Abstract
Background
Expenditures for medications used to treat attention-deficit/hyperactivity disorder (ADHD) in children have increased rapidly. Many employers and health plans have adopted 3-tier formularies in an attempt to control costs for these and other drugs.
Objective
To assess the effect of copayment increases associated with 3-tier formulary adoption on use and spending patterns for ADHD medications for children.
Design and Setting
Observational study using quasi-experimental design to compare effects on ADHD medication use and spending for children enrolled as dependents in an employer-sponsored plan that made major changes to its pharmacy benefit design and a comparison group of children covered by the same insurer. The plan simultaneously moved from a 1-tier (same copayment required for all drugs) to a 3-tier formulary and implemented an across-the-board copayment increase. The plan later moved 3 drugs from tier 3 to tier 2.
Participants
An intervention group of 20326 and a comparison group of 15776 children aged 18 years and younger.
Main Outcome Measures
Monthly probability of using an ADHD medication; plan, enrollee, and total ADHD medication spending; and medication continuation.
Results
A 3-tier formulary implementation resulted in a 17% decrease in the monthly probability of using medication (P<.001), a 20% decrease in expected total medication expenditures, and a substantial shifting of costs from the plan to families (P<.001). Intervention group children using medications in the pre-period were more likely to change to a medication in a different tier after 3-tier adoption, relative to the comparison group (P = .08). The subsequent tier changes resulted in increased plan spending (P<.001) and decreased patient spending (P = .003) for users but no differences in continuation.
Conclusions
The copayment increases associated with 3-tier formulary implementation by 1 employer resulted in lower total ADHD medication spending, sizeable increases in out-of-pocket expenditures for families of children with ADHD, and a significant decrease in the probability of using these medications.
Several safe and efficacious pharmaceutical treatments are now available for attention-deficit/hyperactivity disorder (ADHD), one of the most common chronic conditions of childhood.1 Numerous studies have shown that these medications are effective in controlling the principal symptoms of ADHD, including inattention, hyperactivity, and impulsiveness.1 Although overall efficacy for the various medications is similar, even though some have the same active ingredient but use different pharmacologic delivery systems, the efficacy and effectiveness of different medications may not be the same for a given child with ADHD.1,2 The drugs also differ with respect to their duration of action. For example, the generic drug methylphenidate has a duration of action of 3 to 6 hours, which means that a child would have to take multiple doses during the school day. By contrast, Concerta (ALZA Corp, Mountain View, Calif) has a duration of action of approximately 12 hours, so a single dose could last throughout the day.2 Differences in duration of action have implications for medication compliance, the possibility of breakthrough symptoms, and the potential for medication abuse and stigma.2 These issues suggest that each medication possesses attributes that may affect the drug’s suitability for treating a child given his or her clinical and life circumstances.
Spending on ADHD medications for children has increased rapidly over the past few years. From 2000 to 2003, children’s ADHD medication expenditures increased 183% overall and 369% for children aged 5 years and younger.3 As a result, these medications have become targets for cost-containment efforts by health plans and employers.
In response to recent rapid increases in prescription drug costs, many plans and employers have adopted incentive formularies. These formularies use copayment structures for consumers to encourage them to choose products that are cost-effective for the payer. Incentive formularies also enhance a health plan’s bargaining power in obtaining favorable prices from drug manufacturers by offering them sales volume for preferred drugs.4 A 3-tier formulary, now the most common type of incentive formulary, typically has the lowest copayment for generic drugs in the first tier, a higher copayment for brand-name drugs preferred by the payer in the second tier, and the highest copayment for brand-name drugs not preferred by the payer in the third tier. A 3-tier formulary implementation is often accompanied by copayment increases for drugs in all tiers. In 2004, 65% of workers with prescription drug coverage had a 3-tier formulary.5
Adoption of a 3-tier formulary appears to attenuate increases in drug spending by health plans for adults and leads adult patients to change from nonpreferred to preferred brand-name drugs that treat chronic conditions.6–12 A study of the same intervention examined in this article found that 3-tier formulary adoption and associated copayment increases resulted in medication changes and, more importantly, discontinuation by a sizeable proportion of adult patients using medication for several chronic conditions.6 To our knowledge, no studies have assessed the impact of tiered formularies and accompanying copayment increases on use of psychotropic or other types of medications used commonly by children, for which the parent or guardian is the decision maker rather than the patient. We do not know whether families with children who have a chronic condition like ADHD will continue using medications that now require higher copayments, switch to lower-cost medications, or stop using the drugs altogether in response to the increased cost sharing. To address these questions, we examined a large, national employer’s implementation and management of a 3-tier formulary for ADHD medications.
METHODS
STUDY POPULATION
We studied the use of prescription drugs used to treat ADHD in privately insured children, defined as individuals aged 18 years and younger. The employer who insures the study population contracts with a large managed care organization that offers preferred provider organization and point-of-service plans to its employees and their dependents. The managed care organization subcontracts with Medco Health Solutions Inc (Franklin Lakes, NJ), one of the largest pharmacy benefit managers in the United States, for the management of the pharmacy benefit in this study.
From 1999 to 2001, the employer made 2 important changes to its formulary for ADHD medications. First, in 2000, the employer made a major change to its pharmacy benefits, implementing a 3-tier formulary and an across-the-board increase in copayments. Before the changes, the employer used a 1-tier formulary that required the same copayment, $7 for 30-day prescriptions filled through a retail pharmacy and $15 for 90-day prescriptions filled through the pharmacy benefit manager’s mail-order program, for all prescription drugs. The employer switched to a 3-tier formulary that increased copayment levels for all drugs. Under the new 3-tier formulary, a patient filling a prescription in a retail pharmacy would now pay $8 for generic drugs, $15 for brand-name drugs preferred by the plan, and $30 for brand-name drugs not preferred by the plan. The copayments for the mail-order program were $16 for generic drugs, $30 for preferred brand-name drugs, and $60 for nonpreferred brand-name drugs. The list of drugs covered by insurance did not change, just the copayments required for specific drugs.
The initial assignment of ADHD medications to each of the 3 tiers is presented in the first row of Table 1. When the formulary changes were implemented in 2000, Adderall (Shire Pharmaceuticals Group, Wayne, Pa) was the only brand-name drug listed in tier 2 and approximately 41% of children using ADHD medications were using Adderall. Adderall is a short-acting, immediate-release preparation of d,l-amphetamine with a duration of action of 4 to 6 hours.2 A generic version of Adderall, amphetamine mixture (amphetamine aspartate, amphetamine sulfate, dextroamphetamine saccharate, and dextroamphetamine sulfate), was available in tier 1 at that time. Generic versions of the tier 3 drugs were also available in tier 1, so the new policy provided incentives for families to select the generic version of brand drugs.
Table 1.
ADHD Medications in Each Tier During the Study Period*
| Tier 1 | Tier 2 | Tier 3 | |
|---|---|---|---|
| Tier assignment on date of 3-tier implementation (1/1/00) | Amphetamine mixture
Dextroamphetamine sulfate Methylphenidate SR Methylphenidate HCl Pemoline |
Adderall (Shire Pharmaceuticals Group, Wayne, Pa) | Cylert (Abbott Laboratories, Abbott Park, III)
Dexedrine (Glaxo Smith Kline, Philadelphia, Pa) Metadate ER (Celltech Pharmaceuticals Inc, Rochester, NY) Ritalin (Novartis Pharmaceuticals Corp, East Hanover, NJ) Ritalin SR (Novartis Pharmaceuticals Corp) |
| Tier assignment at end of study period (12/31/01) | Dextroamphetamine
Dextroamphetamine sulfate Methylphenidate SR Methylphenidate HCl Pemoline |
Adderall (Shire Pharmaceuticals Group)
Concerta (ALZA Corp, Mountain View, Calif )† Metadata ER (Celltech Pharmaceuticals Inc)† Methylin ER (Mallinckrodt, St Louis, Mo)† |
Adderall XR (Shire Pharmaceuticals Group)
Cylert (Abbott Laboratories) Dexedrine (Glaxo Smith Kline) Focalin (Novartis Pharmaceuticals Corp) Metadate CD (Celltech Pharmaceuticals Inc) Ritalin (Novartis Pharmaceuticals Corp) Ritalin SR (Novartis Pharmaceuticals Corp) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CD, controlled release; ER, extended release; HCl, hydrochloric acid; SR, slow release; XR, extended release.
Strattera, a nonstimulant medication used to treat ADHD, received approval from the Food and Drug Administration after the study period ended.
Moved from tier 3 to tier 2 in April 2001, more than 1 year after 3-tier implementation.
After 3-tier formulary implementation in 2000, several sustained-release and the first extended-release branded preparations received approval from the Food and Drug Administration and were added to tier 3, as is common with newly approved drugs. On April 7, 2001, the second change to the formulary was implemented. After review by Medco’s pharmacy and therapeutics committee, 3 of the brand-name drugs in tier 3, Metadate ER (Celltech Pharmaceuticals Inc, Rochester, NY), Methylin ER (Mallinckrodt, St Louis, Mo), and Concerta, were given preferred status and moved to tier 2. Metadate ER and Methylin ER are sustained-release preparations with a duration of action of 5 to 8 hours, while Concerta is an extended-release preparation with a duration of action of 12 hours. Several of the other longer-acting stimulants used to treat ADHD, eg, Adderall XR (Shire Pharmaceuticals Group) and Ritalin LA (Novartis Pharmaceuticals Corp, East Hanover, NJ) remained in tier 3.
We studied 20326 children who were enrolled continuously for the 33-month period beginning April 1, 1999, and ending December 31, 2001. Of these children, 839 used an ADHD medication at some point during the 33-month study period.
SELECTION OF COMPARISON GROUP
We identified a comparison group of enrollees from a pool of more than 1000 employer clients of the managed care organization. Inclusion into the comparison group required an employer to have a 2-tier formulary with stable cost sharing throughout the study period; the number of employers using a 1-tier formulary with stable cost sharing was too low to form such a comparison group. A comparison group of employers was identified using the JMP clustering algorithm (SAS version 4.0; SAS Institute Inc, Cary, NC). This process is similar to propensity score matching in that an exact match is not required on each item.13 Matches were made on the basis of overall similarity across several characteristics: type of medical benefits offered (point-of-service and preferred provider organization for the intervention group); copayment levels for the first and second tiers similar to those used in the 3-tier formulary (eg, $8 generic,$ 15 brand for retail); age and sex distribution for the entire enrolled population (not just child enrollees); and geographic distribution of enrollees. The comparison group included 15776 children who were enrolled continuously during the study period. Of these children, 530 used ADHD medication at some point during that period.
DATA
We used eligibility files and pharmacy claims data obtained from Medco for the 33-month period beginning April 1, 1999, and ending December 31, 2001. The study period includes approximately the 9 months before and more than 1 year after 3-tier adoption and approximately the 9 months after the April 2001 tier changes. We did not reveal the exact implementation date during the year 2000 to protect the employer’s anonymity.
STATISTICAL ANALYSIS
To assess the impact of the formulary changes on use and spending on ADHD medications, we estimate multivariate models of drug spending and examine use of specific medications.
Multivariate Analyses of Overall Use and Spending
The pre- and post–nonequivalent control design of the study lends itself to a difference-in-difference approach to control for general trends in pharmacy use and spending.14 With the difference-in-difference approach, we accounted for any change in use and spending experienced by the comparison group in the pre- and post–estimate of change experienced by the intervention group. We estimated the 2-part models because most children do not use any medication for treatment of ADHD.15 The first part consists of a logit model of the probability of using an ADHD medication in a given month. The second part involves estimation of 3 regression models of monthly spending (by the plan, the enrollee, and total) on ADHD medications for children who had some use of an ADHD medication during a given month. A logarithmic transformation of spending for people using the medications was used to address the skewness in the distribution of spending.
The key independent variables were the following: (1) an indicator for the post-formulary change period; (2) an indicator for the intervention group (relative to the comparison group); (3) the interaction of the post-change and intervention group variables; (4) an indicator for the period after the April 2001 tier changes; and (5) the interaction of the post-tier change and intervention group variables. We included several covariates in the statistical models: subject age at the end of the study and its square, study month and its square to account for secular trends in the dependent variable, and sex. The squares of age and month were included to address potential nonlinearity in the impact of these variables on study outcomes. Because many children stop taking ADHD medications when school is out of session, we also include a dummy variable for the summer months of June and July to capture this seasonably in use. The person-month was the unit of analysis. We adjusted standard errors for clustering of multiple observations on the same person using Huber-White corrections.16,17
A key comparison for the intervention and comparison groups is the change in expected spending associated with 3-tier implementation. To estimate this, we combined results from each element of the 2-part model, the logit model on the probability of use, and the regression model of total spending conditional on use, to estimate expected total spending per enrollee.18 From this we were able to calculate the difference-in-difference estimate for expected spending on ADHD drugs.
Descriptive Analyses of Use of Specific Medications
We studied children who filled at least two 30-day prescriptions for an ADHD medication in the 6 months before the formulary changes were adopted, with at least 1 day supplied in the 45 days before 3-tier adoption to ensure current use. We examined whether the intervention group medication users changed or stopped taking ADHD medications at a higher rate than the comparison group users in the 6 months after the changes. We also conducted a similar analysis of the effect of the April 2001 tier changes on medication use continuation, focusing on children using the drugs in the 6 months before the tier changes.
RESULTS
Table 2 reports descriptive information for children in the intervention and comparison groups. The average monthly probability of using an ADHD medication in the 6 months before the formulary changes was higher for the intervention group than for the comparison group (P<.001). There were no statistically significant differences between the 2 groups in age or sex.
Table 2.
Descriptive Statistics for Continuously Enrolled Children
| Characteristic | Intervention Group | Comparison Group | PValue |
|---|---|---|---|
| No. of continuously enrolled children | 20 326 | 15 776 | NA |
| Mean (SD) age as of 12/31/01, y | 10.97(4.43) | 11.00(4.50) | .45 |
| Male, % | 51.66 | 51.12 | .31 |
| Average monthly probability of ADHD medication use in 6 mo before 3-tier adoption, % | 1.2 | 0.8 | <.001 |
| No. of children who used any ADHD medications in 6 mo before 3-tier adoption | 469 | 238 | NA |
| Mean (SD) age as of 12/31/01, y | 12.33(2.82) | 12.84(289) | .03 |
| Male, % | 75.05 | 77.73 | .43 |
Abbreviations: ADHD, attenlion-deficit/hyperactivity disorder; NA, not applicable.
The Figure shows trends in monthly total spending on drugs used to treat ADHD throughout the study period. Trends in pre-period spending per enrollee are similar for the intervention and comparison groups. This is important because a central assumption of the difference-in-difference model is that trends for intervention and comparison groups are similar in the pre-intervention period. Monthly expenditures decrease during the summer months and increase again at the end of each summer, consistent with the fact that some families choose to discontinue ADHD medication use by their children when school is not in session.
Figure.
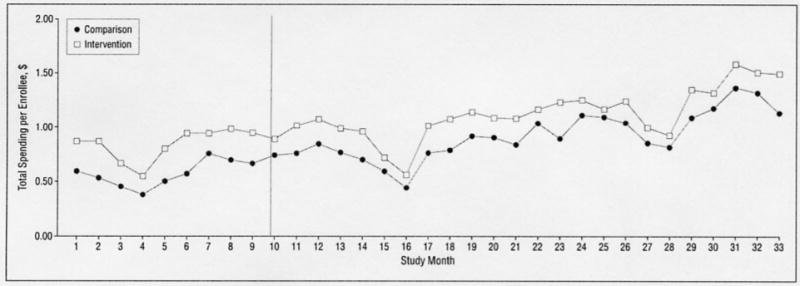
Monthly total spending on attention-deficit/hyperactivity disorder medications for children aged 18 years and younger.
OVERALL ADHD MEDICATION USE AND SPENDING
A 3-tier adoption resulted in a statistically significant slowdown in the rate of growth in the monthly predicted probability of using an ADHD medication for the intervention group relative to the comparison group (−17%) and a 20% decrease in expected total spending per enrollee for ADHD medications (Table 3). A 3-tier adoption did not have a statistically significant effect on total (plan plus enrollee) ADHD medication spending for children who filled a prescription for 1 of these drugs but had a substantial effect on the distribution of medication spending between the plan and the patient’s family. Monthly-plan spending for ADHD medication users decreased by 43% (P<.001) relative to that of the comparison group, while monthly enrollee spending for users increased by 46% (P<.001) relative to that of the comparison group. As a sensitivity analysis, we estimated the same models using data beginning April 1, 1999, and ending March 30, 2001, the month before 3 ADHD brand-name drugs were moved from tier 3 to tier 2. Results were consistent with those from the 33-month study period presented in this article.
Table 3.
Percentage Impacts on Probability of Use and Spending on ADHD Medications Conditional on Use, Relative to Comparison Group*
|
Percent Change |
||
|---|---|---|
| Dependent Variable | 3-Tier Adoption | Tier Changes |
| Expected total spending per enrollee | −20 | NA |
| Predicted probability of using an ADHD medication | −17 | NA |
| Spending for ADHD medication users | ||
| Total spending | −3 (P = .23) | 0.2 (P = .93) |
| Plan spending | −43 (P<.001) | 17 (P<.001) |
| Enrollee spending | 46 (P<.001) | −7 (P = .003) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; NA. not applicable.
The change in expected total spending per enrollee is estimated by combining the results from the 2 parts of the model, the logit model of the probability of use and the regression model of total spending conditional on use. Estimates of expected total spending per enrollee and the predicted probability of using an ADHD medication are not provided in the tier change column because there is no appropriate comparison group (ie. employers with a 3-tier formulary that did not make tier changes during the study period). For the regression models of total, plan, and enrollee spending conditional on use, we transformed the coefficients from the same variable to obtain estimates of the percent change in spending.
The April 2001 tier changes had the opposite effect on the distribution of spending among ADHD medication users as 3-tier formulary adoption. As with 3-tier adoption, the tier changes had no statistically significant effect on total spending among users; however, the tier changes resulted in plan spending increases (17%, P<.001) and enrollee spending decreases (−7%, P<.001), relative to the comparison group.
USE OF SPECIFIC MEDICATIONS
Table 4 shows changes in drug use patterns for children who used an ADHD medication before each of the 2 formulary changes. In the 6 months after 3-tier formulary adoption, a lower proportion of intervention group users than comparison group users stayed with a medication of the same tier (80% vs 85%, P =.17) and a higher proportion of intervention group users changed to a drug of a different tier (11% vs 6%, P =.08), although the differences were not statistically significant at standard levels. Rates of discontinuing use of ADHD medications in the 6 months after adoption were virtually identical (9%) for both groups.
Table 4.
Likelihood of Continuing ADHD Drug Therapy After Formulary Changes*
|
3-Tier Adoption |
Tier Changes (04/07/01) |
|||||
|---|---|---|---|---|---|---|
| Intervention (n = 344) | Comparison (n = 199) | PValue | Intervention (n = 360) | Comparison (n = 250) | PValue | |
| Stay, No. (%) | 276 (80.2) | 169 (84.9) | .17 | 233 (64.7) | 160 (64.0) | .85 |
| Change, No. (%) | 36 (10.5) | 12 (6.0) | .08 | 91 (25.3) | 56 (22.4) | .41 |
| Stop, No. (%) | 32 (9.3) | 18 (9.5) | .92 | 36 (10.0) | 34 (13.6) | .17 |
Abbreviation: ADHD, attention-deficit/hyperactivity disorder.
The first panel of the table includes all children who filled at least two 30-day prescriptions for an ADHD medication, with at least 1 day supplied in the 45 days immediately preceding 3-tier adoption. The second panel includes all children who filled at least two 30-day prescriptions for an ADHD medication, with at least 1 day supplied in the 45 days immediately preceding the April 2001 tier changes. Stay indicates that the child continued using a drug of the same tier: change, that the child changed tiers (ie. switched from using ≥1 drug of a given tier to using ≥1 drug in another tier): and stop, that the child did not use any ADHD medications during the 6 months after the changes. This table does not include children using ADHD medications from more than 1 tier in the 6 months before the respective changes.
After the April 2001 tier changes, the proportion of intervention and comparison group users that continued taking medication of the same tier was the same, approximately 64%. There were also no statistically significant differences in the proportions of users changing and discontinuing use of medications.
COMMENT
Implementation of 3-tier formularies are intended to help control prescription drug costs by encouraging enrollees and their physicians to select drugs that are preferred by the plan. By moving prescription volume toward a subset of drugs in a class, the plan may negotiate larger rebates from manufacturers, further lowering drug costs.
The employer we studied made a major change in its pharmacy benefits by moving from a 1-tier to a 3-tier formulary and implementing an across-the-board copayment increase. These changes resulted in a decrease in expected total ADHD medication expenditures per enrollee and a substantial shifting of ADHD medication costs from the plan to the family. Three-tier adoption also resulted in a decrease in the probability of using an ADHD medication but did not lead to higher rates of medication use discontinuation, which suggests that the decrease in probability of use may be driven by lower rates of medication initiation. An important question to consider is whether such reduction in use was clinically appropriate. We were unable to make this determination using claims data. The results also indicate that 3-tier adoption led to a higher rate of medication changes for pre-period medication users in the intervention group (P = .08), but the absolute proportion of patients who changed medication (11%) was relatively low. The ability to shift demand is important because manufacturers are otherwise unlikely to be willing to negotiate large rebates.
Only 1 brand-name drug, Adderall, was assigned tier 2 status at the same time as the 3-tier formulary implementation, and this drug had a generic version available in tier 1. Since there was a generic version available, this policy may have primarily been intended to provide incentives for patients who took Adderall to switch to the generic amphetamine mixture and pay an $8 copayment instead of $15.
The April 2001 tier changes added 3 longer-acting drugs to tier 2 for which there was not a comparable generic alternative available in tier 1. Although the tier changes resulted in an increase in plan spending and a small decrease in enrollee spending for intervention group users relative to comparison group users, these changes did not appear to affect rates of medication change or discontinuation of use.
These results differ from the results of a similar analysis of 3 drug classes used to treat chronic conditions in adults (angiotensin-converting enzyme inhibitors, proton pump inhibitors, and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or statins).6 Although only 18% of children who used a tier 3 ADHD medication in the 6 months before 3-tier adoption (and thus faced the largest increase in copayments) changed to a lower-tier medication, between one third and one half of pre-period tier 3 users of the 3 adult drug classes changed to a lower-tier medication; the rate of changing medications was significantly higher for the intervention than for the comparison group. One potential explanation is that parents of ongoing users of ADHD medications may be reluctant to request a medication change in response to the cost-sharing increases if the current treatment regimen has proven effective for the child.
Our study had several limitations. We were unable to obtain proprietary data on changes in the magnitude of manufacturer rebates that may have resulted from the formulary changes. As a result, our estimates of the effect of the changes on plan and total spending are likely to be underestimated. The study lacked the statistical power to determine precise differences in patterns of use when using relatively restrictive criteria for identifying a medication user. Ideally, we would compare drugs used to treat ADHD with other classes of long-term–use medications used commonly by children to determine whether parents and physicians are particularly reluctant to change ADHD medications (or perhaps psychotropics more generally) or whether results would be similar for all medications used for children. However, many drug classes often given to children, like antibiotics, are used to treat episodic or acute conditions. The only other class of drugs used to treat chronic conditions with use rates that are high enough to study with our sample is asthma medications. However, the drugs in this class are often not included in incentive formularies since the therapeutic substitutability of different types of agents is considered to be limited. This study examines the experience of a single employer with a primarily hourly workforce that made a dramatic change to its pharmacy benefits and a comparison group of employers with stable pharmacy benefits. Our findings may not be generalizable to other employers, particularly those who make less dramatic changes in formulary and benefit design. We cannot be sure that the groups do not differ in terms of unobservable characteristics, such as income, that could influence the effect of the policy changes. However, the study design was chosen to protect against such Influences if they are stable over time. Finally, the study period ended before the introduction of Strattera (Eli Lilly and Co, Indianapolis, Ind), a nonstimulant medication used to treat ADHD, so we were unable to assess the effect of formulary changes on use of this drug.
In conclusion, the relatively dramatic change in cost-sharing requirements resulting from 1 employer’s adoption of a 3-tier formulary reduced the rate of growth in use of ADHD medications but did not cause most ongoing users to substantially change their patterns of medication use, relative to the comparison group. The overall result of the changes was to shift costs onto families of children receiving ADHD medication therapy. The subsequent movement of 3 longer-acting medications from tier 3 to tier 2 reversed a portion of the cost shifting by increasing plan spending and somewhat decreasing family spending but had no effect on continued medication use. Because incentive formularies differ on a number of key dimensions that are likely to influence drug use and spending patterns for children with ADHD, case studies of other incentive formularies are needed to understand the impact of these arrangements on children with ADHD and their families. These studies should examine the effect of incentive formularies on drug use and spending patterns, as well as other important outcomes including educational outcomes, self-esteem, and relationships with family and peers.
Acknowledgments
We gratefully acknowledge funding from the Robert Wood Johnson Foundation’s Changes in Health Care Financing and Organization Initiative, Princeton, NJ: the National Institute of Mental Health, Bethesda, Md (1 K01 MH66109); Agency for Healthcare Research and Quality, Rockville, Md (5 P01 HS10803); and Alfred P. Sloan Foundation, New York, NY. We are indebted to Kathryn Trainor, MS, Boris Fainstein, MBA, and Hocine Azeni, MA, for expert programming.
Footnotes
Previous Presentation: An earlier version of this analysis was presented at the Workshop on Costs and Assessment in Psychiatry; March 27, 2003; Venice, Italy.
References
- 1.American Academy of Pediatrics. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J. Practical considerations in stimulant drug selection for the attention-deficit/hyperactivity disorder patient–efficacy, potency and titration. Today’s Therapeutic Trends. 2002;20:311–328. [Google Scholar]
- 3.Medco Health Solutions Inc. 2004 Drug Trend Report: Navigating the New Health Economy. Franklin Lakes, NJ: Medco Health Solutions Inc; 2004:5–27.
- 4.Frank R. Prescription drug prices: why do some pay more than others do? Health Aff (Millwood) 2001;20:115–128. doi: 10.1377/hlthaff.20.2.115. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser Family Foundation and the Health Research and Educational Trust. Employer Health Benefits 2004 Annual Report. Available at: http://www.kff.org/insurance/7148/index.cfm Accessed February 1, 2005.
- 6.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription drug utilization and spending. N Engl J Med. 2003;349:2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 7.Motheral B, Fairman KA. Effect of a three-tier prescription copay on pharmaceutical and other medical utilization. MedCare. 2001;39:1293–1304. doi: 10.1097/00005650-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Rector TS, Finch MD. Danzon PM. Pauly MV, Manda BS. Effect of tiered prescription copayments on the use of preferred brand medications. MedCare. 2003;41:398–406. doi: 10.1097/01.MLR.0000053022.47132.82. [DOI] [PubMed] [Google Scholar]
- 9.Nair KV, Wolfe P, Valuck RJ, McCollum MM, Ganther JM, Lewis SJ. Effects of a three-tier pharmacy benefit design on the prescription purchasing behavior of individuals with chronic disease. J Manag Care Pharm. 2003;9:123–133. doi: 10.18553/jmcp.2003.9.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288:1733–1774. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 11.Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff (Millwood) 2004;23:227–236. doi: 10.1377/hlthaff.23.1.227. [DOI] [PubMed] [Google Scholar]
- 12.Goldman DP, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291:2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino RB. Propensity score methods for bias reduction in the comparison of treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Cook TD, Campbell DT. Quasi-experimentation: Design and Analysis Issues for Field Settings. Chicago, III: Rand McNally College Publishing Co; 1979.
- 15.Manning WG, Newhouse JP, Duan N, Keeler EB, Leibowitz A, Marquis MS. Health insurance and the demand for medical care: evidence from a randomized experiment. Am Econ Rev. 1987;77:251–277. [PubMed] [Google Scholar]
- 16.Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. In: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley: University of California Press; 1967:221–233.
- 17.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 18.Duan N, Manning WG, Morris CM, Newhouse JP. A comparison of alternative models for the demand for medical care. J Business Econ Stat. 1983;1:115–126. [Google Scholar]


