is thus not a certainty. Differences between polyenes have also been reported by others (4, 10, 34). Our results suggest that nystatin and its lipid formulation might show a favorable activity against some of the isolates resistant to amphotericin B, although this will require validation in an in vivo system. Our other significant observation was the relatively high amphotericin B MICs for all of the C. krusei isolates tested. Supporting this concept are other reports of relatively high amphotericin B MICs for C. krusei isolates (J. H. Rex, M. Lozano-Chiu, V. Paetznick, A. T. Khyne, S. Nangia, A. Arizmendi, L. Riser, P. G. Pappas, and Coinvestigators of the NIAID MSG Candidiasis Subproject, Program Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 324,1998), reports of failure of amphotericin B in treatment of C. krusei infection in mice (12), and demonstrations of the inability of amphotericin B to decrease the fungal burden of the kidneys (7). Moreover, systemic C. krusei infections, especially those in association with neutropenia, have been reported to be difficult to treat with amphotericin B (15, 19, 22). We detected relatively low nystatin and liposomal nystatin MICs for most of the C. krusei isolates tested. Liposomal nystatin thus might provide improved activity against infections due to C. krusei. The lack of correlation between the MICs of fluconazole and liposomal nystatin was not surprising, given the different structures and mechanisms of action of these two antifungal agents.
On the other hand, our finding of high nystatin MICs for some of the amphotericin B-susceptible isolates was striking and supported the concept that amphotericin B susceptibility is not always associated with nystatin susceptibility. Analysis of this finding together with the previously noted nystatin susceptibility of amphotericin B-resistant isolates showed that variability of the in vitro activities of these agents might operate in both directions. Similarly, in vitro activities of nystatin and liposomal nystatin are not always the same. These findings suggest that, although nystatin, amphotericin B, and liposomal nystatin are all polyenes, variations in their in vitro efficacies against clinical Candida isolates are possible. Whether these in vitro differences have in vivo implications requires clinical investigations.
In summary, we have confirmed that nystatin and liposomal nystatin are similarly active in vitro against a variety of Candida species. Relatively low liposomal nystatin MICs obtained for some amphotericin B-resistant isolates are particularly noteworthy. Further investigations focusing on determination of breakpoint values and the in vivo activity of liposomal nystatin in infections due to amphotericin B-resistant Candida isolates are required.
Acknowledgments
This work was supported in part by a grant to Sevtap Arikan from the Turkish Scientific and Technical Research Council (TÜBITAK), Turkey.
REFERENCES
- 1.Anaissie, E., V. Paetznick, R. Proffitt, M. J. Adler, and G. P. Bodey. 1991. Comparison of the in vitro antifungal activity of free and liposome-encapsulated amphotericin B. Eur. J. Clin. Microbiol. Infect. Dis. 10:665-668. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie, E. J., N. C. Karyotakis, R. Hachem, M. C. Dignani, J. H. Rex, and V. Paetznick. 1994. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J. Infect. Dis. 170:384-389. [DOI] [PubMed] [Google Scholar]
- 3.Anaissie, E. J., V. L. Paetznick, L. G. Ensign, A. Espinel-Ingroff, J. N. Galgiani, C. A. Hitchcock, M. LaRocco, T. Patterson, M. A. Pfaller, J. H. Rex, and M. G. Rinaldi. 1996. Microbroth antifungal susceptibility testing of Candida albicans and Cryptococcus neoformans with and without agitation: an eight-center collaborative study. Antimicrob. Agents Chemother. 40:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broughton, M. C., M. Bard, and N. D. Lees. 1991. Polyene resistance in ergosterol producing strains of Candida albicans. Mycoses 34:75-83. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo-Munoz, A. J., G. Quindos, C. Tur, M. T. Ruesga, Y. Miranda, O. del Valle, P. A. Cossum, and T. L. Wallace. 1999. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother. 44:397-401. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., and P. Warn. 1999. Dose range evaluation of liposomal nystatin and comparisons with amphotericin B and amphotericin B lipid complex in temporarily neutropenic mice infected with an isolate of Aspergillus fumigatus with reduced susceptibility to amphotericin B. Antimicrob. Agents Chemother. 43:2592-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher, M. A., S.-H. Shen, J. Haddad, and W. F. Tarry. 1989. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 33:1443-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galgiani, J. N., and M. L. Lewis. 1997. In vitro studies of activities of the antifungal triazoles SCH 56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob. Agents Chemother. 41:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groll, A. H., V. Petraitis, R. Petraitiene, A. Field-Ridley, M. Calendario, J. Bacher, S. C. Piscitelli, and T. J. Walsh. 1999. Safety and efficacy of multilamellar liposomal nystatin against disseminated candidiasis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 43:2463-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebeka, E. K., and M. Solotorvsky. 1965. Development of resistance to polyene antibiotics in Candida albicans. J. Bacteriol. 89:1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, E. M., J. O. Ojwang, A. Szekely, T. L. Wallace, and D. W. Warnock. 1998. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob. Agents Chemother. 42:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karyotakis, N. C., E. J. Anaissie, R. Hachem, M. C. Dignani, and G. Samonis. 1993. Comparison of the efficacy of polyenes and triazoles against hematogenous Candida krusei infection in neutropenic mice. J. Infect. Dis. 168:1311-1313. [DOI] [PubMed] [Google Scholar]
- 13.Larone, D. H. 1995. Medically important fungi: a guide to identification, 3rd ed. ASM Press, Washington, D.C.
- 14.Law, D., C. B. Moore, and D. W. Denning. 1997. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J. Antimicrob. Chemother. 40:109-112. [DOI] [PubMed] [Google Scholar]
- 15.McQuillen, D. P., B. S. Zingman, F. Meunier, and S. M. Levitz. 1992. Invasive infections due to Candida krusei: report of ten cases of fungemia that include three cases of endophthalmitis. Clin. Infect. Dis. 14:472-478. [DOI] [PubMed] [Google Scholar]
- 16.Meade, R. H. 1979. Drug therapy reviews: clinical pharmacology and therapeutic use of antimycotic drugs. Am. J. Hosp. Pharm. 36:1326-1334. [PubMed] [Google Scholar]
- 17.Mehta, R. T., R. L. Hopfer, L. A. Gunner, R. L. Juliano, and G. Lopez-Berestein. 1987. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Antimicrob. Agents Chemother. 31:1897-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta, R. T., R. L. Hopfer, T. McQueen, R. L. Juliano, and G. Lopez-Berestein. 1987. Toxicity and therapeutic effects in mice of liposome-encapsulated nystatin for systemic fungal infections. Antimicrob. Agents Chemother. 31:1901-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merz, W. G., J. E. Karp, D. Schron, and R. Saral. 1986. Increased incidence of fungemia caused by Candida krusei. J. Clin. Microbiol. 24:581-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Nguyen, M. H., C. J. Clancy, V. L. Yu, Y. V. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi. 1998. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425-430. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen, V. Q., and R. L. Penn. 1987. Candida krusei infectious arthritis. A rare complication of neutropenia. Am. J. Med. 83:963-965. [DOI] [PubMed] [Google Scholar]
- 23.Oakley, K. L., C. B. Moore, and D. W. Denning. 1999. Comparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole. Antimicrob. Agents Chemother. 43:1264-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahls, S., and A. Schaffner. 1994. Comparison of the activity of free and liposomal amphotericin B in vitro and in a model of systemic and localized murine candiasis. J. Infect. Dis. 169:1057-1061. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, and M. G. Rinaldi. 1994. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J. Clin. Microbiol. 32:1650-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, M. G. Rinaldi, C. R. Cooper, and M. R. McGinnis. 1995. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quindos, G., A. J. Carrillo-Munoz, M. T. Ruesga, R. Alonso-Vargas, Y. Miranda, C. Tur-Tur, M. Rubio, T. L. Wallace, P. A. Cossum, E. Martin-Mazuelos, R. Cisterna, and J. Ponton. 2000. In vitro activity of a new liposomal nystatin formulation against opportunistic fungal pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 19:645-648. [DOI] [PubMed] [Google Scholar]
- 28.Rex, J. H., C. R. Cooper, Jr., W. G. Merz, J. N. Galgiani, and E. J. Anaissie. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 39:906-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanglard, D., K. Kuchler, F. Ischer, J.-L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace, T. L., V. Paetznick, P. A. Cossum, G. Lopez-Berenstein, J. H. Rex, and E. Anaissie. 1997. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob. Agents Chemother. 41:2238-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods, R. A., M. Bard, I. E. Jackson, and D. J. Drutz. 1974. Resistance to polyene antibiotics and correlated sterol changes in two isolate of Candida tropicalis from a patient with an amphotericin B-resistant funguria. J. Infect. Dis. 129:53-58. [DOI] [PubMed] [Google Scholar]