Abstract
Candida lipolytica was recovered from the blood and the central venous catheter in a patient receiving allogeneic bone marrow transplantation. Two C. lipolytica strains from different geographical areas and the ATCC 9773 strain of C. lipolytica were used as controls. C. lipolytica was identified by standard methods. MICs indicated antifungal susceptibilities to amphotericin B, fluconazole, and itraconazole for all strains. In vitro testing and scanning electron microscopy showed that C. lipolytica was capable of producing large amounts of viscid slime material in glucose-containing solution, likely responsible for the ability of the yeast to adhere to catheter surfaces. Restriction fragment length polymorphisms revealed an identical profile for all clinical isolates, unrelated to those observed for the control strains. This finding suggested the absence of microevolutionary changes in the population of the infecting strain, despite the length of the sepsis and the potential selective pressure of amphotericin B, which had been administered to the patient for about 20 days. The genomic differences that emerged between the isolates and the control strains were indicative of a certain degree of genetic diversity between C. lipolytica isolates from different geographical areas.
Invasive fungal infections have emerged as a frequent cause of morbidity and mortality in patients with hematological malignancies, especially in patients who are severely immunocompromised, such as those who undergo bone marrow transplantation (BMT) (6, 15). The risk for these infections is quite high during the first 100 days posttransplant. This period corresponds to profound neutropenia of the preengraftment stage and early immune reconstitution postengraftment. Moreover, fungal infections are frequently seen in patients with graft failure or significant delays in immune reconstitution, such as in recipients with severe graft-versus-host disease (GvHD) (5). Candida and Aspergillus species are the most frequently isolated fungal agents from BMT patients. For many years Candida albicans was the principal yeast-like fungus isolated from these infections. More recently, however, other species such as Candida tropicalis, Candida parapsilosis, Candida guillermondii, Candida krusei, Candida glabrata, and Candida inconspicua have emerged as pathogens in BMT patients. These yeasts are often associated with resistance to antifungal azoles and with higher mortality (5, 7, 25, 27).
Candida lipolytica has not been a frequent agent of opportunistic infections (9, 24). It is ubiquitous, having been isolated from refrigerated meat products, petroleum products, agricultural processing plants, and soil (10). C. lipolytica has also been isolated from the mouth, pulmonary tree, and intestines (26). Documented infections caused by C. lipolytica have been described for two patients (alcohol abusers) with candidemia, for three patients with traumatic ocular infection, for one patient with chronic sinusitis, and for one BMT patient with disseminated infection (18, 19, 26, 28). Moreover, we document the production of a biofilm (slime), which may have contributed to colonization of the catheter and subsequent candidemia.
MATERIALS AND METHODS
Patient report.
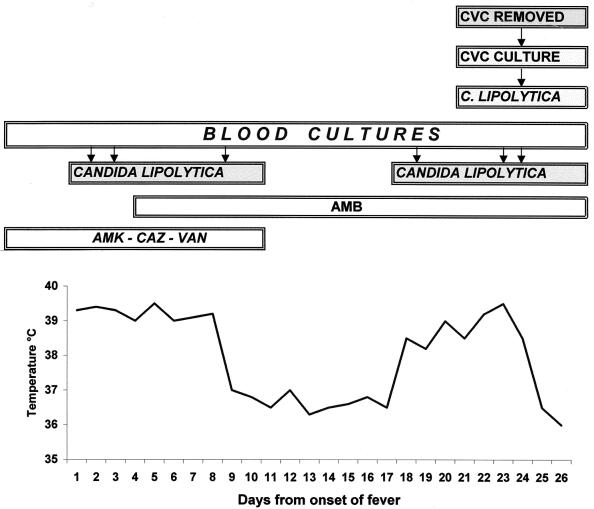
An 18-year-old female with acute lymphoblastic leukemia in stage II complete remission underwent allogeneic BMT from an HLA-identical brother on 26 September 1996. Prior to intensive chemotherapy a central venous catheter (CVC) was positioned. The pretransplant conditioning (myeloablative) regimen consisted of cyclophosphamide, VP16 (antineoplastic and immunosuppressive agents), and fractionated total body irradiation. Her prophylactic treatment consisted of trimethoprim-sulfamethoxazole, oral amphotericin B (AMB), and acyclovir. Prophylaxis of GvHD involved administration of cyclosporine as of day 1 prior to BMT. Engraftment was achieved on day 8 post-BMT. On day 12 post-BMT, an acute grade II GvHD of the skin and alimentary tract developed, requiring the use of high-dose methylprednisolone. On day 46 post-BMT, fever unresponsive to broad-spectrum antimicrobials developed. On day 48 post-BMT, a yeast identified as C. lipolytica was recovered from blood cultures and persisted for 7 days. Intravenous AMB (1 mg/kg of body weight/day) was administered, and for 10 days fever disappeared and blood cultures were negative. On day 65, during AMB treatment, fever reappeared and yeast-like organisms identified as C. lipolytica were isolated from consecutive blood cultures. Despite the absence of erythema, edema, or tenderness at the CVC entry site, and thrombophlebitis, a new central line catheter was inserted and the old CVC was removed and cultured. Cultures of the CVC tip revealed yeast-like organisms identified as C. lipolytica. After CVC removal, the body temperature of the patient became normal and blood cultures were negative. The clinical course and the timing of samples are summarized in Fig. 1. Two weeks later, the patient had rapidly progressive dyspnea and subsequently developed fatal cytomegalovirus interstitial pneumonia that was confirmed with an autopsy. Moreover, the autopsy examination and culturing of autopsy specimens of lungs, pericardium, liver, spleen, and kidneys did not reveal evidence of invasive yeast.
FIG. 1.
Clinical pattern of C. lipolytica catheter-related candidemia. The arrows indicate the day of occurrence of the event in the frame. AMK, amikacin; CAZ, ceftazidime; VAN, vancomycin.
Specimen collection and processing.
Blood samples were inoculated into aerobic and anaerobic bottles (Vital Biomerieux). The bottles were incubated at 37°C and read twice daily with an automatic detector (Biomerieux Italia, Rome, Italy). As bottles became positive, aliquots were removed for Gram staining. Based on the Gram stain results, the samples were subcultured onto appropriate media. Specimens positive for yeast-like cells were streaked for isolation on Sabouraud glucose agar (SGA) plates (Difco Laboratories, Detroit, Mich.), and plates were incubated at 27 and 37°C for 72 h. After removal, the catheter tip was also cultured (12).
Strain identification.
Yeasts grown on SGA plates were identified according to their morphological characteristics and biochemical profiles. The morphological features were examined on cornmeal-Tween 80 agar slide cultures (Unipath S.p.A., Garbagnate, Milan, Italy). Biochemical tests were performed by using ID32C strips with an ATB reader (API System; BioMerieux). Moreover, the following characteristics of the isolates were studied: urease activity with a commercial medium (Liofilchem s.r.l., Roseto degli Abruzzi, Teramo, Italy) and growth in vitamin-free medium (Difco). The in vitro antifungal susceptibility test was performed according to the National Committee for Clinical Laboratory Standards guidelines. The reference method was the broth macrodilution method. The medium used to prepare the 10× drug dilutions and inoculum suspensions was RPMI 1640 medium buffered to pH 7.0 with 0.165 Morpholinepropanesulfonic acid (MOPS) buffer. The MIC of AMB was the lowest concentration at which there was 100% inhibition of growth; that of azoles was defined as the lowest concentration at which there was at least an 80% inhibition of growth compared with that for the growth control tube (16).
Isolates.
Ten strains of C. lipolytica were sequentially isolated from the patient. Nine strains were isolated from blood, and one was isolated from the CVC tip. Three C. lipolytica strains were used as controls: a human isolate (C211) from M. G. Rinaldi, San Antonio, Tex.; an environmental isolate (C202) from E. Manzo, Ancona, Italy; and the strain ATCC 9773, listed as Yarrowia lipolytica.
Slime production test.
Slime production was assayed according to the method proposed by Branchini et al. (2). Briefly, a suspension of 105 cells from Sabouraud agar slants was grown in 5 ml of a liquid medium containing 1% yeast extract (Difco) and glucose at 2 or 8% (wt/vol) (Carlo Erba, Milan, Italy) in 15-ml Falcon 2095 polystyrene conic tubes and incubated at 37°C for 24 h at 150 rpm using an orbital incubator. After removal of liquid medium, each Falcon tube was examined visually for the presence of an adherent slime layer on the internal wall. Slime production was scored as follows: 0 (no production); 1 (weak production), presence of slime at the bottom of the Falcon tube (cone); 2 (moderate production), presence of slime both at the bottom and on the internal wall of the Falcon tube; and 3 (high production), presence of slime at the bottom, on the internal wall, and at the top of the Falcon tube (ring). Each isolate was tested at least three times and read independently by two different observers.
Ultrastructural analysis (SEM).
Isolates were grown in 2 and 8% glucose and studied by scanning electron microscopy (SEM). Samples from different sections of the culture tube (ring, tube, and cone) were examined. For each isolate 105 cells were inoculated in 5 ml of a liquid medium composed of 0.67% (wt/vol) yeast nitrogen base (Difco) and 0.9% (wt/vol) glucose (Carlo Erba), corresponding to a 50 mM concentration, in a 15-ml Falcon 2095 polystyrene tube at 37°C for 24 h at 150 rpm. The liquid medium was removed, tubes were rinsed internally with 5 ml of phosphate-buffered saline containing 1 mM MnCl2 and CaCl2 (pH 7.2), and 2.5 mg of lectin from Canavalia ensiformis (concanavalin A) (Pharmacia) per ml was added for 1 h at room temperature (13, 16). After the solution was discarded, the tubes were rinsed two times with the same buffer; cells adhering to the Falcon tube were fixed in 3% (vol/vol) glutaraldehyde solution in 0.1 M cacodylate buffer, pH 7.4, containing 0.2% ruthenium red for 24 h at room temperature. After three washes in the same solution, the cells were postfixed in 1% osmium tetroxide and 1% thiocarbohydrazide in distilled water for 2 to 4 h at room temperature with gentle agitation (1, 13). The cells were then dehydrated in graded ethanol; 0.5-cm2 sections of tubes were mounted on stubs by means of silver dag glue, covered with gold by means of an SCD040 Balzer sputterer, and observed by SEM (Philips 505).
DNA typing and restriction endonuclease analysis.
Independently, several single colonies of each C. lipolytica isolate were grown to the stationary phase in YPD medium (1% yeast extract, 2% Bacto Peptone, 2% [wt/vol] dextrose) (Difco) at 30°C in a horizontal shaker incubator, and total cellular DNA was extracted (3, 14). The following restriction endonucleases were used: EcoRI, HindIII, HinfI, and HpaII (Boehringer Mannheim GmbH). Approximately 10 μg of total cellular DNA was incubated for 4 to 6 h with 40 to 60 enzyme units, electrophoresed in a 1% agarose gel (Bio-Rad) at 30 V overnight in 1× TBE buffer (49 mM Tris-HCl, 49 mM boric acid, 1.5 mM EDTA, pH 8.2), stained in ethidium bromide, destained in distilled water, and photographed under UV light with Polaroid T57-type film. Bands of interest were sized by using GELS software version 3.1 (Bio-Rad), and lambda phage DNA cut with HindIII endonuclease was used as the molecular size marker.
RESULTS
Morphological and physiological analysis.
The morphological and physiological data gathered on blood and CVC isolates correlated well with those of C. lipolytica. On SGA, the yeasts formed distinctive cerebriform, convoluted firm white colonies. Microscopic examination after 3 days at 25°C showed ellipsoidal yeasts, either single, paired, or single in small clusters. Cultures on cornmeal agar showed features compatible with C. lipolytica such as pseudohyphae and true hyphae. The septa of true hyphae presented a single central pore. The API ID32C system yielded a 2300011011 code with excellent identification of the genus C. lipolytica, and all three control strains yielded an identical profile, assimilating glucose, glycerol, and erythritol and being urease positive. The in vitro antifungal susceptibility results for the clinical isolates and the three control strains are reported in Table 1. All C. lipolytica strains were susceptible to AMB, fluconazole, and itraconazole. No substantial differences in MICs were detected between clinical isolates and control strains.
TABLE 1.
Biofilm production scores and antifungal susceptibilities (16) of all isolates and control strains
| Strain | No. of days from onset of fever | Source | Slime productiona
|
MIC (μg/ml) of drug:
|
|||
|---|---|---|---|---|---|---|---|
| 2% glucose | 8% glucose | AMB | Fluconazole | Itraconazole | |||
| Clinical | |||||||
| 1 | 2 | Blood | 2 | 2 | 0.39 | 2.5 | 0.5 |
| 2 | 4 | Blood | 3 | 2 | 0.78 | 1.25 | 0.5 |
| 3 | 4 | Blood | 2 | 2 | 0.39 | 1.25 | 0.5 |
| 4 | 9 | Blood | 2 | 2 | 0.39 | 1.25 | 0.5 |
| 5 | 9 | Blood | 3 | 3 | 0.78 | 2.5 | 0.5 |
| 6 | 9 | Blood | 3 | 2 | 0.78 | 2.5 | 0.5 |
| 7 | 19 | Blood | 2 | 2 | 0.78 | 1.25 | 0.5 |
| 8 | 23 | Blood | 2 | 2 | 0.39 | 2.5 | 0.5 |
| 9 | 25 | Blood | 2 | 2 | 0.78 | 2.5 | 0.5 |
| 10 | 25 | CVC | 2 | 2 | 0.39 | 2.5 | 0.5 |
| Control | |||||||
| 11 (C211) | 2 | 2 | 0.19 | 2.5 | 0.5 | ||
| 12 (C202) | 2 | 2 | 0.19 | 1.25 | 0.5 | ||
| 13 (ATCC 9773) | 2 | 3 | 0.19 | 1.25 | 0.5 | ||
Score: 1, low; 2, moderate; 3, high.
Slime production.
Table 1 reports the slime score assigned to each strain after growth in 2 or 8% glucose. All isolates and control strains showed moderate to high slime production, and no substantial differences were observed in slime production between 2 and 8% glucose concentrations.
Ultrastructural analysis.
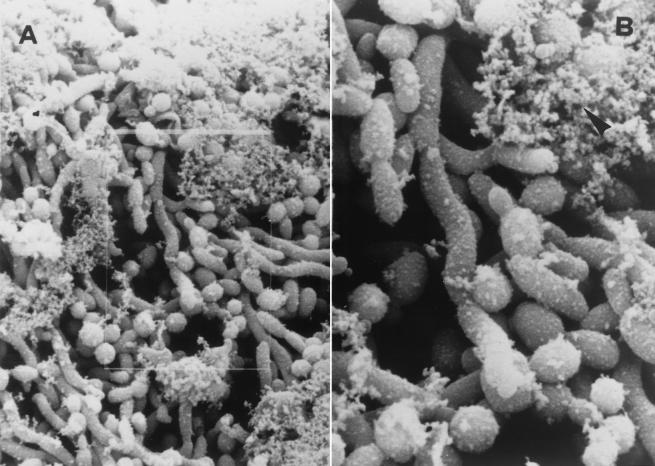
Slime production was confirmed through SEM (Fig. 2), which showed amorphous material (slime) unevenly distributed on the cell surface. The slime seemed to entangle the yeast cells and to contribute both to intrayeast cohesion and to their adhesion to the plastic. SEM also showed that the concentration of yeast cells was quite stable across the different sections of the tube for the strains grown with 2% glucose. When the strains were grown with 8% glucose, a consistent decrease in yeast cell concentration was observed moving from the ring of the culture tube to the cone, and there were no differences in production between our isolates and the control strains.
FIG. 2.
(A) Scanning electron microphotograph of blood isolate. The microphotograph shows microcolonial aggregate intimately associated with an amorphous material which seems to envelope single cells and join them (original magnification, ×950). (B) Enlargement of the image outlined in panel A (original magnification, ×2,150). The arrowhead indicates the amorphous material (slime).
Genomic typing.
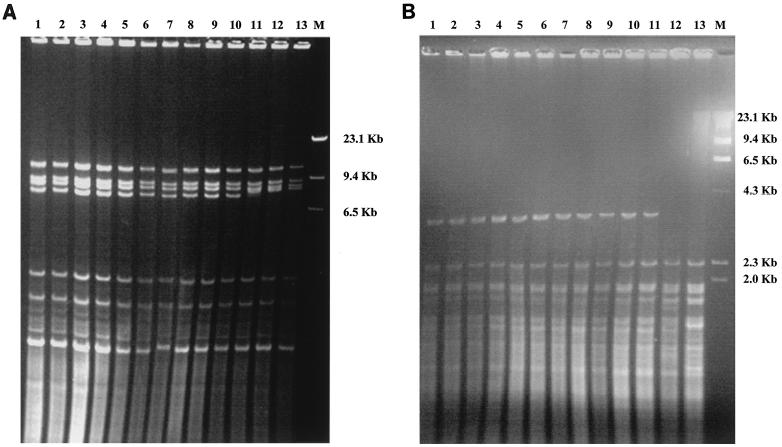
All isolates from the patient had identical restriction profiles with HpaII and HinfI endonucleases, while differences emerged with control strains (Fig. 3A). After restriction with HpaII, the control strains presented the same profile but were clearly different from our isolates, in that the 7,485-kb band was absent. Conversely, HinfI digestion produced substantial differences among the control strains. The C211 strain presented the same profile as did the clinical isolates, the C202 strain showed a unique pattern characterized by the absence of the 2,050-kb band, and the ATCC strain showed a supplementary band of 23,650 kb, compared with the other strains (Fig. 3B).
FIG. 3.
Restriction fragment length polymorphism analysis of whole-cell DNAs of C. lipolytica isolates after digestion with HpaII (A) or HinfI (B). Lanes: 1 to 10, our clinical isolates; 11, control strain C211; 12, control strain C202; 13, ATCC 9773; M, size marker (HindIII digest of bacteriophage lambda DNA).
DISCUSSION
Over the past 25 years the incidence of invasive candidiasis in patients with hematological malignancies has increased dramatically; as well, the variety of AMB-resistant species isolated during infections in these patients has also changed. The proportion of C. albicans strains has decreased, and isolations of non-C. albicans spp. have increased (23; W. G. Merz, Abstr. Invasive Mycoses Crit. Trends Clin. Challenge Focus Itraconazole Congr., 1993). In a recent report, non-C. albicans species accounted for 63.4% of the candidemias studied (20). Of non-C. albicans spp. isolated from opportunistic infections, C. lipolytica has infrequently been identified as a cause. Most of these isolations have been due to line infections (18), as was the case in this study. Positive cultures of the CVC tip from this patient in the absence of other potential sources of infection were very suggestive of CVC-associated candidemia. Furthermore, BMT-related procedures, persistent use of broad-spectrum antimicrobial agents, and GvHD predisposed this patient to infusion-related infections. Finally, after CVC removal, subsequent blood cultures were persistently negative, and the episode of candidemia appeared to be finished.
In all cases of infections reported in the literature C. lipolytica has been shown to be weakly virulent. This is in agreement with the absence of mortality and the very low frequency of visceral lesions observed in mice inoculated with C. lipolytica (26). The weak virulence of this organism is further supported by our findings. In this case, despite the long persistence of candidemia, there was no evidence of deep visceral infection at autopsy examination or through positive autopsy cultures.
The consistent slime production that we observed for this isolate might explain the capability of this organism to adhere to and colonize the plastic CVC. Slime production might also serve to explain the reappearance of a persistent fever indicative of a blood-borne infection during treatment with AMB, despite the susceptibility of our C. lipolytica isolates to this drug. Similar to the inability of antimicrobial agents to penetrate bacterial biofilms (8, 17, 22), extracellular slime of C. lipolytica might have impaired the efficacy of antifungal agents in penetrating the thick biofilm on the catheter surface (11).
The appropriate management of catheter-related C. lipolytica fungemia has yet to be defined. A recent paper suggests that a stand-back approach without catheter removal or antifungal therapy may be acceptable (4). Conversely, according to our findings and in line with those reported by others (24, 26), management of catheter-related C. lipolytica fungemia should include a course of systemic antifungal therapy and removal of the potentially infected vascular catheter.
It has been reported elsewhere that colonizing and infecting Candida populations may undergo microevolution, particularly in immunocompromised individuals such as BMT patients (21). This genetic variation is due primarily to the reorganization of genomic sequences. Therefore, genomic typing was performed to compare strains of C. lipolytica isolated from the patient during the two distinct febrile episodes. Restriction fragment length polymorphism profiles proved to be identical over time for all our isolates. This finding suggests the absence of microevolutionary changes in the population of the infecting strain, despite the length of the sepsis and the potential selective pressure of AMB, which had been administered to the patient for about 20 days. The lack of differences between our isolates did not depend on a low discriminatory power of the typing method. In fact, coupling the results of restriction analyses with HpaII and HinfI we were able both to differentiate our isolates from the control strains and to determine genotype differences among the control strains. These differences between our isolates and the control strains are also suggestive of a certain degree of genetic diversity between C. lipolytica isolates from widely divergent geographical areas. This variability seems to be present also in strains isolated in different places within the same country, as indicated by the diverse genomic profiles observed for our isolates compared to one of the two control strains coming from another Italian city.
In conclusion, the increasing evidence of catheter-related C. lipolytica infections suggests that C. lipolytica should be included in any list of emerging yeast pathogens and also poses the problem of defining the best strategy of patient management, given the current controversy on the most appropriate approach.
Acknowledgments
This work was supported in part by the Associazione Donatori Sangue, Pescara, and Cofinanziamento per Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (Unit G. Carruba).
REFERENCES
- 1.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, and W. J. Martone. 1991. Secular trends in nosocomial primary bloodstream infections in the United States 1980-1989. National Nosocomial Infections Surveillance System. Am. J. Med. 3B:86S-89S. [DOI] [PubMed] [Google Scholar]
- 2.Branchini, M. L., M. A. Pfaller, J. Rhine-Chalberg, T. Frempong, and H. D. Isenberg. 1994. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J. Clin. Microbiol. 32:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carruba, G., E. Pontieri, F. De Bernardis, P. Martino, and A. Cassone. 1991. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J. Clin. Microbiol. 29:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, L., T. H. Park, E. Y. Lee, Y. T. Lim, and H. C. Son. 2001. Recurrent self-limited fungemia caused by Yarrowia lipolytica in a patient with acute myelogenous leukemia. J. Clin. Microbiol. 39:1200-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanock, S. J., and T. D. Walsh. 1996. Evolving concepts of prevention and treatment of invasive fungal infections in pediatric bone marrow transplant recipients. Bone Marrow Transplant. 3:15-20. [PubMed] [Google Scholar]
- 6.D'Antonio, D., A. Iacone, L. Pierelli, T. Bonfini, and the Gruppo Italiano di Studio per la Manipolazione Cellulare in Ematologia. 1995. Patterns of recovery phase infection after autologous blood progenitor cell transplantation in patients with malignancies. Eur. J. Clin. Microbiol. Infect. Dis. 14:552-556. [DOI] [PubMed] [Google Scholar]
- 7.D'Antonio, D., B. Violante, A. Mazzoni, T. Bonfini, M. A. Capuani, F. D'Aloia, A. Iacone, F. Schioppa, and F. Romano. 1998. A nosocomial cluster of Candida inconspicua infections in patients with hematological malignancies. J. Clin. Microbiol. 36:792-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman, D. A., and G. B. Pier. 1993. Pathogenesis of infections related to intravascular catheterization. Clin. Microbiol. Rev. 6:176-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazen, K. C. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 8:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzman, C. P. 1998. Yarrowia van de Walt & von Arx. Descriptions of telomorphic ascomycetous genera and species, p. 420-421. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts: a taxonomic study, 4th revised ed. Elsevier Science, Amsterdam, The Netherlands.
- 11.Levenson, D., M. A. Pfaller, M. A. Smith, R. Hollis, T. Gerarden, C. B. Tucci, and H. D. Isenberg. 1991. Candida zelanoides: another opportunistic yeast. J. Clin. Microbiol. 29:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki, D. G., C. E. Weise, and H. W. Sarafin. 1977. A semi-quantitative culture method for identifying catheter-related infections. N. Engl. J. Med. 296:1305-1309. [DOI] [PubMed] [Google Scholar]
- 13.Malick, L. E., and R. B. Wilson. 1975. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 50:265-269. [DOI] [PubMed] [Google Scholar]
- 14.McCourtie, J., and L. J. Douglas. 1981. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect. Immun. 32:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyers, J. D. 1990. Fungal infections in bone marrow transplant patients. Semin. Oncol. 17:10-13. [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1992. Reference method for broth dilution antifungal susceptibility testing of yeasts. Proposed standard NCCLS document M27-P. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 17.Nikava, H., H. Nishimura, T. Yamamoto, T. Hamada, and L. P. Samaranayake. 1996. The role of saliva and serum in Candida albicans biofilm formation on denture acrylic surfaces. Microb. Ecol. Health Dis. 9:35-48. [Google Scholar]
- 18.Ninin, E., O. Morin, L. E. Tortorec, N. Milpied, P. Moreau, and J. L. Harousseau. 1997. Infection invasive à Candida lipolytica après allogreffe de moelle osseuse. J. Mycol. Med. 7:212-214. [Google Scholar]
- 19.Nitzulescu, V., and M. Niculescu. 1976. Three cases of ocular candidiasis caused by Candida lipolytica. Arch. Roum. Pathol. Exp. Microbiol. 35:269-272. [PubMed] [Google Scholar]
- 20.Nucci, M., A. L. Colombo, F. Silveira, R. Richtmann, R. Salomao, M. L. Branchini, and N. Spector. 1998. Risk factors for death in patients with candidemia. Infect. Control Hosp. Epidemiol. 19:846-850. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., J. Rhine-Chalberg, S. W. Redding, J. Smith, G. Farinacci, A. W. Fothergill, and M. G. Rinaldi. 1994. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J. Clin. Microbiol. 32:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson, M. D., and M. H. Kokki. 1998. Antifungal therapy in bone marrow failure. Br. J. Haematol. 100:619-628. [DOI] [PubMed] [Google Scholar]
- 24.Shin, J. H., H. Kook, D. H. Shin, T. J. Hwang, M. Kim, S. P. Suh, and D. W. Ryang. 2000. Nosocomial cluster of Candida lipolytica fungemia in pediatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 19:344-349. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez, J. A., T. Lundstrom, L. Dembry, P. Chandrasekar, D. Boikov, M. B. Parri, and M. J. Zervos. 1995. Invasive Candida guillermondii infection: in vitro susceptibility studies and molecular analysis. Bone Marrow Transplant. 16:849-853. [PubMed] [Google Scholar]
- 26.Walsh, T. J., I. F. Salkin, D. M. Dixon, and N. J. Hurd. 1989. Clinical, microbiological, and experimental animal studies of Candida lipolytica. J. Clin. Microbiol. 27:927-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter, E., and R. Bowden. 1995. Infection in the bone marrow transplant recipient. Infect. Dis. Clin. N. Am. 9:823-847. [PubMed] [Google Scholar]
- 28.Wehrspann, P., and U. Fullbrandt. 1985. Report of a case of Yarrowia lipolytica (Wickerham et al.) van der Walt and von Arx isolated from a blood culture. Mykosen 28:217-222. [PubMed] [Google Scholar]