Abstract
CD4+ T cells control the effector function, memory, and maintenance of CD8+ T cells. Paradoxically, we found that absence of CD4+ T cells enhanced adoptive immunotherapy of cancer when using CD8+ T cells directed against a persisting tumor/self-antigen. However, adoptive transfer of CD4+CD25- T cells (Thelper) with tumor/self-reactive CD8+ T cells and vaccination into CD4+ T cell-deficient hosts induced autoimmunity and regression of established melanoma. Transfer of CD4+ T cells that contained a mixture of Thelper and CD4+CD25+ T regulatory cells (Treg) or Treg cells alone prevented effective adoptive immunotherapy. Maintenance of CD8+ T cell numbers and function was dependent on Thelper cells that were capable of interleukin-2 production as therapy failed when Thelper cells were derived from IL-2-/- mice. These findings reveal that Thelper cells can help break tolerance to a persisting self-antigen and treat established tumors through an IL-2-dependent mechanism, but requires simultaneous absence of naturally occurring Treg cells to be effective.
Introduction
Although CD8+ T cells have been shown to be potent mediators of antitumor immunity, the role of CD4+ T cells is largely undefined (1, 2). In the absence of CD4+ T cell help, CD8+ T cells against viral or foreign antigens can become lethargic(3), deleted(4), or lose the capacity to become and remain memory CD8+ T cells upon rechallenge(5-7). Therefore, the use of self-reactive CD8+ T cells in the adoptive immunotherapy of cancer may face similar fates, as T cells must remove tumor-antigen in the context of persisting self-antigen. One theoretical means of improving immunotherapy to self may involve the provision of CD4+ T cell help, since helper cells facilitate CD8+ T cell activation, function and survival(1, 6, 8). Nonetheless, naturally occurring CD4+ T cells represent a double-edged immunological sword: in addition to their helper functions, one T cell subset, naturally occurring CD4+CD25+ T regulatory cells (hereafter referred to as Treg), suppresses T cells and controls immunological tolerance to self-antigens (9-12).
In recent years, naturally occurring CD4+CD25+ T regulatory (Treg) cells have emerged as the dominant T cell population governing peripheral self-tolerance (13-15). CD4+CD25+ T cells develop in the thymus and represent 5-10% of the peripheral CD4+ T cell compartment. They constitutively express the high affinity IL-2 receptor or CD25 (IL-2Rα), glucocorticoid-induced TNFR (GITR), CTLA-4, and the transcription factor Foxp3 (forkhead box P3)(13). The mechanism of suppression is through cell-cell contact but how Treg cells induce and maintain self-tolerance in vivo is still unknown.
In mice, autoimmune destruction of a variety of tissues can be triggered by the removal of Treg cells(12, 16). Organ-specific destruction of tissue expressing a self-antigen can be further enhanced by self-antigen vaccination or through the provision of inflammatory signals when functional Treg cells are absent(17). When lymphopenic mice are reconstituted with a normal repertoire of CD4+CD25- T cells (hereafter referred to as Thelper), autoimmunity is observed in multiple tissues and co-transfer of Treg cells abrogates these effects(18). Furthermore, depletion of Treg cells can enhance tumor protection to tumor-associated antigens that are expressed as self-antigens(19, 20).
Spontaneous autoimmunity is also seen in IL-2-/-, IL-2 receptor (IL-2R) α-/-, IL-2 receptor (IL-2R) β-/-, JAK-3-/-, STAT-5-/-, and Foxp3 deficient mice(15, 21, 22). The CD4+ T cell subset that remains in each of these knockouts is devoid of functional Treg cells. Together, these observations suggest a role for IL-2 and Treg cells in controlling effector T lymphocytes specific for self-antigens in vivo.
Because Treg cells are able to control autoimmunity to naturally expressed self-antigens, they may play a role in T cell tolerance to self-antigens naturally or over-expressed by tumors. We hypothesized that by removing the Treg cell subset we could use the autoimmune potential present within the normal repertoire of CD4+ T cells to help self-reactive CD8+ T cells mediate antitumor immunity against tumors expressing self-antigens.
Previously we have shown that adoptive transfer of CD8+ T cells from pmel-1 transgenic mice which recognize the melanocyte differentiation antigen, gp100, could cause autoimmunity and the regression of established tumors in tumor-bearing syngeneic animals. This therapy was successful after vaccination with a virus encoding a tumor-antigen, and exogenous administration of a γc-signaling cytokine, either IL-2 or IL-15(23, 24). In the present report, we transferred different CD4+ T cell subsets along with pmel-1 CD8+ T cells, and vaccination into CD4+ T cell-deficient tumor-bearing animals. Although help against foreign and viral antigens has been described numerous times (reviewed in(2)), we sought to understand the effect of different CD4+ T cell subpopulations on adoptive immunotherapy of established tumors as well as the mechanisms that cause and break tolerance to tumors in an environment of persisting self-antigen. We conclude that naturally occurring Thelper cells can help self-reactive CD8+ T cells break tolerance to self through an IL-2-dependent mechanism, but require the absence of naturally occurring Treg cells to be effective.
Material and Methods
Mice and tumor cells. Pmel-1 TCR transgenic mice have been described previously (23). Pmel-1 TCR transgenic and pmel-1 Thy1.1+ mice were bred and kept at the NIH animal facilities. C57BL/6, C57BL/6 CD45.1, CD4-/-, CD8-/-, RAG-1-/-, IL-2+/- and Thy1.1+ mice were obtained from Jackson laboratories (Bar Harbor, ME) and bred at the NIH animal facility. IL-2-/- mice were obtained by crossing IL-2+/- mice together and verified by PCR (Jackson Laboratories). B16.F10 (H-2b), hereafter called B16, is a gp100+ spontaneous murine melanoma obtained from the National Cancer Institute tumor repository and was maintained in culture media (CM) as previously described (23, 62).
Peptides and recombinant fowlpox and vaccinia viruses. All synthetic peptides were synthesized using regular F-MOC chemistry. The synthetic H-2Db-restricted peptide, hgp10025-33, KVPRNQDWL, was synthesized by Peptide Technologies (Washington, D.C.) to a purity > 99% by HPLC and amino acid analysis. All recombinant viruses encoding hgp100 have been described before and were kindly provided by Therion Biologics (Cambridge, MA) (23).
In vitro activation of pmel-1 T cells and cytokine release assay. Splenocytes from mice were depleted of erythrocytes by hypotonic lysis, cultured in CM with 30 IU/ml rhIL-2 in the presence of 1 μM hgp10025-33 peptide and used on day 6 after start of the culture. For cytokine release assays, sorted Thy1.1+ pmel-1 T cells (5.0 × 104) were cocultured in CM with 105 irradiated (3000 rads) splenocytes pulsed with 1μM hgp10025-33 peptide. Supernatants were collected after 24 hrs and tested using a mIFN-γ ELISA kit (Endogen, Cambridge, MA) according to manufacturer's protocol.
Flow cytometry and CFSE staining. From fresh splenocytes, erythrocytes were removed by hypotonic lysis and cells were stained with the indicated mAbs: CD8α-Allophycocyanin (53-6.7), CD4-Allophycocyanin (H129.19), Vb13-FITC (MR12-3), CD25-PE (PC61), Thy1.1-PE (OX-7). For IFN-γ intracellular staining, splenocytes were activated with lymphocyte activating cocktail (BD Biosciences) for 6 hours, and then fixed and stained using cytofix and cytoperm intracellularing staining protocol (BD Biosciencs). All antibodies were purchased from BD Biosciences. Propidium iodide staining cells were excluded from analysis. Samples were analyzed using a FACScalibur™ flow cytometer and CELLquest™ software. For CFSE staining, pmel-1 T cells were activated and cultured for 1 week before being sorted on CD8+ enrichment columns (R&D systems) and labeled with 2uM CFSE dye (CFDA SE Cell Tracer Kit; Molecular Probes). Pmel-1 T cells were prepared by resuspending in prewarmed PBS (37°C) containing the appropriate concentration of CFSE dye for 15 minutes. Cells were then washed and incubated with fresh PBS for an additional 30 minutes to allow complete modification of the probe before adoptive cell transfer. 4 days later, pmel-1 T cells were isolated from mouse splenocytes and analyzed by FACS.
Purification of CD4+ T cell subsets and pmel-1 T cells. Unfractionated CD4+ T cells were purified from single cell suspensions of IL-2-/-, C57BL/6, or C57BL/6 CD45.1 spleens, using a CD4+ enrichment column (R&D Systems). Treg cells were subsequently purified using MACS CD4+CD25+ Isolation Kits (Miltenyi Biotech) to a purity >95%. Thelper cells were purified on a LS+ selection column twice (Miltenyi Biotech) to obtain >98% depletion of CD4+CD25+ T cells. Cells were either cultured overnight in CM or transferred immediately. Cells were also used for suppression assays to confirm their function. Pmel-1 Thy1.1+ T cells were purified from splenocytes of RAG-1-/- mice by labeling with Thy1.1-PE (15 μg/ 1.0 × 108 cells/mL) for 10 minutes. Cells were subsequently washed and sorted from whole splenocytes with anti-PE microbeads using LS+ selection columns (Miltenyi Biotech).
In vitro suppression assays. CD4+CD25+ and CD4+CD25- T cells were isolated from peripheral lymph nodes of C57BL/6 mice by FACS sorting as previously described (28). Subsequently, they were activated with irradiated TΔS (T-depleted splenocytes; 1:1 ratio), soluble anti-CD3 (0.5 μg/mL), and human IL-2 (5 ng/mL, 100 U/mL) for 72 h and then were split and maintained in IL-2 medium for 7-14 days (28). Pmel-1 transgenics were activated with 1 μM of hgp10025-33 peptide, which was pulsed onto γ-irradiated (3000 rads) TΔS for 30 minutes and washed twice before co-culture. In vitro suppression assays were performed by stimulating pmel-1 CD8+ cells (5.0 × 104) alone or in the presence of titrated numbers of either freshly isolated or activated CD4+CD25+ or CD4+CD25- T cells. Cultures were stimulated with either soluble anti-CD3 (0.5ug/ml) or in the presence of peptide-pulsed TΔS for 72hrs, as previously described (28). Supernatants were taken on day 3 of co-culture for IFN-γ release and 1 μCi [3H]-TdR was added for the last 8 hours. All data represent the average cpm of triplicate determinations. IFN-γ was measured using an ELISA kit (R&D systems).
Adoptive cell transfer. Mice were injected subcutaneously with 1.0-5.0 × 105 B16 melanoma cells as depicted. The standard treatment regimen consisted of the intravenous administration of 1.0 × 106 pmel-1 T cells activated for 1 week in vitro with 1 μM hgp100 peptide and subsequently purified using CD8+ enrichment kits (R&D Systems) to a purity > 98%. CD4+CD25+ (1.0 × 105), CD4+CD25- (1.0 × 106), unfractionated CD4+ T cells (1.0 ×106), IL-2-/- CD4+CD25- T cells (1.0 × 106), or a mixture of Treg to Thelper (1:10) were co-injected with pmel-1 T cells as indicated. One day before adoptive cell transfer of T cells, C57BL/6 mice underwent sub-lethal whole-body irradiation (500 cGy) (24). Mice were vaccinated by intravenous injection with 2.0 × 107 PFU of a recombinant fowlpox virus encoding human gp100 (rFPVhgp100) on the same day of transfer. IL-2 (Chiron) was administered for 4 days directly following vaccination by daily intraperitoneal injections of 600,000 IU rhIL-2 in PBS. Tumors were measured in a blinded fashion using calipers and the products of perpendicular diameters were recorded.
Masked uveitis score. Eyes were enucleated from mice and placed in 4% gluteraldehyde for 30 minutes. Subsequently, eyes were transferred in 10% formalin for 48 hours, and then embedded in methylarcylate. Four to five micron sections were taken along pupillary-optical axis. Sections were evaluated by a masked ophthalmic pathologist using the score as follows: minimal = 0.5, mild = 1, moderate = 2, severe = 3. Scores where given for iridiocyclitis, choroiditis, vitritis, and retinal involvement. The grading was then combined for a final masked uveitis score.
Statistics. Tumor graphs were compared using Wilcoxon rank sum test. Factorial ANOVA was used to compare autoimmunity in the eye. The t-test for means was used to analyze IFN-γ ELISA results.
Results
Naturally occurring CD4+ T cells prevent immunotherapy to established tumors.
We have previously demonstrated that adoptive cell therapy using either 107 naïve or activated pmel-1 T cells, recombinant fowl-pox virus encoding human gp100 (rFPVhgp100) vaccination, and exogenous IL-2 could effectively cure established B16 melanoma in wild-type (WT) syngeneic mice(23). This therapy was not dependent on host T- or B-lymphocytes for its effectiveness. However, because the transfer of a large precursor frequency of CD8+ transgenic T cells can be independent of the effects of CD4+ T cells(25), we evaluated whether host lymphocytes could have a positive or negative effect by transferring a smaller dose of pmel-1 T cells (1.0 × 106/mouse).
To evaluate the impact of host lymphocytes on a CD8+ T cell-mediated adoptive cell therapy, we tested the relative efficacy of treatment in mice with either a selected loss of lymphocyte subsets through genetic knockouts or through whole body irradiation. Recombination-activating gene-1 deficient mice (RAG)-1-/-, CD4-/- mice, CD8-/-, or C57BL/6 mice were inoculated with the highly aggressive, poorly immunogenic B16 melanoma and treated intravenously 14 d later with the tripartite treatment regimen comprised of activated pmel-1 T cells (CD25+, CD44high, CD62Llow, CD69high), rFPVhgp100, and IL-2(23). Treatment of B16 melanoma in mice either sub-lethally irradiated (500 cGy) or on a RAG-1-/- background was markedly enhanced when compared to non-irradiated WT C57BL/6 mice (Fig. 1a). Importantly, B16 melanoma grew at the same rate in the no treatment controls indicating that the absence of lymphocytes did not alter the growth kinetics of the B16 tumor.
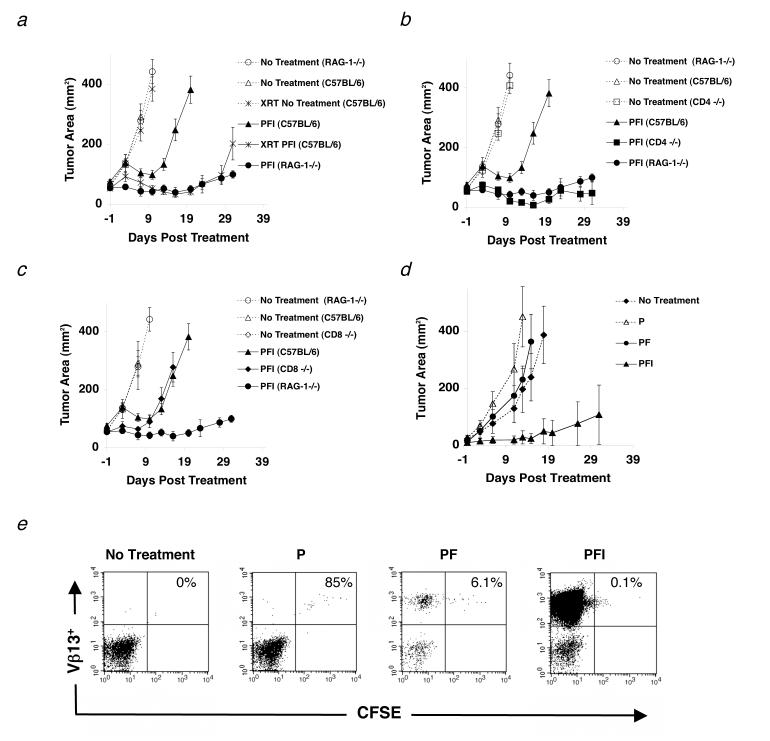
Figure 1.
Naturally occurring CD4+ T cells inhibit effective immunotherapy to established tumors. (a-d) Mice were inoculated with 1.0 × 105 cells of B16 melanoma on day -14 before adoptive cell transfer with 1.0 × 106 pmel-1 T cells (P), 2.0 × 107 PFU rFPVhgp100 (F), and 600,000 IU of exogenous IL-2 (I), which was given daily for 3-4 days. (a) Tumor regression in C57BL/6 mice (□) is compared with RAG-1-/- mice (•) and C57BL/6 mice receiving 500 (cGy) whole body irradiation on day -1 of treatment (□). (b) Tumor regression in C57BL/6 mice (□) is compared with RAG-1-/- mice (•) and CD4-/- mice (□). (c) Tumor regression in C57BL/6 mice (□) is compared with RAG-1-/- mice (•) and CD8-/- mice (◆) in the same experiment. Data are represented as mean tumor size ± S.E.M. Experiments were independently repeated twice. (d-e) Tumor regression is IL-2 dependent. (d) Transfer of pmel-1 T cells alone (Δ) or pmel-1 T cells and rFPVhgp100 vaccine (•) into tumor-bearing RAG-1-/- hosts is similar to no treatment (◆). Addition of exogenous IL-2 with cells and vaccine is required for tumor regression (□). (e) CFSE profile of adoptively transferred pmel-1 T cells into RAG-1-/- hosts. Pmel-1 CD8+ T cells were labeled with CFSE and adoptively transferred into tumor-bearing RAG-1-/- hosts alone (P), with vaccination (PF), or with vaccination and exogenous IL-2 (PFI). 4 days later, splenocytes from treated mice were analyzed by flow cytometry. Gated on CD8+ T cells and displayed as Vβ13-PE vs. CFSE.
Next, we continued with a genetic dissection of the adaptive immune system by using selective knockout mice for different T cell subsets. In the same experiment, tumor regression was augmented in CD4-/- mice, similar to that seen in RAG-1-/- mice (Fig. 1b). Because both RAG-1-/- and CD4-/- do not develop CD4+ T cells, we employed our adoptive cell transfer regimen in CD8-/- mice, whose immune system contains CD4+ T cells. As shown in figure 1c, there was no augmentation of tumor treatment in CD8-/- mice when compared with WT C57BL/6 controls. Tumor regression in MHC class II-/-, athymic nude, and severe combined immunodeficient (SCID) mice was also similar to RAG-1-/- mice and CD4-/- mice in the same experiment (data not shown). Therefore, the endogenous CD4+ T cell repertoire is capable of suppressing antitumor immunity to established tumors as demonstrated 20 years earlier(26).
Regression of self-antigen expressing tumors is independent of homeostatic proliferation
Because it has been reported that homeostatic proliferation of adoptively transferred CD8+ T lymphocytes can protect mice from tumor challenge (27), we evaluated whether or not regression of established B16.F10 tumors (expressing gp100) in RAG-1-/- mice, which also express the gp100 antigen in their skin and eyes, was due to nonspecific activation of CD8+ T cells by adoptive transfer. In figure 1d, RAG-1-/- mice bearing established tumors were treated with carboxyl-fluorescein succinimidyl ester (CFSE)-labeled pmel-1 T cells and given rFPVhgp100 vaccine and/or exogenous IL-2. Four days later, splenocytes were isolated from recipient mice and analyzed by flow cytometry. CFSE staining revealed that pmel-1 T cells, designated here as Vβ13+, transferred alone (P) divided minimally when compared to mice receiving cells and vaccination (PF) or cells, vaccination, and IL-2 (PFI) (Fig. 1e). Surprisingly, even though pmel-1 T cells from mice that received cells and vaccine (PF) had more T cell divisions (Fig. 1e) and T cell numbers (Table I) than mice receiving cells alone (P), tumor regression was similar (Fig. 1d). Durable tumor regression was only seen in mice receiving pmel-1 T cells, vaccine, and exogenous IL-2 (PFI; Fig. 1d). In this group, CFSE staining demonstrated that pmel-1 T cells proliferated extensively (PFI; Fig. 1e). The frequency of tumor-reactive pmel-1 T cells as indicated by CD8+Vβ13+ staining was substantial, when compared with transfer of cells alone (>3000 fold increase; Table I). These results showed that IL-2 not only enhances T cell function in vivo(23) but also increases their T cell numbers. Thus in this model, enhanced tumor regression (i.e. autoimmunity) seen in RAG-1-/- mice was dependent on exogenous IL-2 administration not homeostatic proliferation.
Table I.
Absolute number of pmel-1 CD8+T cell after transfer into RAG-1 deficient hosts. Mice were inoculated with 1.0 × 105 cells of B16 melanoma on day -7 before adoptive cell transfer with pmel-1 T cells (1.0 × 106), plus/minus 2.0 × 107 PFU rFPVhgp100, and/or exogenous IL-2 (600,000 IU) given daily for 3-4 days. Four days later, splenocytes were harvested and the frequency of pmel-1 T cells was determined by flow cytometry. Absolute number of cells was calculated by multiplying the frequency of pmel-1 T cells by the splenocyte count (n = 2). Fold increase is determined by dividing the absolute number of each group by the absolute number of pmel-1 T cells from the transfer of cells alone (pmel-1 T cells only).
| Treatment group | Absolute# X 103 | +/- S.E.M. X 103 | Fold Increase |
|---|---|---|---|
| No Treatment | 0 | 0 | 0 |
| Pmel-1 T cells only (P) | 7 | 0.3 | 1 |
| Pmel-1 T cells and vaccine (PF) | 393 | 19 | 55 |
| Pmel-1 T cells, vaccine, and IL-2 (PFI) | 26913 | 780 | 3753 |
Treg cells suppress self-reactive CD8+ T cells in vitro.
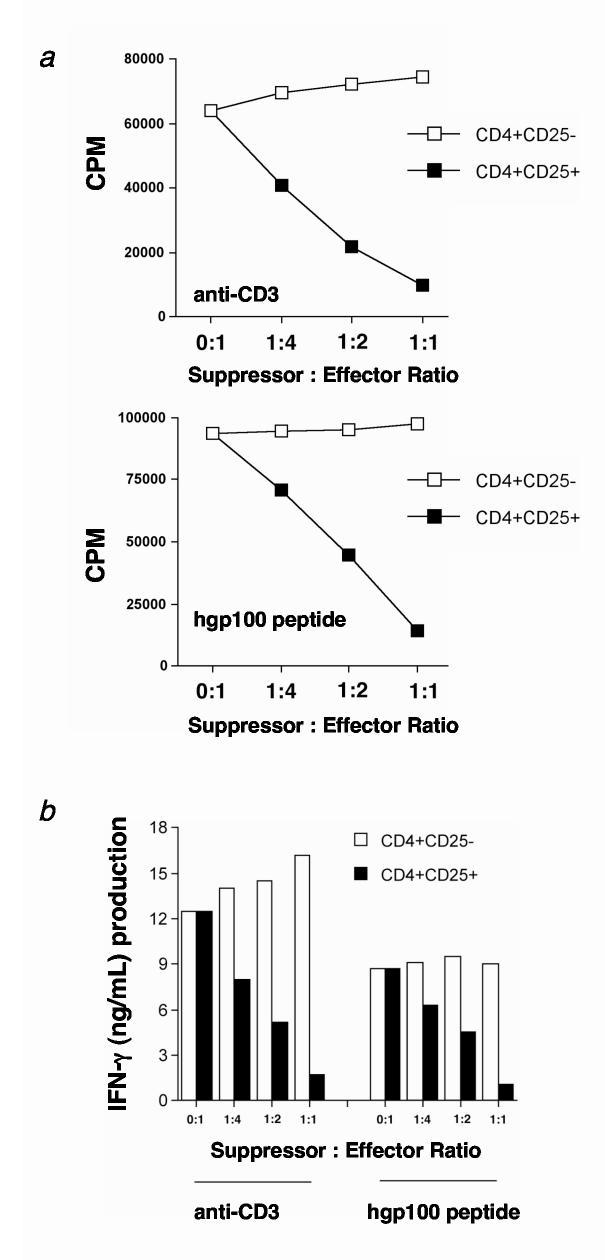
Because Treg cells can suppress CD4+ and CD8+ T cells(28), we evaluated whether Treg cells could suppress transgenic pmel-1 CD8+ T cells. Therefore, naturally occurring CD4+ T cells were purified from lymph nodes of C57BL/6 mice and fractionated into Treg and Thelper cells. Sorted Treg and Thelper cell subsets were separately activated with anti-CD3 and IL-2 as previously described(28) and then cocultured with either anti-CD3 activated (0.5 μg/mL) or peptide stimulated (1 μM hgp100) pmel-1 T cells. From this assay, we observed a dose-dependent suppression of proliferation (Fig. 2a) and IFN-γ production (Fig. 2b) when pmel-1 T cells were mixed with Treg cells. By contrast, coculture with CD4+CD25- Thelper cells did not suppress proliferation or IFN-γ production. As reported in the literature, similar results were found when Treg cells were cocultured with Thelper cells (data not shown and (28)). Treg cells cultured alone proliferated minimally (data not shown). These results indicated that Treg cells could profoundly suppress tumor/self-antigen-specific pmel-1 T cells and naturally occurring CD4+CD25- Thelper cells in vitro.
Figure 2.
CD4+CD25+ Treg cells suppress pmel-1 CD8+ T cell proliferation and function in vitro. (a-b) Highly purified Treg cells from C57BL/6 lymph nodes suppress (a) proliferation and (b) IFN-γ production of pmel-1 T cells in vitro. Treg or Thelper cells were activated with irradiated APC (1:1 ratio) and soluble anti-CD3 antibody (0.5 μg/mL) for 3 days in the presence of 100 IU human IL-2. Cells were then split and maintained for an additional 7-14 days in 100 IU human IL-2. (a) Pmel-1 T cells were stimulated with soluble anti-CD3 or 1 μM hgp10025-33 peptide-pulsed T-depleted spleen cells in the presence of activated Treg (■) or Thelper (□) cells for 3 days in the absence of IL-2 at the indicated ratios. Maximum suppression of pmel-1 T cell proliferation is seen at a 1:1 suppressor to effector ratio. (b) Pmel-1 T cells were stimulated with anti-CD3 or 1 μM hgp10025-33 peptide-pulsed TΔS cells in the presence of activated Treg (■) or Thelper (□) cells for 3 days in the absence of IL-2 at the indicated ratios. Maximal suppression of IFN-γ production by pmel-1 T cells for both modes of stimulation is seen at a 1:1 suppressor to effector ratio. Experiments were independently repeated twice.
Thelper cells are required to maintain effector self-reactive CD8+ T cells in vivo
We observed that transfer of pmel-1 T cells with or without rFPVhgp100 vaccination into tumor-bearing RAG-1-/- hosts could not induce regression of B16 melanoma, despite the fact that RAG-1-/- hosts lack Treg cells (Fig. 1d). As seen in figure 1d, the addition of exogenous IL-2 was necessary for full therapeutic effectiveness. Therefore, we hypothesized that exogenous IL-2, in this setting, was substituting for a T helper cell. Because absence of T cell help can hinder the in vivo maintenance of CD8+ T cells and development of memory T cells(4, 7, 29, 30), we surmised that effector CD8+ T cells also needed help to induce the regression of established tumors, in addition to removal of Treg cells.
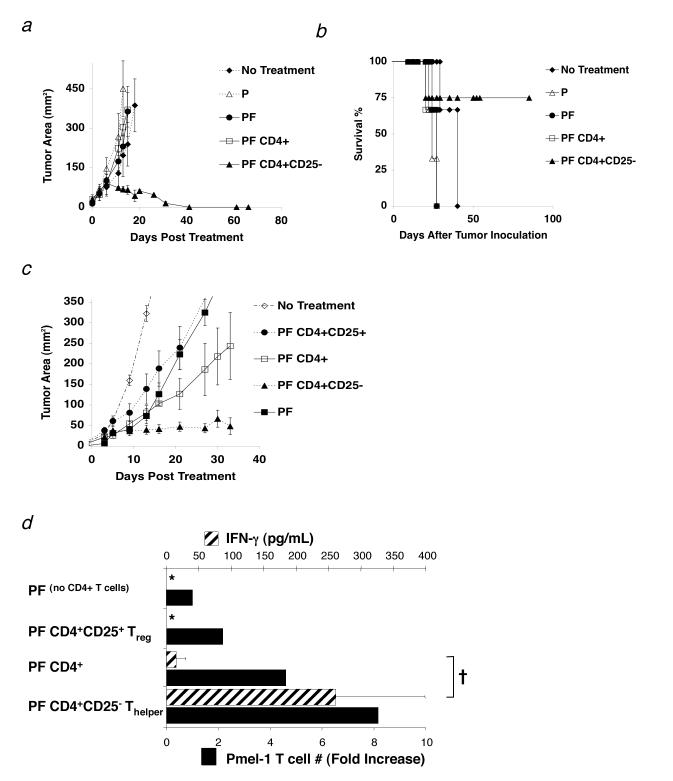
To test if the transfer of CD4+ T cells might replace the requirement for exogenous IL-2 and help pmel-1 T cells eradicate tumors, we transferred unfractionated CD4+ T cells and sorted CD4+CD25- T cells with pmel-1 T cells and rFPVhgp100 vaccine into tumor-bearing RAG-1-/- hosts. The combination of pmel-1 T cells, vaccine, and CD4+CD25- T cells induced tumor regression and long-term survival without exogenous IL-2 (Fig. 3a and 3b), whereas no or minimal therapeutic effect was seen with unfractionated CD4+ T cells (Fig. 3a). Furthermore, adoptive transfer of CD4+CD25- T cells alone or in combination with pmel-1 cells without vaccine did not induce durable and stable tumor regression, showing the requirement for vaccination in this treatment model (data not shown).
Figure 3.
Thelper cells maintain self-reactive effector CD8+ T cells in vivo. (a-d) RAG-1-/-mice were inoculated with 3.0 × 105 cells of B16 melanoma between day -7 and -14 before adoptive cell transfer. (a) Thelper cells help CD8+ T cell mediated antitumor immunity to established B16 melanoma. Mice receiving 1.0 × 106 pmel-1 T cells (P), 2.0 × 107 PFU fowlpox virus encoding human gp100 (F), and 1.0 × 106 Thelper cells maintained long-term, durable regression of established B16 melanoma (□). Data are represented as mean tumor size ± S.E.M. Data represent 6 independent experiments. (b) Survival of mice in (a) treated with pmel-1 T cells (P), rFPVhgp100 vaccine (F), and Thelper cells was maintained up to 80 days post treatment (□). (c) Adoptive cell transfer of sorted 1.0 × 105 Treg cells (•) or a mixture of 1.0 × 105 Treg and 1.0 × 106 Thelper cells (1:10 ratio; CD4+ T cells; □) with pmel-1 T cells and vaccination does not maintain tumor regression of B16 melanoma. Removal of the Treg subset from the unfractionated CD4+ T cell pool allows the helper function of the remaining Thelper cells to become apparent (□). Data represent 5 independent experiments. Experiment in (c) was stopped at 35 days post treatment to allow for analysis of adoptively transferred pmel-1 T cells. (d) Thelper cells maintain maximal pmel-1 T cell numbers (solid black bars) and function (cross-hatched bars) in the absence of IL-2. †, P <0.037. Proliferation bar graphs represent fold increase in the absolute number of pmel-1 T cells (% CD8+ Thy1.1+ Vβ13+ T cells × splenocyte count) taken from pooled spleens 5 wks after transfer (n=2). Fold increase is defined as: (absolute #pmel-T cells in a group divided by absolute #pmel-1 T cells from the group which received no CD4+ T cells (PF)). For all functional assays, pmel-1 Thy1.1+ CD8+ T cells were purified from spleens of treated mice 5 wks after transfer and stimulated with 1μM hgp10025-33 peptide-pulsed γ-irradiated spleen cells for 24 hours (n = 2). All groups were also tested against non-peptide-pulsed targets, which resulted in no production of IFN-g (data not shown). Data are represented as IFN-γ (pg/mL) ± S.E.M. Experiments were repeated twice. The symbol, *, represents an undetectable value.
Although in figure 3a there was no tumor regression observed when unfractionated CD4+ T cells were used, we found in repeated experiments that the transfer of unfractionated CD4+ T cells had a variable effect on tumor regression; ranging from negligible (Fig. 3a) to a modest suppression of tumor growth (Fig. 3c). We found in sorted CD4+ T cell preparations that the CD4+CD25+ T cell population varied from 2-13% (data not shown). Therefore, we hypothesized that the variability of the antitumor responses was due to the relative percentages of regulatory and helper T cell subsets. To solve the variability between these subsets, we prepared Treg and Thelper cells from a common pool of CD4+ T cells or from different congenic strains (CD4+CD25+CD45.1+ or CD4+CD25-CD45.2+ T cells) and fixed the ratio at 1:10 (Treg: Thelper) for subsequent experiments, which is the accepted physiological ratio in vivo(13). This ratio was verified in vivo by flow cytometry (data not shown).
Adoptive transfer of a mixture of Treg and Thelper cells at a 1:10 ratio resulted in tumor growth that was similar to transfer of unfractionated CD4+ T cells that had a comparable ratio (Fig. 3c and data not shown). As seen in figure 3a, the transfer of sorted Thelper cells (1.0 × 106) with pmel-1 T cells and rFPVhgp100 vaccine resulted in durable and stable tumor regression (Fig. 3c). However, transfer of sorted Treg cells (1.0 × 105) with pmel-1 T cells and vaccine resulted in minimal tumor regression that was similar to pmel-1 T cells and vaccination alone (Fig. 3c).
To understand the differences between the treatment groups, we analyzed the impact of these CD4+ T cell subpopulations on the persistence and function of adoptively transferred pmel-1 T cells. By using a congenic marker system (Thy1.1+/Thy1.2+), we were able to measure the persistence of Thy1.1+ pmel-1 T cells 5 wks after vaccination. We found that the absolute numbers of pmel-1 T cells was enhanced by cotransfer with Thelper cells, but not with a mixture of Treg: Thelper (1:10 ratio) (Fig. 3d). Function of sorted Thy1.1+ pmel-1 T cells as measured by IFN-γ enzyme-linked immunosorbent assay (ELISA) after peptide stimulation (1 μM hgp100) ex vivo was significantly enhanced with the addition of Thelper cells (Fig. 3d; †; P = 0.037).
Next, pmel-1 T cells were taken from mice treated with Treg cells and analyzed ex vivo for T cell numbers and IFN-γ secretion. We found that pmel-1 T cell numbers were decreased approximately 4-fold when compared with Thelper cells and function was similar to the transfer of no CD4+ T cells as measured by IFN-γ ELISA (Fig. 3d). Suppression could only be observed when Treg cells were cotransferred with the Thelper population, which contained approximately 90% CD4+CD25- T cells (Fig. 3d; †; P = 0.037). Thus, Treg cells suppressed adoptive immunotherapy of an established tumor, but Thelper cells improved tumor immunotherapy by maintaining the functionality of tumor/self-reactive CD8+ T cells.
Thelper cells enhance exogenous IL-2 therapy
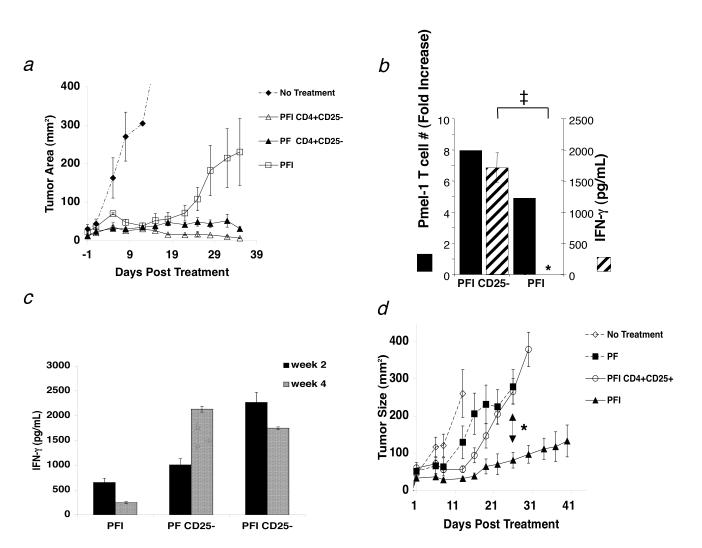
In the current report, we noticed that lower dosages of pmel-1 T cells (106/mouse) could induce stable tumor regression when given in combination with vaccination and Thelper cells but not with exogenous IL-2 (Fig. 4a). IL-2 treatment induced transient tumor regression that lasted for approximately 20 d after adoptive transfer. However, in the presence of Thelper cells, IL-2 therapy was enhanced and induced stable tumor regression beyond 20 d (Fig. 4a).
Figure 4.
Thelper cells can replace exogenous IL-2 and maintain function of tumor-reactive CD8+ T cells but exogenous IL-2 therapy fails in the presence of Treg cells. (a) The combination of 1.0 × 106 pmel-1 T cells, 2.0 × 107 PFU rFPVhgp100 vaccination, and exogenous IL-2 (600,000 IU) given daily for 3 days in RAG-1-/- hosts enhances but does not maintain tumor regression (□). Only cotransfer of 1.0 × 106 Thelper cells with pmel-1 T cells and vaccination into RAG-1-/- hosts helped maintain tumor regression with (Δ) or without (□) exogenous IL-2. Data represent 3 independent experiments. (b) Exogenous IL-2 does not maintain function (crosshatched bars) of pmel-1 T cells unless given in combination with Thelper cells (PFI CD4+CD25- vs PFI, ‡, P = 0.001). Pmel-1 T cell absolute numbers were increased approximately 2-fold in the presence of Thelper cells (solid black bars). Fold increase is defined as: (absolute #pmel-T cells in a group divided by absolute #pmel-1 T cells from the group which received no CD4+ T cells (PF)). The symbol, *, represents an undetectable value. (c) Function of adoptively transferred pmel-1 T cells 2 weeks (solid black bars) and 4 weeks (crosshatched bars) after transfer. Function (IFN-g (pg/mL)) declines with time unless Thelper cells are also transferred: PFI vs. PF CD25- or PFI CD25- (d) Activated pmel-1 CD8+ T cells (CD25+, CD44high, CD62Llow, CD69high; 1.0 × 106) were transferred with 1.0 × 105 sorted Treg cells, rFPVhgp100 vaccination (2.0 × 107 pfu), and exogenous IL-2 on day 7 after tumor inoculation. CD8+ T cells required vaccine and IL-2 for tumor treatment (□). Treg cells inhibited tumor treatment by effector CD8+ T cells in the presence of exogenous IL-2 (Ο) and treatment was similar to groups receiving no exogenous IL-2 (■). Experiments repeated independently three times.
Next, to understand how IL-2 and Thelper cells affected the adoptive immunotherapy differently, we analyzed the function of pmel-1 CD8+ T cells co-transferred with vaccination, exogenous IL-2 and/or Thelper cells ex vivo beyond 20 days after transfer (week 5). We found that the co-transfer of Thelper cells resulted in a significant increase in pmel-1 T cell function that was maintained in vivo, as measured by IFN-γ ELISA (PFI CD25-; ‡, P < 0.001; Fig. 4b). Surprisingly, the provision of pmel-1 T cells, vaccine, and exogenous IL-2 in the absence of Thelper cells was insufficient to maintain long-term CD8+ T cell function (5 wks after transfer) (PFI; Fig. 4b). A more detailed analysis revealed that T cell function goes down with time in the absence of Thelper cells (Fig. 4c). Thus, adoptive cell transfer of Thelper cells could replace and/or enhance exogenous IL-2 therapy of established tumors by maintaining the effector function and numbers of adoptively transferred CD8+ T cells.
Exogenous IL-2 therapy fails in the presence of Treg cells
Next we investigated the effects of exogenous IL-2 on both CD8+ T cells and Treg cells together in vivo. We transferred effector (CD25+, CD44high, CD62Llow, CD69high) pmel-1 T cells along with Treg cells into tumor-bearing RAG-1-/- mice. Vaccination with rFPhgp100 and exogenous IL-2 were also administered and tumor size was monitored for 41 days. Again, as shown earlier, pmel-1 T cells, vaccination with rFPVhgp100, and exogenous IL-2 was required for treatment of established tumors (Figure 4d). However, IL-2 therapy failed when activated pmel-1 T cells were adoptively transferred with Treg cells. These results were similar to groups that received cells and vaccine alone (*, P < 0.007; Figure 4d) and were similar to groups that had endogenous Treg cells (C57BL/6 and CD8-/- mice; Fig. 1a,c). Thus, exogenous IL-2 therapy was only effective when Treg cells were absent.
T cell help is IL-2 dependent, not programmed, and lost in the presence of Treg cells
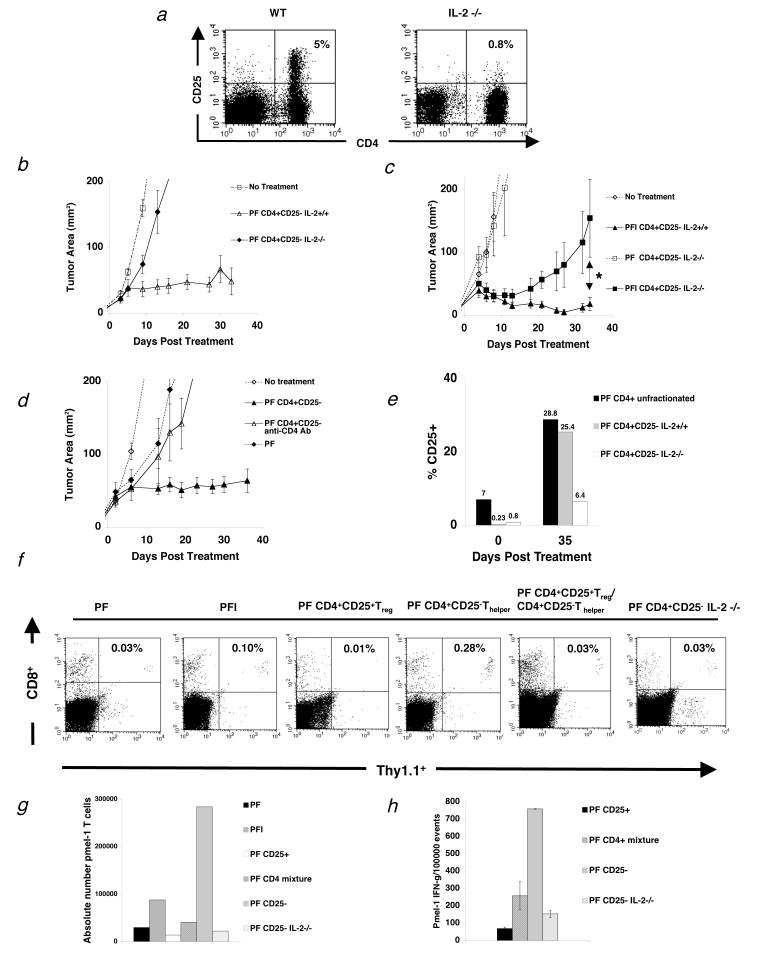
We showed earlier that treatment was either IL-2 dependent or required Thelper cells to be effective. Therefore we hypothesized that tumor regression observed following adoptive transfer of Thelper cells was the result of IL-2 production by the Thelper cell population. To be able to test this hypothesis we derived T helper cells from IL-2-/- mice. However, because precursor Treg cells may be resident in IL-2-/- mice(31) (Fig. 5a), we transferred sorted Thelper cells from IL-2-/- mice together with pmel-1 T cells and vaccine into tumor-bearing RAG-1-/- hosts. In more than 5 independent experiments, we did not observe stable tumor regression using Thelper cells derived from IL-2-/- mice, whereas sorted Thelper cells from IL-2+/+ mice effectively enhanced tumor regression (Fig. 5b).
Figure 5.
T cell help is IL-2 dependent and lost in the presence of Treg cells. (a) Flow cytometry analysis of mouse splenocytes shows that IL-2-/- mice do not develop Treg cells (n=3). (b-c) RAG-1-/- mice were inoculated with 3.0 × 105 cells of B16 melanoma between day -7 before adoptive cell transfer with 1.0 × 106 pmel-1 T cells (P), 2.0 × 107PFU rFPVhgp100 (F), plus Thelper cells from IL-2-/- mice or naturally occurring Thelper cells plus/minus exogenous IL-2 (I). (b) Transfer of Thelper cells from IL-2-/- mice with pmel-1 T cells and vaccination into tumor-bearing RAG-1-/- hosts does not help treatment of established B16 melanoma (◆). (c) Addition of exogenous IL-2 does not restore the helper function of Thelper cells from IL-2-/- mice (■). *, P = 0.021. Data are derived from a single experiment that was independently repeated 3 times. (d) Thelper cells do not program tumor-reactive CD8+ T cells. Depletion of Thelper cells 4 days after transfer with 500 mg of GK1.5 CD4 depleting mAb (Δ). Data represents 3 independent experiments with similar results. Isotype control antibody had no effect on CD4+ T cells and depletion of CD4+ T cells was confirmed by flow cytometry. (e) Thelper cells utilize IL-2 in vivo. CD25 expression on adoptively transferred Thelper cells alone, Thelper cells with Treg(CD4+unfractionated), and Thelper cells derived from IL-2-/- mice, 35 d after treatment. (f) Spleens were taken from tumor-bearing RAG-1-/- mice and analyzed by flow cytometry for the congenic marker Thy1.1 and CD8, which represents the transferred pmel-1 T cells 3 weeks after treatment with the indicated regimen. Two mice were used per group. Data is indicative of 3 independent experiments. (g) Absolute number of pmel-1 T cells 3 weeks after transfer from the same experiment in (f). (h) Intracellular IFN-γ 3 wks after adoptive cell therapy. Cells were activated with lymphocyte activating kit and analyzed by flow cytometry 6 hours later. Two mice were analyzed per group. Data is indicative of 3 independent experiments.
In an attempt to understand the kinetics of IL-2 dependency in this system, we gave exogenous IL-2 for 4 days after treatment together with Thelper cells derived from IL-2-/- mice. Initially, we observed tumor regression, but this regression was not maintained (Fig. 5c; * P = 0.021).
To assess whether Thelper cells could program CD8+ T cells to treat established tumors, we transferred Thelper cells for 4 days and then depleted with injection of 500 μg of CD4+ T cell depleting mAb (GK1.5; Fig. 5d), which was confirmed by flow cytometry (data not shown). Tumor treatment in mice receiving depleting mAb was similar to mice that had received no Thelper cells or exogenous IL-2 (Fig. 5d) or Thelper cells derived from IL-2-/- mice (Fig. 5b). Isotype control antibody had no effect on adoptively transferred Thelper cells in vivo (data not shown).
Next, the expression of CD25 on transferred CD4+ T cells was determined. IL-2 upregulates it's own receptor expression(32). Therefore, to determine whether the major source of IL-2 was from the transferred cells or from the host, CD4+ T cells were analyzed by flow cytometry 35 days after transfer into tumor-bearing RAG-1-/- hosts. CD25 expression was between 5-10% as expected for whole CD4+ T cells (Fig. 5e). Sorted Thelper cells had 0.23% CD25 expression and Thelper cells from IL-2-/- mice had 0.8% CD25 expression (Fig. 5e). After 35 days in vivo, whole CD4+ T cells and sorted Thelper cells upregulated their receptor (Fig. 5e). Thelper cells from IL-2-/- mice also upregulated their receptor, but at a much lower level (Fig. 5e). Thus, even though whole CD4+ cells, which contain Treg cells, upregulated their CD25 expression to comparable levels as Thelper cells; tumor regression was minimal. Together, these results indicated that IL-2 mainly comes from transferred activated Thelper cells.
Next we looked at the persistence of tumor-reactive CD8+ T cells after adoptive transfer with different CD4+ T cell subsets by flow cytometry 3 wks after treatment. As shown in Figure 5f, Thy1.1+ pmel-1 T cells required the presence of Thelper cells in order to persist. A 10-fold reduction in Thy1.1+ pmel-1 T cell frequency was seen in groups that received Treg cells and Thelper cells at a 1:10 ratio when compared to groups receiving pmel-1 T cells and Thelper cells alone. The same reduction in Thy1.1+ pmel-1 T cell frequency was seen in groups that received Thelper cells from IL-2-/- mice or no Thelper cells. Persistence of Thy1.1+ pmel-1 T cells was even more depressed in groups receiving only Treg cells, approximately a 30-fold reduction when compared to the Thelper cell group. As a comparison, absolute number of pmel-1 CD8+ T cells was also calculated for the same experiment (Fig. 5g). Function, as measured by intracellular IFN-γ, of adoptively transferred pmel-1 T cells with Thelper cells was also assessed and shown to be suppressed when Treg cells were cotransferred at 1:10 ratio, or when Thelper cells were derived from IL-2-/- mice (Fig. 5h). Thus, these results highlight that IL-2 from Thelper cells was essential for the induction of antitumor immunity to a self-antigen, and this effect was lost in the presence of Treg cells.
Breakdown of tolerance to the gp100 self-antigen is IL-2 dependent.
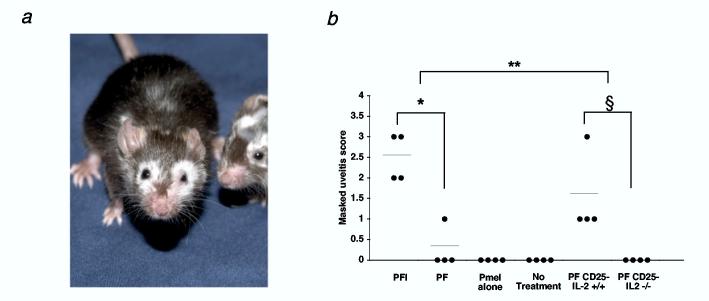
We notice mice treated with pmel-1 T cells, vaccine, and IL-2 or Thelper cells developed profound autoimmune vitiligo following 5 weeks after adoptive cell transfer. This vitiligo usually started periorbitally and spread in a random fashion as shown in figure 6a (n = 26). Conversely, limited or no autoimmune vitiligo was seen in mice that didn't receive exogenous IL-2 (n = 25) or received Thelper cells derived from IL-2-/- mice (n = 25).
Figure 6.
Breakdown of self-tolerance to the gp100 antigen requires IL-2. (a) Mice receiving pmel-1 T cells, rFPVhgp100 vaccination, and Thelper cells develop autoimmune vitiligo that spreads in an unpredictable fashion 5 wks after adoptive cell transfer. Mice receiving pmel-1 T cells, rFPVhgp100 vaccination, and exogenous IL-2 also develop vitiligo (as shown in (23)). Two representative mice receiving pmel-1 T cells, rFPVhgp100, and Thelper cells are shown (n = 25). (b) Uveitis during immunotherapy of B16 melanoma is only present with the administration of pmel-1 T cells, rFPVhgp100 vaccine, and exogenous IL-2, *, P < 0.05, or with cotransfer of Thelper cells without exogenous IL-2, §, P < 0.05. Uveitis is not observed in mice receiving pmel-1 T cells, rFPVhgp100 vaccine, and Thelper cells derived from IL-2-/- mice. Uveitis in the eyes of treated mice was scored as follows: (0 = none, 1 = mild, 2 = moderate, 3 = severe). Data represent 2 independent experiments.
Since the eyes of C57BL/6 mice also express gp100 tumor/self-antigen(33), we evaluated the requirement for IL-2 production in the destruction of normal eye tissue. We looked for the induction of autoimmunity in the eye, as evidenced by uveitis. We found in repeated experiments that exogenous IL-2 caused significant uveitis (10 fold increase) when compare to no exogenous IL-2 treatment (PFI vs. PF; *, P < 0.05; Fig. 6b and manuscript in preparation; Palmer, D. et al.). We also found that the addition of Thelper cells induced uveitis that was similar to groups receiving IL-2 (PF CD25- IL-2+/+ vs PFI; **, P > 0.05; Fig. 6b). Importantly, as seen with autoimmune vitiligo, no uveitis was observed when Thelper cells were derived from IL-2-/- mice (PF CD25- IL-2-/-; Fig. 6b; §, P < 0.05). Together, these results indicated that naturally occurring Thelper cells facilitated the induction of tumor regression and autoimmunity against a tumor/self-antigen through an IL-2-dependent mechanism.
Discussion
A fundamental question unanswered in immunology is how to raise T cell help against a persisting self-antigen, which subsequently results in the breakdown of self-tolerance(2). We describe here the requirements for the initiation of autoimmunity and thus the induction of anti-tumor immunity to established tumors expressing the gp100 melanocyte differentiation antigen, an antigen also expressed in the skin and eyes of C57BL/6 mice(33).
Recently depletion of Treg cells has been shown to augment reactivity to tumor/self-antigens in tumor prevention models(19, 20, 34, 35), but we show for the first time that Treg cells can inhibit help of self-reactive CD4+ T cells and prevent effector CD8+ T cells from initiating autoimmunity. Treg cells control peripheral self-tolerance through yet unknown mechanisms, but we believe that progressively growing tumors shed or secrete self-antigens that subsequently activate naturally occurring Treg cells(9-11, 36). Although depletion of Treg cells enhances tumor protection in many models using artificial self-antigens, we show that complete absence of Treg cells is not enough for treatment of established tumors against a self-antigen when using adoptive immunotherapy(37). Even in the absence of Treg cells, treatment still required CD8+ T cells, vaccination, and some type of help either provided through exogenous cytokines or through Thelper cells. Therefore we suspect as with acute infections(6) that ongoing tumor regression will need continuous T cell help to eradicate established tumors, in addition to removing Treg cells, because Thelper cells were unable to program self-reactive CD8+ T cells.
Since self-antigens can activate T regulatory cells(38), their role may have been over looked in artificial systems modeling self-antigens(36). The modeling of self-reactivity is likely to be important in the development of new immunotherapies that target tumor/self-antigens. Many of the currently available tumor models show the complete destruction of established tumors targeting ″foreign″ antigens and represent tumors given for only a short period of time(8, 39-41). These models have shed valuable light on basic immunologic principles, but it is unclear to what extent the results obtained from these models reflects immune responses against true self/tumor antigens(42). The present study was designed to elucidate the requirements for raising help to a tumor-antigen that was also expressed in normal tissues, a situation that models the clinical scenario in patients with cancer.
We found that CD8+ T cell-mediated immunotherapy and vaccination was ineffective in CD4+ T cell-deficient hosts unless given in combination with IL-2, and this effect was dramatically diminished in the presence of Treg cells. Additionally, we showed that Thelper cells were superior to exogenous IL-2 therapy but that cotransfer of Treg cells also inhibited this effect. Importantly, Thelper cells derived from IL-2-/- mice contributed to neither antitumor immunity nor autoimmunity, even in mice lacking Treg cells. All together these findings show the importance of Thelper cell derived IL-2 in the help of CD8+ T cells in vivo.
Next we showed that adoptive cell therapy with Thelper cells or exogenous IL-2 failed in the presence of Treg cells. Since Treg cells constitutively express the high affinity IL-2R, it is plausible that Treg cells may be preferentially using IL-2 as shown in vitro(43), as they require IL-2 for their function and maintenance in vivo(44). It is also feasible that Treg cells suppress CD8+ T cells or CD4+ T cells by decreasing their access to IL-2 either by suppressing the production of IL-2 by Thelper cells(45, 46), or decreasing the surface expression of the IL-2 receptor(28). Alternatively, Treg cells may condition the antigen presenting cell towards tolerance(47). Thus, the durable induction of an antitumor (anti-self) response by Thelper cells may be dependent on their continuous production of IL-2, which is lost in the presence of Treg cells constitutively expressing high affinity IL-2R. Whether Thelper cells that become CD25+ T cells are bona fide Foxp3 expressing Treg cells in unknown in this model, but a recent paper suggests that this may be the case during expansion in a lymphopenic environment(48). Regardless, we still see maintenance of tumor regression and therefore a converted Thelper to an induced Treg cell may not play a role during treatment of established tumors.
It has been argued that Thelper cells from IL-2-/- mice have a secondary deficiency that diminishes their helper effect. However, it has been reported in the literature that T cell ontogeny and function in IL-2-/- mice is not affected(49, 50). Furthermore, mixed bone marrow chimeras of IL-2-/- and CD25-/- cells used to reconstitute lethally irradiated hosts resulted in normal T cell homeostasis and engraftment of a stable CD4+CD25+ Tregpopulation(51). In addition, transfer of Treg cells into CD25-/- mice, which have the same phenotype as IL-2-/- mice, lead to recoverable levels of Treg cells and suppression of autoimmune disease since CD25-/- mice still have a cellular source of IL-2(21). However, transfer of Treg cells into IL-2-/- mice did not prevent autoimmune disease(44). Taken together, these findings show that the main deficiency in IL-2-/- mice is the complete absence of functional Treg cells and not an intrinsic functional T cell defect(44). Lack of Treg cells, due to absence of IL-2 signaling, leads to uncontrolled CD4+ T cell proliferation and activation, which paradoxically is not dependent on IL-2 (15, 21, 31, 51)
However, even though IL-2-/- mice get autoimmune disease, adoptive transfer of IL-2 deficient Thelper cells were unable to help CD8+ T cells treat an established tumor or cause autoimmunity in IL-2+/+ mice. Autoimmunity in our model is dependent on IL-2 production whereas in IL-2-/- mice it is independent of IL-2(15). This is an important finding as it shows a disparity between how these two types of autoimmunity can manifest. Most importantly, it shows the risk of using self-antigens to immunize against self-antigen expressing tumors(52, 53).
These findings also point to the deficiencies in the use of high-dose exogenous IL-2 in cancer clinical trials(54). Already known for its toxicity, another danger inherent in the administration of exogenous IL-2 may be the induction of Treg cell function(21, 44, 46, 55). Thus, depletion of Treg cells with either ONTAK(56) or another method before adoptive cell transfer may enable unencumbered delivery of IL-2 by Thelper cells to tumor-reactive T cells or to Thelper cells themselves.
A key feature of this immunotherapy regimen is that Thelper cells are derived from wild-type mice, obviating the need for the development of Thelper cells with specificities for tumor-antigens a priori. The identities of the antigens recognized by Thelper cells in this setting remains of considerable interest because isolation of tumor-reactive Thelper cells can lead to more effective class-II-restricted vaccines(57). However, exactly what the requirements of T cell help are in vivo are still being debated(2), but we report here that IL-2 plays an important role in the breakdown of self-tolerance to a persisting antigen. Whether IL-2 is acting on CD8+ T cells or Thelper cells or both is unknown(2). It is possible that IL-2 secreted by Thelper cells leads to down-stream events that participate in T cell help, such as release of other cytokines or activation of costimulatory molecules, which license the APC to initiate help of CD8+ T cells. Thus, exogenous IL-2 therapy may lose these contributions by Thelper cells when used alone. However, whatever the mechanism of help in vivo, transfer of naturally occurring Thelper cells in combination with tumor-reactive CD8+ T cells plus vaccination represents a clinically feasible approach to the immunotherapy of established, progressing tumors in humans, since isolation of tumor-reactive CD4+ T cells has been difficult.
One currently used and approved immunotherapeutic approach in humans involves lymphodepletion prior to adoptive transfer(58). The immune-enhancing effects of lymphodepletion can be accomplished through irradiation, chemoablation(27, 58) or through genetic means as demonstrated here. The mechanisms of how lymphodepletion enhances adoptive immunotherapy remain incompletely understood (Klebanoff et al., Trends Immunology, in press), but our data suggests that the removal of Treg cells is a major contributing factor. But as shown here, removing Treg cells is not enough to treat established tumors, T cell help must be provided. The mechanisms of lymphodepletion are multifactorial because antitumor immunotherapies in CD4+ T cell-deficient mice can be further enhanced with total body γ-irradiation (data not shown). As has been shown in other systems(59), the increased availability of homeostatic γc-signaling cytokines such as IL-7, IL-15, or IL-21 could be enhancing T cell function in this model(24, 60, 61).
Nevertheless, we show here that naturally occurring Thelper cells can initiate autoimmunity and tumor regression in an environment of persisting self-antigen through self-reactive CD8+ T cells and that naturally occurring Treg cells represent a formidable barrier to the breakdown of self-tolerance. Therefore, the future of immunotherapy against self-antigens will rely on ways of removing this population and augmenting T cell help of tumor-reactive T cells or tumor-infiltrating lymphocytes(54) isolated from patients. Together, these findings form a new approach for studying T cell help and suppression in vivo against self-antigens and form the basis of a new treatment for many types of cancers expressing self-antigens and chronic persisting infections.
Acknowledgements
The authors would like to thank A. Mixon and S. Farid of the Flow Cytometry Unit for FACS sorting and analysis, the NCI, CCR Fellows Editorial Board, W. Leitner, B. Reines and G. Lizee for critical review of the manuscript.
Nonstandard abbreviations used:
- Foxp3
Forkhead box P3
- rFPVhgp100
fowlpox virus expressing human gp100
- hgp100
human melanocyte differentiation antigen gp100
- IL-2Rα
interleukin-2 receptor alpha chain
- IL-2Rβ
interleukin-2 receptor beta chain
- mgp100
mouse melanocyte differentiation antigen gp100
- P or pmel-1
pmel-1 transgenic CD8+ T cells
- Treg cell
CD4+CD25+ T regulatory cell
- Thelper cell
CD4+ CD25- Thelper cell
References
- 1.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. CD8 lethargy in the absence of CD4 help. Eur J Immunol. 2002;32:2199. doi: 10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 8.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major ″post-licensing″ role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 9.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon B. Continuous Activation of autoreactive CD4+CD25+ Regulatory T cells in the Steady State. J Exp Med. 2003;198:737. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 12.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. Journal of Experimental Medicine. 1996;184:387. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally Arising CD4 Regulatory T Cells for Immunological Self-Tolerance and Negative Control of Immune Responses. Annu Rev Immunol. 2004;22:531. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 15.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 16.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4(+)CD25(+) T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. Journal of Immunology. 1998;160:1212. [PubMed] [Google Scholar]
- 17.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 18.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: Production of CD25(+)CD4(+)naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. Journal of Immunology. 1999;162:5317. [PubMed] [Google Scholar]
- 19.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malek T, Yu A, Vincek V, Scibelli P, Kong L. CD4 Regulatory T Cells Prevent Lethal Autoimmunity in IL-2Rbeta-Deficient Mice. Implications for the Nonredundant Function of IL-2. Immunity. 2002;17:167. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 22.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol. 2003;171:3435. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 23.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, De Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8+ T Cells. J Exp Med. 2003;198:569. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T Cells. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mintern JD, Davey GM, Belz GT, Carbone FR, Heath WR. Cutting edge: precursor frequency affects the helper dependence of cytotoxic T cells. J Immunol. 2002;168:977. doi: 10.4049/jimmunol.168.3.977. [DOI] [PubMed] [Google Scholar]
- 26.Berendt MJ, North RJ. T-Cell-Mediated Suppression of Anti-Tumor Immunity - Explanation for Progressive Growth of an Immunogenic Tumor. Journal of Experimental Medicine. 1980;151:69. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccirillo CA, Shevach EM. Cutting Edge: Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 29.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun JC, Williams MA, Bevan MJ. CD4(+) T cells are required for the maintenance, not programming, of memory CD8(+) T cells after acute infection. Nat Immunol. 2004 doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985;82:4230. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreurs MW, de Boer AJ, Schmidt A, Figdor CG, Adema GJ. Cloning, expression and tissue distribution of the murine homologue of the melanocyte lineage-specific antigen gp100. Melanoma Res. 1997;7:463. doi: 10.1097/00008390-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 35.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25(+)CD4(+) T cells: A common basis between tumor immunity and autoimmunity. Journal of Immunology. 1999;163:5211. [PubMed] [Google Scholar]
- 36.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 37.Antony PA, Restifo NP. Do CD4+ CD25+ Immunoregulatory T Cells Hinder Tumor Immunotherapy? J Immunother. 2002;25:202. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cozzo C, Larkin J, 3rd, Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 39.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 40.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 41.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De La Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4(+)CD25(+) regulatory T cell function. Eur J Immunol. 2004;34:2480. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 44.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 45.Thornton AM, Shevach EM. CD4(+)CD25(+) immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. Journal of Experimental Medicine. 1998;188:287. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 47.Cederbom L, Hall H, Ivars F. CD4(+)CD25(+) regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. European Journal of Immunology. 2000;30:1538. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T Cells Generate CD25+Foxp3+ Regulatory T Cells by Peripheral Expansion. J Immunol. 2004;173:7259. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 49.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 50.Horak I, Lohler J, Ma A, Smith KA. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol Rev. 1995;148:35. doi: 10.1111/j.1600-065x.1995.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 51.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 52.Overwijk WW, Restifo NP. Autoimmunity and the immunotherapy of cancer: targeting the ″self″ to destroy the ″other″. 2000;20:433. [PMC free article] [PubMed] [Google Scholar]
- 53.Gilboa E. The risk of autoimmunity associated with tumor immunotherapy. Nat Immunol. 2001;2:789. doi: 10.1038/ni0901-789. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 55.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 56.Foss FM. Interleukin-2 fusion toxin: targeted therapy for cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941:166. doi: 10.1111/j.1749-6632.2001.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269. doi: 10.1016/s1471-4906(01)01896-8. [DOI] [PubMed] [Google Scholar]
- 58.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 61.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 62.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a ″self″ antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]