Abstract
Unfed Ixodes ricinus nymphs were infected with eight different strains and clones of Borrelia afzelii and B. garinii by capillary feeding. Except one B. afzelii clone, all expressed OspC in culture. Tick midguts and salivary glands were investigated at different time intervals for the presence of borreliae and for OspA and OspC phenotypes by immunofluorescence with simultaneous staining of OspA and OspC with monoclonal antibodies. Both species were transmittable to I. ricinus. All OspC-expressing strains and clones were able to disseminate into the salivary glands. In contrast, the OspC-negative B. afzelii clone was not detectable in the salivary glands, an indication that OspC plays an important role in dissemination. OspA-positive borreliae prevailed in the midgut. OspC positives were more frequent in the salivary glands than in the midgut. Notably, simultaneously OspA- and OspC-negative borreliae were detected in both organs. Kinetics of dissemination varied with the strains. The OspC-positive B. afzelii clone and all B. garinii OspA type 4 strains were detectable in the salivary glands right after feeding, while one B. garinii OspA type 6 strain invaded the salivary glands with a delay of 24 h. These findings support the hypothesis that OspA is abundantly expressed in unfed ticks while upregulation of OspC is also a prerequisite for dissemination in the vector for the Eurasian species B. afzelii and B. garinii. However, we found strain-specific dynamics of Osp expression and strain-specific kinetics of systemic infection in the vector tick and it appears that additional factors are involved in the initiation and regulation of the dissemination process.
The Borrelia burgdorferi sensu lato complex comprises at least three human-pathogenic species, all of which are present in Europe: B. burgdorferi sensu stricto, the only species causing Lyme borreliosis in the United States; B. afzelii; and B. garinii (1, 3). These spirochetes alternate in nature between endothermic hosts (mammals) and poikilothermic vectors (hard ticks within the genus Ixodes). In the vector, the spirochetes restart replication during the feeding process, migrate through the gut wall, and invade various tissues, including the salivary glands, wherefrom they are transmitted to the host via saliva (2, 7, 11, 25, 27, 36). The borreliae are confronted with abrupt environmental changes during this cycling, such as differences in temperature, pH, the immune system, or osmotic pressure. To cope with these rapid changes, effective regulatory mechanisms for adaptation are required. Alteration of the outer surface protein (Osp) expression pattern—especially that of OspA and OspC—seems to be crucial for this adaptation process; expression of these two proteins varies even under routine culture conditions and seems to be inversely correlated (5, 29, 30, 31). Elevated temperature and cocultivation with tick cells have been shown to induce OspC expression (10, 20, 26, 28). OspA is abundantly expressed in unfed ticks, possibly mediating adherence to midgut cells and thus enabling borreliae to survive in the vector for prolonged periods without tick feeding, while during the blood meal, up-regulation of OspC is associated with borrelial invasion of the tick salivary glands and infection of the warm-blooded host (7, 8, 9, 16, 18, 19, 25, 26, 27). These data have been obtained mainly with B. burgdorferi sensu stricto strains, the only human-pathogenic species present in the United States. In Europe, ticks in nature are likely to be infected with Lyme borreliae of all three known human-pathogenic species.
We were therefore interested in the performance of different strains of B. afzelii and B. garinii in the natural vector tick, Ixodes ricinus, with respect to their Osp phenotypes and their ability to cause a systemic infection after capillary feeding. The results presented herein indicate strain-specific dynamics of Osp expression and a strain-specific ability to disseminate in the tick vector.
MATERIALS AND METHODS
Ticks.
All of the nymphal I. ricinus ticks used in this study were derived from a colony maintained at the Institut für Angewandte Zoologie und Ökologie der Tiere, Freie Universität Berlin, Berlin, Germany. The laboratory-reared ticks had been free of B. burgdorferi sensu lato infection for at least two generations and were used, as a rule, 8 months post nymphal ecdysis at the earliest. The nymphs were kept shaded at room temperature and 95% relative humidity for at least 2 weeks before the start of the experiments.
Borrelia strains and clones (Table 1).
TABLE 1.
Expression of OspA and OspC of the borrelial strains and clones studied in culture
| Species and strain or clone | Expressiona of:
|
|
|---|---|---|
| OspA | OspC | |
| B. afzelii | ||
| cPKo97 | + | + |
| cPKo345 | + | − |
| B. garinii (OspA type 4) | ||
| PBi | + | + |
| PBaeII | + | + |
| PMue | + | + |
| B. garinii (OspA type 6) | ||
| PSoR | + | + |
| IS2r | + | + |
As determined by IFA and immunoblot assay.
B. afzelii clones PKo97 K37 (cPKo97) and PKo345 II-2-3 (cPKo345) were derived by triple-colony selection of reisolates from a gerbil--cPKo345 from a joint and cPKo97 from a kidney--infected with low-passage B. afzelii strain PKo (a human skin isolate) (10). As described previously (10), cPKo345 has an insertion of a guanine in the ospC gene at position 200, leading to a frame shift with a stop codon after position 222 and an inability to produce OspC. As B. garinii OspA serotype 4 strains, PBi102, a reisolate from a gerbil infected with low-passage strain PBi (a cerebrospinal fluid [CSF] isolate) (32, 35), and low-passage PBaeII (34) and PMue (17, 34), both human CSF isolates, were included in this study. B. garinii OspA serotype 6 strains comprised the low-passage human CSF isolate PSoR (34) and the reisolate IS2r (unpublished) from a gerbil infected with the tick isolate IS2. All serotype 4 and 6 strains and cPKo97 were positive for OspA and OspC by immunofluorescence assay (IFA) and immunoblot assay (Table 1).
Strains and clones were grown as previously described (24) to a density of 106/ml in 100 ml of MKP medium (24) at 33°C and tested by IFA and Western blot assay for expression of OspA and OspC, and at least 20 vials of each culture were frozen at −70°C as a stock of cultures with identical passage and cultivation histories. Before each experiment, the concerning borreliae were regenerated and cultured for 1 week as described above.
Capillary feeding and preparation of nymphs.
Nymphal ticks were artificially infected with the different strains or clones by the capillary feeding method as described previously (11). The ticks were allowed to feed for 3 to 4 h at 33°C in a humid chamber on capillaries containing MKP with 107 to 108 borreliae/ml. Indications of successful feeding were excretion of droplets via the anus and a more transparent appearance of the tick body. The infected nymphs were kept at room temperature in a chamber at 95% relative humidity until dissection. Ticks were dissected at different time intervals after capillary feeding (0, 6, 12, 18, 24, 48, 72, and 96 h and 14 days) under a Zeiss Stemi SV11 binocular microscope (Zeiss, Leipzig, Germany) at magnifications of ×6 to ×66. Prepared salivary glands and midguts were separately rinsed twice in 15 μl of phosphate-buffered saline (pH 7.4), transferred to a spot of a 12-well multitest slide (ICN Biomedicals) with 5 μl (for salivary glands) or 10 μl (for midguts) of distilled water, carefully homogenized with fine needles and forceps, and then distributed onto two spots each. During homogenization, small amounts of distilled water were added from time to time to prevent desiccation. After air drying, the smears were fixed with methanol for 15 min and stored at −20°C until use for IFA.
MAbs.
OspA-specific monoclonal antibody (MAb) L32 1F11 (immunoglobulin G1 [IgG1] subclass) recognizes an epitope conserved among B. burgdorferi sensu lato strains and could therefore be used for all OspA IFAs (32). MAb L22 1F8 was used for OspC detection in PKo clones, as well as in serotype 4 strains, and L22 2B8 was used for OspC detection in serotype 6 strains (31, 33). Both of the anti-OspC MAbs belong to the IgG2a subclass.
IFAs.
The IFA protocol used was essentially that described previously (9). To achieve simultaneous labeling of OspA and OspC of the borreliae, slides were incubated for 30 min with a mixture of anti-OspA MAb L32 1F11 (IgG1 subclass; final dilution, 1:4) and MAb L22 1F8 or L22 2B8 (both IgG2a subclass; final dilution, 1:4) (33) against OspC. To differentiate the two antibodies, slides were incubated for 30 min with a mixture of a fluorescein isothiocyanate (FITC)-conjugated antibody to mouse IgG1 (Caltag, San Francisco, Calif.) and rhodamine-phycoerythrin (R-PE)-conjugated antibody to mouse IgG2a (Caltag) at a final dilution of 1:100 each. The slides were finally incubated for 30 s with 4′,6′-diamidino-2-phenylindole (DAPI; 1:10,000), a blue fluorescent, DNA-intercalating dye, to visualize all borreliae (Fig. 1). A defined OspA- and OspC-expressing passage of skin isolate PKo was used as a positive control. An OspC-negative variant of CSF isolate PKa2 (33) and an OspA-negative variant of skin isolate PPop (32) served as negative controls. Entire individual spots were carefully examined with a Leitz Laborlux 12 microscope fitted for epifluorescence imaging at a magnification of ×400. Osp expression of individual spirochetes was visualized with filters suitable for FITC (green), R-PE (red), FITC and R-PE (for photodocumentation), or DAPI (blue). All visual fields were screened with the filters suitable for FITC, R-PE, and DAPI.
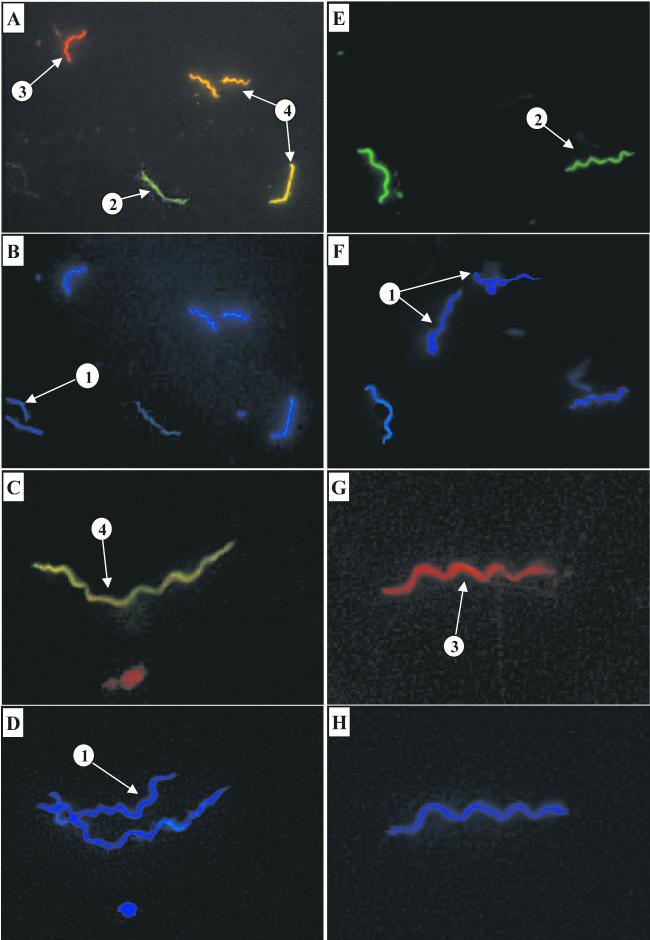
FIG. 1.
Examples of the appearance of different borrelial phenotypes under a microscope. Blue pictures were taken with a filter suitable for DAPI, and red and green pictures were taken with a filter suitable for FITC and R-PE. Arrows: 1, OspA- and OspC-negative borreliae; 2, borreliae positive for only OspA; 3, borreliae positive for only OspC; 4, borreliae positive for both OspA and OspC. Panels A to F show strain PMue. The same field of a culture preparation (A and B), a midgut preparation (C and D), and a salivary gland preparation (E and F) is shown in the indicated pairs of panels. Samples C to F were from a tick 48 h after capillary feeding. Panels G and H show the same field of a salivary gland preparation directly after capillary feeding with strain IS2r.
To determine the detection limit of the IFA test, 10-fold serial dilutions from 105 down to 101 borreliae/ml were produced from cPKo97, PBi, and PSoR. The density of borreliae was determined by dark-field microscopy since insufficient growth of our borreliae on solid media prevents CFU counting. Ten microliters of each dilution, corresponding to 103 to 10−1 borreliae per spot, was fixed in triplicate on 12-well slides and tested by IFA as described above. Furthermore, tick homogenates—produced as described above—on 12-well slides were spiked with 10 and 100 borreliae of cPKo97, PBi, and PSoR, each in duplicate, to determine the influence of tick material on the detection limit.
Statistical analysis.
For statistical analysis, Fisher′s exact test was performed. P < 0.05 was regarded as significant. Only preparations with ≥10 spirochetes were used for calculations.
RESULTS
Detection limit of the IFA.
Borreliae were detectable in preparations with only cultured material in all spots with 100 or more cells and in five of the nine spots with 10 borreliae. We then spiked homogenized tick material with 100 or 10 spirochetes to assess their influence on the assay's detection limit. Borreliae were visible in all six spots spiked with 100 borreliae but in none of the six spots spiked with 10 borreliae.
Dissemination of borreliae (Table 2).
TABLE 2.
Numbers of borreliae found in the midguts versus the salivary glands of infected individual I. ricinus nymphsa
| Time after CFb and tick no. |
B. afzelii
|
B. garinii OspA type 4
|
B. garinii OspA type 6
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cPKo97
|
cPKo345
|
PBaeII
|
PBi102
|
PMue
|
PSoR
|
IS2r
|
||||||||
| MG | SG | MG | SG | MG | SG | MG | SG | MG | SG | MG | SG | MG | SG | |
| 0 h | ||||||||||||||
| 1 | 54 | 15 | 52 | 0c | 17 | 1 | 10 | 1 | 20 | 0c | 0c | 0c | 67 | 8 |
| 2 | 85 | 8 | 26 | 0c | 145 | 13 | 9 | 3 | 92 | 25 | 64 | 0c | 7 | 53 |
| 3 | NDd | ND | 32 | 0c | 6 | 25 | 26 | 4 | 132 | 0c | 7 | 0c | 52 | 4 |
| 6 h | ||||||||||||||
| 1 | 131 | 23 | 9 | 0c | 72 | 7 | 60 | 1 | 9 | 0c | 0c | 0c | 34 | 17 |
| 2 | 67 | 11 | 38 | 0c | 41 | 9 | 7 | 3 | 35 | 22 | 19 | 0c | 6 | 5 |
| 3 | ND | ND | 19 | 0c | 137 | 57 | 37 | 0c | 26 | 3 | 7 | 0c | 2 | 25 |
| 12 h | ||||||||||||||
| 1 | 30 | 15 | 44 | 0c | 140 | 116 | 227 | COe | 4 | 1 | 0c | 0c | 16 | 11 |
| 2 | 99 | 21 | 18 | 0c | 28 | 1 | 30 | 13 | 17 | 22 | 7 | 0c | 6 | 2 |
| 3 | ND | ND | 17 | 0c | 5 | 3 | 39 | 0c | 123 | 9 | 14 | 0c | 2 | 11 |
| 18 h | ||||||||||||||
| 1 | 54 | 14 | 13 | 0c | 24 | 0c | 75 | 5 | 162 | 0c | 0c | 0c | 5 | 30 |
| 2 | 26 | 12 | 29 | 0c | 2 | 11 | 11 | 3 | 7 | 22 | 6 | 0c | 11 | 0c |
| 3 | ND | ND | 8 | 0c | 1 | 0c | 5 | 0c | 56 | 10 | 10 | 0c | 0c | 6f |
| 24 h | ||||||||||||||
| 1 | 125 | 35 | 25 | 0c | 39 | 12 | 13 | 1 | 39 | 2 | 130 | 53 | 16 | 0c |
| 2 | 56 | 18 | 122 | 0c | 2 | 2 | 16 | 1 | 9 | 6 | 22 | 0c | 172 | 1 |
| 3 | ND | ND | 20 | 0c | 2 | 8 | 5 | 10 | 11 | 5 | 62 | 0c | 7 | 1 |
| 48 h | ||||||||||||||
| 1 | 76 | 41 | 9 | 0c | 11 | 0c | 21 | 17 | 301 | 7 | 9 | 10 | 0c | 0c |
| 2 | 38 | 18 | 168 | 0c | 10 | 2 | 5 | 7 | 7 | 25 | 21 | 19 | 0c | 4f |
| 3 | ND | ND | ND | ND | 55 | 19 | 21 | 6 | 43 | 9 | 2 | 2 | 10 | 46 |
| 72 h | ||||||||||||||
| 1 | 29 | 8 | 21 | 0c | 9 | 10 | 26 | 4 | 34 | 3 | 236 | CO | 3 | 12 |
| 2 | 87 | 37 | 13 | 0c | 0c | 0c | 14 | CO | 12 | 13 | 24 | 5 | 23 | 0c |
| 3 | ND | ND | 22 | 0c | 11 | 11 | 13 | 2 | ND | ND | 1 | 1 | 0c | 7f |
| 96 h | ||||||||||||||
| 1 | 93 | 54 | 54 | 0c | 13 | 0c | 4 | 1 | 419 | 1 | 36.5 | 312 | 2 | 14 |
| 2 | 91 | 25 | 9 | 0c | 3 | 3 | 43 | 7 | 15 | 5 | 7 | 14 | 64 | 7 |
| 3 | ND | ND | 30 | 0c | 66 | 5 | 0c | 0c | 17 | 14 | 2 | 0c | 1 | 36 |
| 2 wks | ||||||||||||||
| 1 | ND | ND | ND | ND | 1 | 0c | 299 | 0c | 0c | 4f | 43 | 28 | 0c | 12f |
| 2 | ND | ND | ND | ND | 38 | 4 | 55 | 1 | 7 | CO | 2 | 18 | 0c | 0c |
| 3 | ND | ND | ND | ND | 17 | 0c | 17 | 0c | ND | ND | 5 | 0c | 5 | 1 |
Osp phenotypes are given in Fig. 2.
CF, capillary feeding.
Material without borreliae.
Nd, not done.
CO, salivary glands contaminated with bacteria from the midgut.
Salivary gland infection without any borreliae detected in the midgut.
OspC-positive, B. afzelii-derived cPKo97 was present in the salivary glands immediately after the feeding process and throughout the investigation period. In contrast, OspC-negative cPKo345 was not detectable in the salivary glands during the whole investigation period of 96 h, although this clone was always found in the midgut. The B. garinii strains were monitored for 14 days. All of the OspA type 4 strains investigated—PBaeII, PBi, and PMue—were able to disseminate to the salivary glands during capillary feeding. However, there were differences in salivary gland infection. Strain PMue caused salivary gland infection in only one of three ticks immediately after capillary feeding but was found in all salivary glands 12 h after capillary feeding. Strain PBaeII was observed in the salivary glands of all of the ticks examined at 0, 6, and 12 h after capillary feeding, but 6 out of 17 salivary glands were found to be negative at 18 h postinfection and later although borreliae were concurrently present in the ticks' midguts. OspA type 6 strains showed an even greater difference in behavior. PSoR entered the salivary glands with a delay of 24 h but was regularly present afterward, while strain IS2r was present in the salivary glands just after capillary feeding and in most of the salivary glands throughout the whole investigation period. Moreover, borreliae were present in the salivary glands of four ticks infected with IS2r although no borreliae were found in their midguts.
Expression of OspA and OspC (Fig. 2). (i) B. afzelii clones.
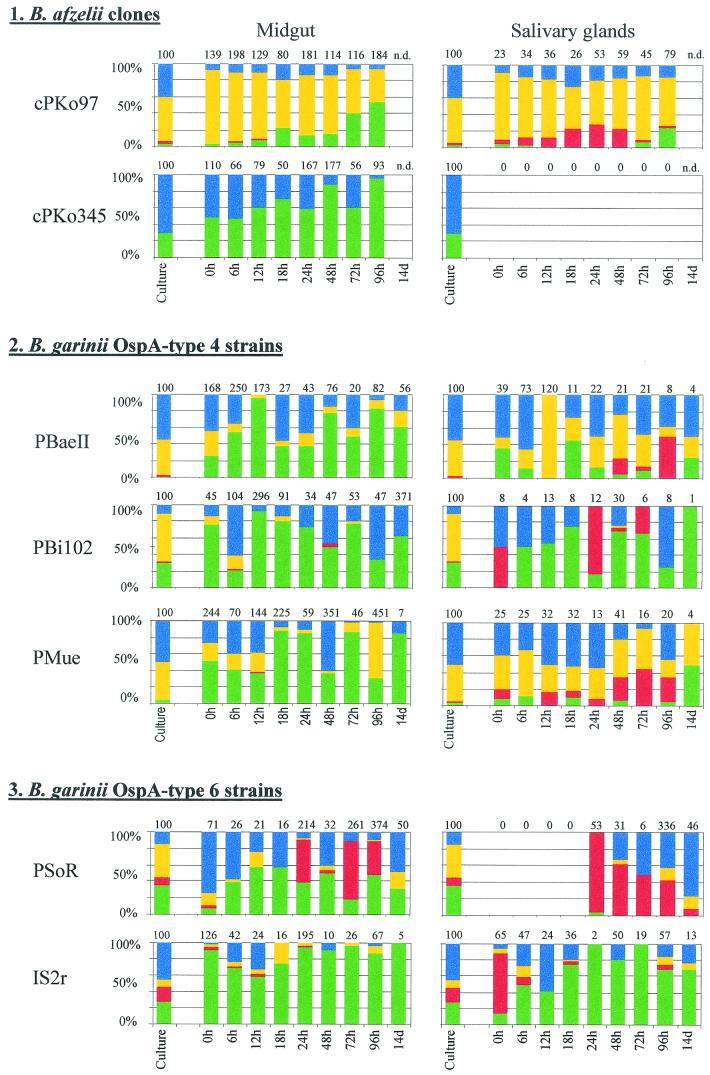
FIG. 2.
Osp expression patterns of the investigated Borrelia strains and clones in ticks. Nymphal I. ricinus ticks were infected by capillary feeding with different strains and clones of B. afzelii and B. garinii and investigated at various intervals after capillary feeding for the presence and Osp phenotypes of borreliae in the midgut and salivary glands. Individual bars show the proportions of the different phenotypes. The first bar in each diagram represents the percentage of Osp phenotypes in the culture used for capillary feeding. The following bars represent the percentages of the Osp phenotypes present in all of the ticks investigated at the respective time point. The value above each bar indicates the number of borreliae the Osp phenotype relationship is based upon. For the numbers of borreliae in individual ticks, see Table 2. n.d., not done. Colors: green, positive for only OspA; red, positive for only OspC; yellow, positive for OspA and OspC; blue, positive for neither OspA nor OspC.
The cultured borreliae of cPKo97 used for capillary feeding showed predominantly simultaneous expression of OspA and OspC or no such expression. Few spirochetes had only OspA or only OspC detectable on the surface. Immediately after capillary feeding, significantly more of the borreliae in the tick gut simultaneously expressed OspA and OspC. The fraction positive for only OspA increased significantly during the investigation period, while the proportion of doubly expressing borreliae decreased. The borrelia population in the salivary glands just after capillary feeding consisted mainly of doubly expressing bacteria, comparable to those found in the midgut. The fraction positive for only OspC increased significantly to nearly 30% at 24 h post capillary feeding, while those positive for only OspA had disappeared until 6 h after capillary feeding. The fraction of those positive for only OspC had decreased significantly at 96 h post capillary feeding, while OspA was up-regulated again on the borrelial surface. In cPKo345, most of the cultured borreliae expressed neither OspA nor OspC and a minor fraction expressed only OspA. A significant increase in the fraction of those positive for only OspA was observed in the tick midgut during the study period, while OspC was never present.
(ii) B. garinii OspA type 4 strains.
The OspA type 4 strains used for capillary feeding in culture consisted predominantly of borreliae that expressed both OspA and OspC combined with borreliae that expressed no Osp (PBaeII and PMue) or only OspA (PBi102). A significantly higher percentage of bacteria of all three strains that were positive for only OspA was found in the midgut during the investigation period compared to the culture. There was no recognizable development of specific phenotypes in the tick salivary glands. However, the proportion of OspC-positive borreliae was higher there in all three strains than in the midgut.
(iii) B. garinii OspA type 6 strains.
Strain PSoR in culture consisted mainly of borreliae expressing only OspA or both OspA and OspC. The borreliae showed no development of a specific phenotype in the midgut during the study period. In contrast, in the salivary glands, where the borreliae were first detectable 24 h after capillary feeding, initially only OspC-positive cells dominated. This phenotype significantly decreased over time, and most borreliae were both OspA and OspC negative at the end of the investigation period. Strain IS2r in culture consisted mainly of OspA- and OspC-negative borreliae, followed by fractions presenting only OspA or only OspC. Most of the borreliae in the midgut were positive for only OspA immediately after capillary feeding and throughout the whole investigation period. In the salivary glands, the majority of the borreliae were at first positive for only OspC. OspC was then downregulated and those positive for only OspA prevailed.
DISCUSSION
Current hypotheses about the events that happen during dissemination of borreliae from the midgut to the salivary glands of the tick vector are based on experiments with the species B. burgdorferi sensu stricto and the North American vector I. scapularis. However, the genetic diversity among human-pathogenic Borrelia species and even among strains within a particular species in Europe (1, 32, 33, 34) in the context of a different vector, I. ricinus, raised the question of whether there are differences in the adaptation strategies of different borreliae in different tick vectors. We therefore investigated B. afzelii and B. garinii strains and clones for the ability to disseminate in I. ricinus after artificial infection via capillary feeding. The results of the present study strongly suggest that Osp expression and dissemination dynamics may vary even among strains of the same genospecies.
We demonstrated in the present study that the European vector tick I. ricinus can be infected with B. afzelii and B. garinii via capillary feeding. Except for OspC-negative B. afzelii clone PKo345, all of the strains tested were able to disseminate into the salivary glands without prior blood contact. These results parallel the findings of Hu et al. (12) on B. garinii and show that the same is true of B. afzelii.
Immediately after capillary feeding, the proportion of OspA-positive borreliae in the midgut was—except for strain PSoR--consistently higher than that of such borreliae in the culture used for feeding and it increased further over time. OspA is expressed by the bacteria primarily in the midguts of unfed ticks (4, 9, 26). It has been shown that OspA-positive borreliae in vitro adhere better to tick cells than do OspC-positive ones, indicating that OspA works as an adhesin (10). Pal et al. (22) recently found that OspA in B. burgdorferi sensu stricto mediates attachment to the tick gut by binding to an I. scapularis protein. The present results further underscore these findings and suggest that OspA of B. afzelii and B. garinii also acts as an adhesin in the European vector I. ricinus. Furthermore, culture-derived borreliae seem to be able to readapt to the natural situation in that they upregulate OspA when being reintroduced into the vector tick midgut.
Studies on OspC expression during tick engorgement revealed that dissemination of borreliae in the vector and infectivity for the mammalian host coincide with the up-regulation of this protein on the borrelial surface (7, 8, 18, 19, 26, 27). This has led to the hypothesis that, while OspA serves to retain the borreliae in the tick midgut between blood meals, up-regulation of OspC allows the bacteria to leave the midgut during feeding and to enter the salivary gland alveoli in order to infect a new host. In the present study, all of the borrelial strains that disseminated to the salivary glands were OspC positive. Notably, the proportion of OspC-positive borreliae was usually greater when the borreliae reached the salivary glands than that in the culture fed to the ticks. This was especially pronounced in strain PSoR, where nearly all of the borreliae were OspC positive but OspA negative when first detectable in the salivary glands. In contrast, OspC-negative cPKo345 was not detectable in the salivary glands although infection of the midgut could be demonstrated. As described recently (10), this clone has an insertion of a guanine nucleotide at position 200 of the ospC gene, leading to a stop codon after position 222, and is therefore unable to produce OspC. The fact that the OspC-negative clone was not able to disseminate further argues for a prominent role of OspC in borrelial dissemination in the tick vector. However, in most organs, borreliae expressing neither OspA nor OspC were also present. In a former study with I. ricinus ticks removed from humans, we detected spirochetes exhibiting all of the possible expression patterns. Either OspC or OspA alone, both of them, or neither of them could be found in ticks at different stages of engorgement as determined by tick weight (8). Notably, borreliae with only OspA on their surface were detectable in the salivary glands of an almost fully engorged nymph whose bite resulted in multiple erythema migrans. Recently, Ohnishi et al. (21) reported similar observations with I. scapularis nymphs infected with a clonal B. burgdorferi sensu stricto strain. They described a gradually developing heterogeneous borrelial population consisting of all possible Osp phenotypes in the midgut and salivary glands during a blood meal. Interestingly, the main phenotype found in the salivary glands was the OspA- and OspC-negative one. Taken together, these and the present results suggest that factors other than OspC must also be involved in the dissemination process and that these factors might differ, at least in part, between different strains. Coleman et al. (6) have shown that plasminogen binding to the borrelial cell—which was not determined in the present study—is an essential factor for dissemination in the tick. Possibly, differences in the abilities of different strains to bind plasminogen may also account for differences in dissemination.
A crucial question regarding risk of infection is the time gap between the beginning of the blood meal and transmission of the borreliae to the host. The results presented here indicate that speed of dissemination in the vector I. ricinus and regulation of Osp expression vary among different borrelia strains. Animal experiments with the American vector I. scapularis and B. burgdorferi sensu stricto suggest that at least 36 h is necessary for successful transmission (21, 23). In contrast, a study by Kahl et al. (13) with B. burgdorferi sensu lato-infected I. ricinus revealed that infection of Mongolian gerbils occurred as early as 16.7 h after the start of a tick blood meal. Further evidence that early transmission may occur in Europe is given by the high salivary gland infection rates found in unfed I. ricinus: 36% in adult ticks and up to 22% in nymphal ticks collected in different parts of Switzerland (14, 15). Borreliae already present in the salivary glands might be transmitted much earlier to a host than those residing in the midgut. In the present study, we found evidence that velocity of dissemination in the vector depends on the strain and might even vary among strains with the same OspA type. Notably, all of the strains that disseminated into the salivary glands were still detectable there at the end of the investigation period. However, the question of how long the borreliae can persist in the salivary glands remains open.
It is important to note that tick infection in the present study was achieved artificially by capillary feeding, which certainly finds the tick in a condition physiologically different from that which occurs when it is feeding on a vertebrate host. This model is certainly limited and must be regarded as an approach to the natural conditions; e.g., the borreliae were grown in vitro, they had a different antigen composition compared to that in the natural situation, and the ticks were probably infected with a number of pathogens higher than that encountered during blood meals on reservoir hosts. However, even if this system does not exactly mirror the natural situation, it offers several advantages. It allows testing of strains defined with respect to their actual protein expression, testing of mutants for the ability to survive and disseminate within the vector, and the use of mixed infections with different strains to test competitive or supporting behavior in the tick. Thus, this model offers the opportunity to gain significant insight into the adaptation process of borreliae required for survival and dissemination in the tick and for transmission to the vertebrate host. Our findings suggest that Osp regulation and velocity of dissemination in the vector I. ricinus may differ even among borrelial strains.
Further isolates comprising all of the human-pathogenic species and OspA serotypes should be investigated for OspA and OspC regulation, as well as for the ability to cause a permanent systemic infection in I. ricinus. This is important in view of the development of a human vaccine for Europe in a complex situation with marked variability on both the species and strain levels.
Acknowledgments
Sandra Rauser and Volker Fingerle contributed equally to this work.
We thank C. Hizo-Teufel and G. Lehnert for excellent technical work and Jürgen Heesemann for generous support.
This work was supported by Deutsche Forschungs-Gesellschaft grant Wi 894/4-1 and SmithKline Beecham, Rixensart, Belgium.
REFERENCES
- 1.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. D. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 2.Benach, J. L., J. L. Coleman, R. A. Skinner, and E. M. Bosler. 1987. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J. Infect. Dis. 155:1300-1306. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwald, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 4.Burkot, T. R., L. Patrican, and J. Piesman. 1994. Field trial of an outer surface protein A (OspA) antigen-capture enzyme-linked immunosorbent assay (ELISA) to detect Borrelia burgdorferi in Ixodes scapularis. Am. J. Trop. Med. Hyg. 50:354-358. [DOI] [PubMed] [Google Scholar]
- 5.Busch, U., G. Will, C. Hizo-Teufel, B. Wilske, and V. Preac-Mursic. 1997. Long-term in vitro cultivation of Borrelia burgdorferi sensu lato strains: influence on plasmid patterns, genome stability and expression of proteins. Res. Microbiol. 148:109-118. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 7.de Silva, A. M., and E. Fikrig. 1995. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53:397-404. [DOI] [PubMed] [Google Scholar]
- 8.Fingerle, V., G. Liegl, U. Munderloh, and B. Wilske. 1998. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus ticks removed from humans. Med. Microbiol. Immunol. 187:121-126. [DOI] [PubMed] [Google Scholar]
- 9.Fingerle, V., U. Hauser, G. Liegl, B. Petko, V. Preac-Mursic, and B. Wilske. 1995. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J. Clin. Microbiol. 33:1867-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fingerle, V., H. Laux, U. G. Munderloh, U. Schulte-Spechtel, and B. Wilske. 2000. Differential expression of outer surface proteins A and C by individual Borrelia burgdorferi in different genospecies. Med. Microbiol. Immunol. 189:59-66. [DOI] [PubMed] [Google Scholar]
- 11.Gern, L., Z. Zhu, and A. Aeschlimann. 1990. Development of Borrelia burgdorferi in Ixodes ricinus females during blood feeding. Ann. Parasitol. Hum. Comp. 65:89-93.2264691 [Google Scholar]
- 12.Hu, C. M., M. M. Simon, M. D. Kramer, and L. Gern. 1996. Tick-factors and in vitro cultivation influence the protein profile, antigenicity and pathogenicity of a cloned Borrelia garinii isolate from Ixodes ricinus hemolymph. Infection 24:251-257. [DOI] [PubMed] [Google Scholar]
- 13.Kahl, O., C. Janetzki-Mittmann, J. S. Gray, R. Jonas, J. Stein, and R. de Boer. 1998. Risk of infection with Borrelia burgdorferi sensu lato for a host in relation to the duration of nymphal Ixodes ricinus feeding and the method of tick removal. Zentbl. Bakteriol. Hyg. A 287:41-52. [DOI] [PubMed] [Google Scholar]
- 14.Lebet, N., and L. Gern. 1994. Histological examination of Borrelia burgdorferi infections in unfed Ixodes ricinus nymphs. Exp. Appl. Acarol. 18:177-183. [DOI] [PubMed] [Google Scholar]
- 15.Leuba-Garcia, S., M. D. Kramer, R. Wallich, and L. Gern. 1994. Characterization of Borrelia burgdorferi isolated from different organs of Ixodes ricinus ticks collected in nature. Zentbl. Bakteriol. 280:468-475. [DOI] [PubMed] [Google Scholar]
- 16.Leuba-Garcia, S., R. Martinez, and L. Gern. 1998. Expression of outer surface proteins A and C of Borrelia afzelii in Ixodes ricinus ticks and in the skin of mice. Zentbl. Bakteriol. 287:475-484. [DOI] [PubMed] [Google Scholar]
- 17.Marconi, R. T., S. Hohenberger, S. Jauris-Heipke, U. Schulte-Spechtel, C. P. LaVoie, D. Rössler, and B. Wilske. 1999. Genetic analysis of Borrelia garinii OspA serotype 4 strains associated with neuroborreliosis: evidence for extensive genetic homogeneity. J. Clin. Microbiol. 37:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuzawa, T., T. Kurita, H. Kawabata, and Y. Yanagihara. 1994. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol. Lett. 123:319-324. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery, R. R., S. E. Malawista, K. J. M. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2001. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piesman, J. 1993. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J. Infect. Dis. 167:1082-1085. [DOI] [PubMed] [Google Scholar]
- 24.Preac-Mursic, V., B. Wilske, and S. Reinhardt. 1991. Culture of Borrelia burgdorferi on six solid media. Eur. J. Microbiol. Infect. Dis. 10:1076-1079. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro, J. M. C., T. N. Mather, J. Piesman, and A. Spielman. 1987. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 24:201-205. [DOI] [PubMed] [Google Scholar]
- 26.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilske, B., V. Preac-Mursic, G. Schierz, and K. von Busch. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentbl. Bakteriol. Hyg. A 263:92-102. [DOI] [PubMed] [Google Scholar]
- 30.Wilske, B., V. Preac-Mursic, G. Schierz, R. Kühbeck, A. G. Barbour, and M. Kramer. 1988. Antigenic variability of Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 539:126-143. [DOI] [PubMed] [Google Scholar]
- 31.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilske, B., V. Preac-Mursic, U. B. Göbel, S. Graf, S. Jauris, E. Soutschek, E. Schwab, and G. Zumstein. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J. Clin. Microbiol. 31:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilske, B., S. Jauris-Heipke, R. Lobentanzer, I. Pradel, V. Preac-Mursic, D. Rössler, E. Soutschek, and R. C. Johnson. 1995. Phenotypic analysis of the outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J. Clin. Microbiol. 33:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilske, B., U. Busch, H. Eiffert, V. Fingerle, H. W. Pfister, D. Rössler, and V. Preac-Mursic. 1996. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med. Microbiol. Immunol. 184:195-201. [DOI] [PubMed] [Google Scholar]
- 35.Zumstein, G., R. Fuchs, A. Hofmann, V. Preac-Mursic, E. Soutschek, and B. Wilske. 1992. Genetic polymorphism of the gene encoding the outer surface protein A (OspA) of Borrelia burgdorferi. Med. Microbiol. Immunol. 181:57-70. [DOI] [PubMed] [Google Scholar]
- 36.Zung, J. L., S. Lewengrub, M. A. Rudzinska, A. Spielman, S. R. Telford, and J. Piesman. 1989. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can. J. Zool. 67:1737-1748 [Google Scholar]