Abstract
Over a 6-year period, Burkholderia cepacia complex species were isolated from cystic fibrosis (CF) patients receiving care at The University of North Carolina Hospitals (clinic CF patients) and from those referred from other treatment centers. Fifty-six isolates collected from 30 referred patients and 26 clinic CF patients were characterized by pulsed-field gel electrophoresis (PFGE) and were assayed by PCR to detect the cable pilin gene, cblA. PFGE results indicated that six separate clusters (clusters A to F) were present among the 56 isolates and that three clusters (clusters A, B, and E) consisted only of isolates from referred patients infected with B. cepacia complex isolates prior to referral. However, one cluster (cluster C) consisted of isolates from four CF patients, and hospital records indicate that this cluster began with an isolate that came from a referred patient and that spread to three clinic CF patients. Cluster D consisted of two isolates from clinic CF patients, and hospitalization records are consistent with nosocomial, patient-to-patient spread. cblA was present in only 4 of the 56 isolates and included isolates in cluster E from the referred patients. Our results indicate a lack of spread of a previously characterized, transmissible clone from referred patients to our clinic CF population. Only two instances of nosocomial, patient-to-patient spread could be documented over the 6-year period. An additional spread of an isolate (cluster F) from a referred patient to a clinic patient could not be documented as nosocomial and may have been the result of spread in a nonhospitalized setting. The majority (36 of 56) of our B. cepacia complex-infected CF patients harbor isolates with unique genotypes, indicating that a diversity of sources account for infection. These data suggest that CF patients infected with B. cepacia complex and referred for lung transplantation evaluation were not a major source of B. cepacia complex strains that infected our resident CF clinic population.
The Burkholderia cepacia complex is composed of at least seven closely related species or genomovars consisting of B. cepacia (genomovar I), B. multivorans (genomovar II), B. stabilis (genomovar IV), B. vietnamiensis (genomovar V), and B. ambifaria (genomovar VII), and genomovars III and VI, with species designations for genomovars III and VI still pending (4, 8).
Nearly 4% of cystic fibrosis (CF) patients in the United States are infected with a member of the B. cepacia complex (13). Of these patients, some will develop the cepacia syndrome, a rapidly fatal necrotizing pneumonia with bacteremia (9, 10, 26). Previous studies have shown that transmissible clones of B. cepacia exist, and subsequent infection with these clones of both healthy uninfected CF patients and CF patients seeking lung transplantation can be devastating (9, 11, 12, 22, 25). Therefore, screening of CF patients for the detection of B. cepacia complex and management of patients once infection is documented are extremely important. Furthermore, CF patients infected with B. cepacia complex isolates are stigmatized, being segregated from the general CF patient population in terms of clinical care and social interaction. In many North American transplant centers, infection with B. cepacia complex is a strict contraindication for lung transplantation in CF patients (14).
There are more than 120 lung transplantation centers in North America. The University of North Carolina (UNC) Hospitals is one of the few CF centers in North America that will perform lung transplantation for CF patients infected with B. cepacia complex. For this reason, about 14% of adult CF patients (i.e., roughly three times the national percentage) referred to the UNC Hospitals for double lung transplantation are infected with B. cepacia complex.
Some transmissible clones of B. cepacia complex exist, such as the cable pilin-positive (cblA+) electropherotype (ET) ET 12 clone responsible for epidemic transmission in both Canada and the United Kingdom (7, 9, 11, 21, 22, 25). Therefore, we believed that it was important to study the CF patient population at the UNC Hospitals by asking three questions. First, have referred patients brought a previously characterized, transmissible clone into our (the UNC Hospitals) center, and has it been transmitted to our clinic CF patient population? Second, are any new, previously uncharacterized, transmissible clones evident among our referred patients, and has transmission from referred patients to our local clinic CF population occurred? Third, how much intracenter spread of B. cepacia complex has occurred?
MATERIALS AND METHODS
Characterization of UNC Hospitals CF center and infection control.
All B. cepacia complex isolates were recovered from CF patients between 1 January 1995 and 1 March 2001. We studied two distinct patient populations from which B. cepacia complex organisms were isolated. One consisted of 30 CF patients who were referred to the UNC Hospitals, often for lung transplantation evaluation, already infected with B. cepacia complex. The second population was of 26 CF patients who received their routine care at the UNC Hospitals and who became newly infected during a study period from 1 January 1997 to March 2001. For these patients to be considered “newly infected,” prior cultures of samples from these patients at our institution had to be negative for B. cepacia and the organism had to be isolated during the study period.
For the year 2000, 451 CF patients received care at the UNC Hospitals, with 251 of these patients being pediatric patients, while 200 were adult patients. Nineteen (9.5%) of the 200 adult patients and 8 (3.2%) of the 251 pediatric patients were infected with B. cepacia complex. A total of 107 CF patients are awaiting lung transplantation at the UNC Hospitals, with 15 (14%) of these patients infected with B. cepacia complex. As part of the evaluation of possible nosocomial transmission among CF patients whose isolates were in clusters C, D, and F, all patient charts were reviewed by a nurse trained in infection control. The review included production of a time line of all hospital admissions including hospital location, clinic visits, physical and occupational therapy visits, pulmonary rehabilitation visits, and evaluations in specialty clinics (e.g., pulmonary function laboratory, radiology, and transplantation clinics). Patients with known B. cepacia complex colonization requiring inpatient hospitalization were admitted to private rooms and placed on contact precautions. When pediatric patients with B. cepacia complex were seen in the outpatient clinic, they either were scheduled to be seen on a different day than other CF patients or were scheduled to be seen at the end of the day, after all patients not infected or colonized with B. cepacia had left the facility. Until 1998, adult CF patients were seen in the general pulmonary clinics in order to minimize contact with each other. In 1998, the formation of a dedicated adult CF clinic prompted the institution of several infection control measures, including patient education, masking of all patients upon arrival at the clinic and in all common areas, strict adherence to hand-washing procedures, and disinfection of rooms between patients. The UNC Hospitals Lung Transplant Clinic adopted similar infection control guidelines in 2000.
B. cepacia isolation, PFGE, and cblA analysis.
All strains were isolated on either Pseudomonas cepacia agar or B. cepacia selective agar prepared in-house and were further characterized biochemically and by PCR genomovar analysis as described previously (6, 16, 19). Once a member of the B. cepacia complex was isolated from a CF patient, a subculture was prepared from a single colony and stocks were prepared in skim milk and frozen at −70°C.
Pulsed-field gel electrophoresis (PFGE) was conducted as described previously, with the following revisions (7). All cultures were grown overnight in tryptic soy broth and adjusted to an optical density at 600 nm of 1. One milliliter was removed and centrifuged for 5 min to pellet the cells. The cells were then resuspended in formalin (3.8% [vol/vol]) (5) and allowed to sit on ice for 1 h. The cells were pelleted and rinsed three times in TE (10 mM Tris-HCl, 10 mM EDTA [pH 8.0]) before being placed in agarose blocks. Genomic DNA was restricted with SpeI (New England Biolabs); and electrophoresis was conducted for 24 h in 0.5× TBE (Tris-borate, EDTA), with initial and final pulse times of 1.2 and 54 s, respectively, by using a CHEF Mapper system (Bio-Rad). An ET 12 strain of B. cepacia complex was kindly provided by R. Goldstein, Boston University School of Medicine (25). Gels were analyzed visually according to the criteria of Tenover et al. (27).
All isolates of B. cepacia complex were tested by PCR for the presence of the cable pilin gene, cblA, as described previously (22).
RESULTS
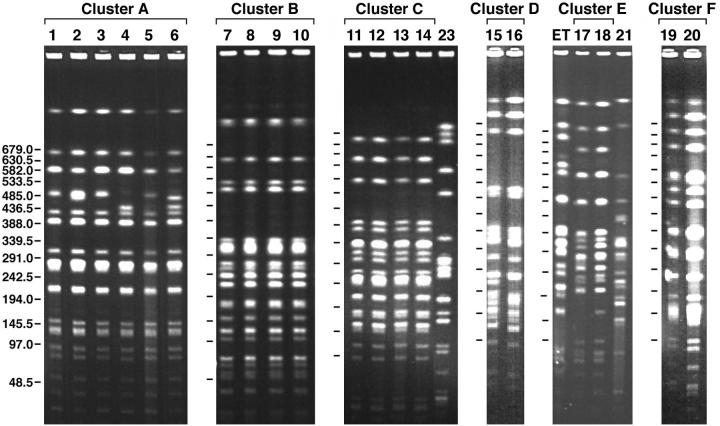
Fifty-six B. cepacia complex isolates cultured from 56 CF patients were characterized for their PFGE patterns (genotypes) after digestion with SpeI (Table 1). Genotypes were considered related if they differed by three or fewer bands (27). In total, 42 distinct genotypes were present, which likely indicates a wide diversity in the sources of infection. There were 26 genomovar II (B. multivorans) isolates, 25 genomovar III isolates, two genomovar I (B. cepacia) isolates, and three genomovar V (B. vietnamiensis) isolates. Our PFGE analysis revealed that six clusters of PFGE patterns were present within the CF population at the UNC Hospitals during this study period (Fig. 1). Three clusters consisted of strains from multiple patients (clusters A to C), while clusters D to F consisted of isolates from only two patients each. The cluster A genotype occurred in six patients, five of whom had previously been seen at CF centers in Michigan and who were infected prior to care at the UNC Hospitals. The fifth patient whose isolate was in this cluster was also infected with B. cepacia prior to referral, but the patient was originally from Nebraska and it is not known whether there is a link between this patient and the other patients whose isolates were in this cluster. Isolates from patients 1 through 3 and 5 shared identical genotypes, while isolates from patient 4 differed from those from patients 1 through 3 and 5 by two bands and isolates from patient 6 differed from those from patients 1 through 3 and 5 by three bands. All were genomovar III isolates.
TABLE 1.
Summary of genotypes and clusters of B. cepacia complex isolates recovered from January 1995 to March 2001
| Genotype | Patient no. | Genomovar | Cluster | Area | cblAa |
|---|---|---|---|---|---|
| 1 | 1 | III | A | Michigan | Neg |
| 1 | 2 | III | A | Michigan | Neg |
| 1 | 3 | III | A | Michigan | Neg |
| 1a | 4 | III | A | Michigan | Neg |
| 1 | 5 | III | A | Nebraska | Neg |
| 1b | 6 | III | A | Michigan | Neg |
| 2 | 7 | III | B | New York | Neg |
| 2 | 8 | III | B | New York | Neg |
| 2 | 9 | III | B | New York | Neg |
| 2 | 10 | III | B | New Jersey | Neg |
| 3 | 11 | B. multivorans (II) | C | Florida | Neg |
| 3 | 12 | B. multivorans (II) | C | UNC Hospitals | Neg |
| 3 | 13 | B. multivorans (II) | C | UNC Hospitals | Neg |
| 3 | 14 | B. multivorans (II) | C | UNC Hospitals | Neg |
| 4 | 15 | III | D | UNC Hospitals | Neg |
| 4 | 16 | III | D | UNC Hospitals | Neg |
| 5 | 17 | III | E | Nova Scotia | Pos |
| 5 | 18 | III | E | Nova Scotia | Pos |
| 6 | 19 | III | F | UNC Hospitals | Neg |
| 6 | 20 | III | F | New Jersey | Neg |
| 7 | 21 | III | Distinct genotype | Pos | |
| 8 | 22 | B. cepacia (I) | Distinct genotype | Pos | |
| 9-30 | 23-44 | B. multivorans (II) | Distinct genotypes | Neg | |
| 31-38 | 45-52 | III | Distinct genotypes | Neg | |
| 39-41 | 53-55 | B. vietnamiensis (V) | Distinct genotypes | Neg | |
| 42 | 56 | B. cepacia (I) | Distinct genotype | Neg |
Neg, negative; Pos, positive.
FIG. 1.
PFGE banding patterns of six clusters (clusters A to F) of B. cepacia complex isolates recovered from CF patients at the UNC Hospitals CF center. The cluster C gel contains a B. multivorans strain designated 23 for purposes of comparison. The cluster E gel contains the Toronto strain, designated ET, and a cblA+ genomovar III strain, designated 21, for the purposes of comparison. Numbers on the left are molecular weights.
Likewise, cluster B isolates (Fig. 1) occurred in those referred patients seen previously at CF centers within the metropolitan New York City-New Jersey area and infected prior to care at the UNC Hospitals. Again, all were genomovar III isolates, and there was no evidence of the spread of this cluster to any of our clinic CF patients.
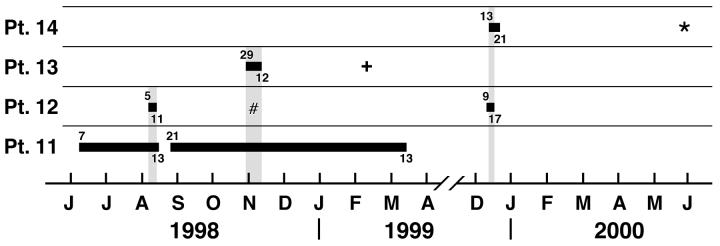
Interestingly, cluster C (Fig. 1) began with patient 11 from Florida, who sought care at the UNC Hospitals for a possible double lung transplantation and who was already infected with B. multivorans. This patient had a lengthy hospitalization from 7 June 1998 until 13 August 1998 and again from 21 August 1998 until 13 March 1999 (Fig. 2). Patient 12 was also hospitalized from 5 through 11 August 1998, during the hospitalization of patient 11, and subsequently became culture positive for B. multivorans on 5 November 1998. On the same date, patient 12 was also seen in the adult CF clinic. Patient 13 was a pediatric patient hospitalized from 29 October 1998 until 12 November 1998 and was later (11 February 1999) culture positive for B. multivorans. Patient 14 was hospitalized from 13 through 21 December 1999 and had an overlapping hospitalization with patient 12. Patient 14 was then culture positive for B. multivorans on 25 May 2000. Although these patients did not share the same hospital floor, they did share common hospital services (physical therapy, pulmonary function laboratory, and radiology). Since the B. multivorans isolates from all of these patients share the same genotype and overlapping hospitalizations were evident, we conclude that nosocomial, patient-to-patient spread occurred among these patients either directly or indirectly.
FIG. 2.
Time line graph showing the hospitalization course of patients (Pt.) 11 to 14. Horizontal bars represent inpatient status, with the admission date given at the beginning of the bars and the discharge date given at the ends. Vertical bars represent dates of concurrent admission resulting in possible cross infection. #, +, and ∗, the dates that patients 12, 13, and 14, respectively, first became positive for B. multivorans infection (see text).
Three small clusters (clusters D to F) (Fig. 1) consisting of isolates from two patients each were also detected by PFGE, and isolates in one of these clusters (cluster E) were from siblings who were infected with B. cepacia and who had been referred to the UNC Hospitals (Fig. 1). As with cluster C isolates, the isolates in cluster D were from two patients who also had overlapping hospitalizations on the same hospital unit that were clearly documented (data not shown). These two patients represent part of the UNC Hospitals CF clinic population. The isolates in cluster F consisted of isolates from patient 19, who became infected while receiving care at the UNC Hospitals, and patient 20, who was from New Jersey and who came to the UNC Hospitals already infected with B. cepacia genomovar III. We could find no evidence of overlapping hospitalizations or shared hospital services between these two patients. Therefore, we suspect that patient 19 may have become infected with an isolate of the same genotype as that from patient 20 in a nonhospitalized setting (23).
Epidemic clone ET 12, isolated from CF centers in both the United Kingdom and North America, expresses cable pilin on its surface (25). For this reason, we performed PCR on our isolates to see if the cblA gene was present, especially in our cluster isolates. Our results show that the cblA gene was present in only four isolates, two of which were from cluster E (isolates from a sibling pair). A comparison of the SpeI restriction patterns between the B. cepacia cblA+ isolates from the siblings (patients 17 and 18) and patient 21 with that of the highly transmissible Toronto strain is shown in Fig. 1. The results indicate that while the SpeI patterns for the isolates from the siblings are an exact match, the genotypes produced by isolates from the siblings, patient 21, and the ET 12 clone differ from each other by more than seven fragments and are therefore unrelated (25). A fourth patient infected with a cblA+ strain also showed a unique SpeI genotype, as expected, since this isolate was a genomovar I strain (data not shown), while the other cblA+ isolates were genomovar III strains (Table 1).
DISCUSSION
In an earlier study, we analyzed B. cepacia complex isolates from five transplant patients and 17 clinic patients and found no evidence of transmission of B. cepacia complex isolates among these patients (24). However, we did find a strain of B. cepacia complex in a patient referred for transplantation evaluation that resulted in the recognition of a large nosocomial outbreak at the institution from which he was referred (9). This caused us to be concerned that patients referred for transplantation might transmit B. cepacia complex strains to patients receiving their routine care for CF at our institution.
Of the six clones found in clusters in this study, isolates of four previously uncharacterized clones (clusters B, C, E, and F) were present in our referred patients, while cluster A has been reported previously (12) and cluster D isolates were not present in patients referred for transplantation. Cluster B isolates were present in four referred patients from the metropolitan New York City-New Jersey area. To our knowledge, there is no published study of an epidemic clone of the B. cepacia complex from CF patients from that geographic area. Therefore, we cannot comment on the transmissibility of this clone except to say that it was present in these four referred CF patients and did not spread to our clinic CF patients.
Cluster C comprised a clone of B. multivorans. Among the isolates in the B. cepacia complex, transmissibility is typically thought to be associated with B. cepacia genomovar III organisms (2, 7, 9, 11, 21, 22, 25). Person-to-person spread of B. multivorans is not as frequently described, and most patients infected with B. multivorans are infected with unique strains (1, 14). Overlapping hospitalizations could be traced for all four patients infected with cluster C isolates. Cluster E consisted of only two isolates from referred patients who were siblings, but the isolates were cblA+. Again, there was no evidence of spread from these referred patients to our clinic CF patient population. Cluster F consisted of isolates from two patients and represented the spread of B. cepacia genomovar III from a referred patient to a clinic CF patient. However, we were unable to show any link between these two patients through overlapping hospitalizations, clinic visits, or hospital services and therefore consider this cluster not to have occurred nosocomially.
A closer analysis of the isolates in cluster A indicated that four of five referred patients from Michigan were infected with isolates with identical genotypes (cluster A) when they arrived at the UNC Hospitals and that the isolate from the fifth patient (Fig. 1, patient 4) possessed a genotype that differed from that of the isolates from the first four patients by only two bands. Digestion with XbaI and PFGE of the isolate from patient 4 allowed us to confirm by direct comparison with the PFGE results of Kumar et al. (12) that these isolates were part of the cluster of genetically related isolates from five CF centers in Michigan (data not shown). We were initially alarmed at finding this cluster among our referred patients. However, we found no evidence of the spread of isolates in this cluster to any of the other CF patients screened in this study. Cluster A also included an isolate from a single referred patient from Nebraska. We have not been able to verify whether this patient attended some of the same CF camps or CF centers as the rest of the patients whose isolates were in cluster A.
One of the shortcomings of our study design was that we studied only a single isolate from each patient. We had two instances in which isolates from sibling pairs had different genotypes. Patient 14 is a sibling of patient 23, and both patients were infected with the same genomovar (B. multivorans) but the genotypes of the isolates were not related. In another instance, we noted that isolates from siblings (patient 10, whose isolate was in cluster B, and patient 20, whose isolate was in cluster F) differed in their genotypic patterns. Since we studied only one isolate from each patient, it is possible that the siblings may have been infected with isolates with common genotypes that were not detected. We previously showed that over time patients harbor B. cepacia complex isolates with the same genotype (24).
Overall, the results of our genotype analysis indicate that the spread of a previously characterized, transmissible clone of B. cepacia from referred patients to our clinic CF patient population has not occurred. Other studies have indicated that a high percentage of patients at CF centers often harbor endemic, transmissible clones (2, 12, 15, 17, 23, 25, 29). In contrast, our PFGE results did not indicate the presence of a common, transmissible clone among our clinic CF patient population. Our results more closely parallel those of a recent study (20) indicating that hospitals with a segregation policy tend to have patients infected with unique strains. Documented nosocomial spread involved 4 of 26 (15%) of our clinic B. cepacia complex-infected patients (patients 12 to 14, whose isolates were in cluster C, and patient 16, whose isolate was in cluster D).
A recent publication indicated that in the United Kingdom the cblA gene may be used as a marker to identify strains with an enhanced capacity for spread (3). We found the cblA gene in only 4 of the 56 B. cepacia isolates that we examined. Two of the four isolates were from referred siblings from Canada and were in cluster E. However, the genotype for cluster E isolates differed from that of the epidemic, cblA+ ET 12 strain, also isolated from Canadian patients, by more than seven fragments, and they are therefore considered genetically unrelated. There was no evidence of spread of cblA+ clones in our patient population. Our data are consistent with those of LiPuma and colleagues (18), who found that only 1 of 606 isolates carried the cblA gene. These data suggest that other factors are important in the transmissibility of the genomovar III organisms.
The most frequently recovered B. cepacia complex species from CF patients are genomovar III (18), and recent data indicate that CF transplant patients infected with genomovar III suffer higher rates of mortality than those infected with another genomovar (1). However, it is apparent from our study and those of others that B. multivorans can also be frequently recovered (8, 18, 28). In fact, our results indicate that B. multivorans was slightly more prevalent than genomovar III among our CF patient population (26 and 25 patients, respectively). Our results also revealed that 36 of the 56 CF patients seeking care at the UNC Hospitals harbor strains with unique genotypes, so their sources of infection are likely to be diverse.
In conclusion, our study of B. cepacia complex-infected CF patients indicated that transmission of an isolate from a referred patient to our clinic CF patient population occurred in only two instances (with isolates in clusters C and F), but nosocomial transmission could clearly be documented for only one of these isolates (a cluster C isolate). Intracenter transmission of isolates in one, two-patient cluster (cluster D) occurred within our clinic patient population. Our clinic patients were infected with a variety of different genotypes of the B. cepacia complex. These data suggest that CF patients who were infected with B. cepacia complex isolates and who were referred for lung transplantation evaluation were not a major source of the B. cepacia complex organisms that infected our resident CF clinic population.
Acknowledgments
This work was supported in part by a grant from the Cystic Fibrosis Foundation (to J.J.L.).
REFERENCES
- 1.Aris, R. M., J. Routh, J. LiPuma, D. G. Heath, and P. H. Gilligan. 2001. Increased mortality due to Burkholderia cepacia complex infection in cystic fibrosis patients after lung transplantation. Am. J. Respir. Crit. Care Med. 164:2102-2106. [DOI] [PubMed] [Google Scholar]
- 2.Chen, J. S., K. A. Witzmann, T. Spilker, R. J. Fink, and J. J. LiPuma. 2001. Endemicity and inter-city spread of Burkholdria cepacia genomovar III in cystic fibrosis patients. J. Pediatr. 139:643-649. [DOI] [PubMed] [Google Scholar]
- 3.Clode, F. E., M. E. Kaufmann, H. Malnick, and T. L. Pitt. 2000. Distribution of genes encoding putative transmissibility factors among epidemic and nonepidemic strains of Burkholderia cepacia from cystic fibrosis patients in the United Kingdom. J. Clin. Microbiol. 38:1763-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coenye, T., E. Mahenthiralingam, D. Henry, J. J. LiPuma, S. Laevens, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. Int. J. Syst. E vol. Microbiol. 51:1481-1490. [DOI] [PubMed] [Google Scholar]
- 5.Gibson, J. R., K. Sutherland, and R. J. Owen. 1994. Inhibition of DNase activity in PFGE analysis of DNA from Campylobacter jejuni. Lett. Appl. Microbiol. 19:357-358. [DOI] [PubMed] [Google Scholar]
- 6.Gilligan, P. H., and S. Whittier. 1999. Burkholderia, Stentotrophomonas, Ralstonia, Brevundomonas, Comamonas, and Acidovorax, p. 526-538. In P. R. Murray, E. J. Baron, M. A. Pfaller, F.C. Tenover, and R.H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 7.Goldstein, R., L. Sun, R.-Z. Jiang, U. Sajjan, J. F. Forstner, and C. Campanelli. 1995. Structurally variant classes of pilus appendage fibers coexpressed from Burkholderia (Pseudomonas) cepacia. J. Bacteriol. 177:1039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry, D. A., E. Mahenthiralingham, P. Vandamme, T. Coenye, and D. Speert. 2001. Phenotypic methods for determining genomovar status of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:1073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes, A., R. Nolan, R. Taylor, R. Finley, M. Riley, R. Z. Jiang, S. Steinbach, and R. Goldstein. 1999. An epidemic of Burkholderia cepacia transmitted between patients with and without cystic fibrosis. J. Infect. Dis. 179:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, W. M., S. D. Tyler, and K. R. Rozee. 1994. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J. Clin. Microbiol. 31:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, A., S. Dietrich, W. Schneider, R. Jacobson, F. P. Downes, B. E. Robinson-Dunn, R. Honicky, J. Smith, and R. Martin. 1997. Genetic relatedness of Burkholderia (Pseudomonas) cepacia isolates from five cystic fibrosis centers in Michigan. Respir. Med. 91:485-492. [DOI] [PubMed] [Google Scholar]
- 13.LiPuma, J. J. 1998. Burkholderia cepacia: management issues and new insights. Clin. Chest Med. 19:473-486. [DOI] [PubMed] [Google Scholar]
- 14.LiPuma, J. J. 2001. Burkholderia cepacia: a contraindication to lung transplantation in CF? Transplant. Infect. Dis. 3:150-161. [DOI] [PubMed] [Google Scholar]
- 15.LiPuma, J. J., S. E. Dasen, D. W. Nielson, R. C. Stern, and T. L. Stull. 1990. Person-to-transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336:1094-1096. [DOI] [PubMed] [Google Scholar]
- 16.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 37:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LiPuma, J. J., J. E. Mortensen, S. E. Dasen, T. D. Edlind, D. V. Schidlow, J. Burns, and T. L. Stull. 1988. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J. Pediatr. 113:859-862. [DOI] [PubMed] [Google Scholar]
- 18.LiPuma, J. J., T. Spiker, L. Gill, P. W. Campbell, L. Liu, and E. Mahenthiralingham. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility factors in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 19.Mahenthiralingham, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for the identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul, M., M. Pegler, and R. Benn. 1998. Molecular epidemiology of Burkholderia cepacia in two Australian cystic fibrosis centres. J. Hosp. Infect. 38:19-26. [DOI] [PubMed] [Google Scholar]
- 21.Pitt, T. L., M. E. Kaufmann, P. S. Patel, L. C. Gaskin, and D. M. Livermore. 1996. Type characterisation and antibiotic susceptibility of Burkholderia (Pseudomonas) cepacia isolates from patients with cystic fibrosis in the United Kingdom and the Republic of Ireland. J. Med. Microbiol. 44:203-210. [DOI] [PubMed] [Google Scholar]
- 22.Sajjan, U. S., L. Sun, R. Goldstein, and J. F. Forstner. 1995. Cable (Cbl) type II pili of cystic fibrosis-associated Burkholderia (Pseudomonas) cepacia: nucleotide sequence of the cblA major subunit pilin gene and novel morphology of the assembled appendage fibers. J. Bacteriol. 177:1030-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, D. L., L. B. Gumery, E. G. Smith, D. E. Stableforth, M. E. Kaufmann, and T. L. Pitt. 1993. Epidemic of Pseudomonas cepacia in an adult cystic fibrosis unit: evidence of person-to-person transmission. J. Clin. Microbiol. 31:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach, S., L. Sun, R.-U. Jiang, P. Flume, P. Gilligan, T. M. Egan, and R. Goldstein. 1994. Transmissibility of Pseudomonas cepacia infection in clinic patients and lung transplant recipients with cystic fibrosis. N. Engl. J. Med. 331:981-987. [DOI] [PubMed] [Google Scholar]
- 25.Sun, L., R. Z. Jiang, S. Steinbach, A. Holmes, C. Campanelli, J. Forstner, U. Sajjan, Y. Tan, M. Riley, and R. Goldstein. 1995. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat. Med. 1:661-666. [DOI] [PubMed] [Google Scholar]
- 26.Tablan, O. C., T. L. Chorba, D. V. Scidlow, J. W. White, K. A. Hardy, P. H. Gilligan, W. M., Morgan, L. A. Carson, W. J. Martone, J. M. Jason, et al. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J. Pediatr. 107:382-387. [DOI] [PubMed] [Google Scholar]
- 27.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. A. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, T. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. W. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi, Y., J. Fujita, K. Takigawa, K. Negayama, T. Nakazawa, and J. Takahara. 1993. Clinical features of Pseudomonas cepacia pneumonia in an epidemic among immunocompromised patients. Chest 103:1706-1709. [DOI] [PubMed] [Google Scholar]