Abstract
To isolate Babesia equi genes encoding immunodominant proteins, a cDNA expression library prepared from B. equi mRNA was immunoscreened with B. equi-infected horse serum. Eighteen positive cDNA clones were obtained, and the clone that showed the strongest immunoreactivity, designated Be82, was further characterized. The Be82 gene consisted of 1,953 bp and contained a partial open reading frame lacking the 5′-terminal sequence. As shown by Western blot analyses, immune sera from mice intraperitoneally injected with the Be82 gene product recognized the 82- and 52-kDa proteins of B. equi but not those of Babesia caballi. The glutathione S-transferase fusion protein expressed in Escherichia coli that was purified and used as the antigen in the enzyme-linked immunosorbent assay reacted specifically with B. equi-infected horse sera. These results suggest that the Be82 gene product is a potential diagnostic antigen candidate in the detection of B. equi infection in horses that will be useful both in the performance of epidemiological studies and in the granting of quarantine passes.
Equine piroplasmosis is an economically important tick-borne protozoan disease of horses reported worldwide. This disease is caused by Babesia equi and Babesia caballi (18). Babesia parasites destroy erythrocytes and induce fever, anemia, and icterus in infected horses (12). These parasites are usually detected in blood smears only during the acute stage of the infection, and animals that recover from the disease remain parasite carriers. These carriers, as well as those previously exposed or infected, can be identified serologically (6).
Standard serological tests for babesiosis are the complement fixation test (CFT) and the indirect fluorescent antibody test (IFAT) (5), both of which require large amounts of parasite antigens, particularly in large-scale seroepidemiological surveys. Because of its low sensitivity and specificity, the CFT is unable to detect latent infection and fails to accurately discriminate between negative and carrier animals (24). With the IFAT, on the other hand, standardization is difficult, considering the subjectivity of the reader in assessing the results (3, 4). Weiland (25) has demonstrated the strong cross-reactivity of anti-B. caballi horse serum with the lysate of B. equi-infected erythrocytes using an enzyme-linked immunosorbent assay (ELISA). However, with Western blot analysis, Ikadai et al. (8) have noted an erratic or inconsistent cross-reactivity between the anti-B. equi serum and the lysate of B. caballi-infected erythrocytes. In view of the absence of equine piroplasmosis in Japan and the increasing number of horses imported to the country from places where the infection is endemic, the development of a highly specific and sensitive diagnostic system for B. equi is of urgent necessity.
In this study, we isolated and determined the nucleotide sequence of a cDNA clone from a cDNA expression library prepared from B. equi mRNA and we attempted to develop and assess the efficacy of the ELISA system using the gene product in the diagnosis of B. equi infection.
MATERIALS AND METHODS
Parasites.
U.S. Department of Agriculture strains of B. equi and B. caballi were grown in horse erythrocytes in vitro as described by Avarzed et al. (1, 2). Parasite development in vitro was monitored by microscopic observation of Giemsa stain-treated thin smears.
Construction and immunoscreening of a B. equi cDNA expression library.
Total RNA was extracted from the B. equi-infected horse erythrocytes with acid guanidinium thiocyanate-phenol-chloroform, and the polyadenylated mRNA was purified with Oligotex-dT 30 latex beads (Takara, Tokyo, Japan). Double-stranded cDNA with an EcoRI or XhoI linker at both terminal sites was synthesized from the mRNA by using a Zap cDNA synthesis kit (Stratagene, La Jolla, Calif.) and then ligated to the EcoRI and XhoI sites of a λZap II phage gene expression vector (Stratagene). A Gigapack III packaging system (Stratagene) was used for the ligation mixture.
The cDNA library (2.5 × 105 PFU) was immunoscreened with anti-B. equi horse serum according to the method of Ikadai et al. (10). In brief, the packed phage were grown on NZYM agar, and the phage plaques were transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) with isopropyl-β-d-thiogalactopyranoside. The membranes were incubated with anti-B. equi horse serum for 60 min at room temperature and subsequently with alkaline phosphate-conjugated goat anti-horse immunoglobulin G (Stratagene) for 60 min at room temperature and then visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Stratagene). Positive phage were plaque-purified to obtain a 100% clonal population. The insert cDNA of the phage was subcloned into a pBluescript SK (+) vector (Stratagene) by the in vivo excision capability of λZAP II (20).
DNA sequencing.
The cDNA clone was digested with the appropriate restriction enzymes, and the smaller inserts of deleted sequence were subcloned into the pBluescript SK (+) vector. The cDNA deletion clones were sequenced with an ABI PRISM 377 DNA sequencer (Perkin-Elmer, Foster City, Calif.) and a dye primer cycle sequencing ready-reaction kit (Perkin-Elmer). Nucleic acid and protein homology searches were performed with the MacVector program (Oxford Molecular, Ltd., Oxford, United Kingdom) on sequences in the National Center for Biotechnology Information (NCBI) database.
Escherichia coli expression of the Be82 gene.
After digestion of pBS/Be82 with EcoRI and XhoI, a 1,953-bp EcoRI and XhoI fragment containing the complete cDNA insert was subcloned into the EcoRI and XhoI sites of the pGEMEX-2 (Promega Corp., Madison, Wis.) and pGEX-4T (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) E. coli expression plasmid vectors, and the resulting plasmids were designated pGEMEX-2/Be82 and pGEX/Be82, respectively, and later used to transform the E. coli strain BL21 (Stratagene) according to standard techniques (17). After the transformation, the recombinant products were expressed as T7 gene 10 (gene 10-Be82) or glutathione S-transferase (GST; GST-Be82) fusion proteins in E. coli. The insoluble gene 10-Be82 protein was used in the immunization of mice to produce antibodies against the Be82 gene product, while the soluble GST-Be82 protein was purified and used as the antigen in the ELISA.
Production of antibodies against the Be82 gene product in mice.
Six-week-old ddY mice were immunized intraperitoneally with transformed E. coli containing pGEMEX-2/Be82 in complete Freund's adjuvant (Difco, Detroit, Mich.) on day 0. On days 14 and 28, the same mice were given booster shots of the same antigen in Freund's incomplete adjuvant (Difco). Immune mouse sera were collected 10 days after the last immunization.
IFAT.
SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Purification of the B. equi cells was performed according to methods described previously (14, 15). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blot analysis of purified antigens were performed as described previously (27)
ELISA.
The transformed E. coli cells containing pGEX/Be82 were washed three times with phosphate-bufferd saline, lysed in 1% Triton X-100-phosphate-buffered saline, sonicated, and then centrifuged at 18,000 × g for 10 min at 4°C. The purified supernatant containing the soluble GST-Be82 protein was purified with glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) as described previously (21). An ELISA using the purified GST-Be82 protein was performed as previously described (10, 26).
Serum samples.
For the ELISA, 10 sera from uninfected horses, 13 sera from horses experimentally infected with B. equi, and 3 sera from horses experimentally infected with B. caballi were used. Four sequential sera from horses experimentally infected with either B. equi (E3 and E4) or B. caballi (C3 and C4) were also used. All sera were obtained from the Equine Research Institute of the Japan Racing Association. They were kept at −80°C until use.
RESULTS
Cloning and DNA sequencing of the Be82 cDNA clone.
Eighteen positive cDNA clones were obtained, and the one cDNA clone which showed the strongest reactivity in the immunoscreening was analyzed further. With computer-aided analysis, it was determined that this cDNA had a total of 1,953 bp (accession number AB062788 in GenBank, EMBL, and DDBJ) (Fig. 1) and contained a truncated open reading frame lacking the starting codon sequence at the 5′ terminus, which we designated Be82. The sequence from position 1 to the terminal codon contained 1,907 bp, corresponding to 634 amino acid residues, and had a predicted molecular size of 69.7 kDa. The predicted protein had an isoelectric point (pI) of 4.91, which was suggestive of the acidic nature of the Be82 protein. Of the deduced amino acids at positions 1 to 634, 30% were charged. We did not find the signal sequence or any significant homology of the Be82 gene product with protein sequences registered in the NCBI database.
FIG. 1.
Complete nucleotide sequence, including the 3′ untranslated region, and the deduced amino acid sequence of the Be82 gene.
E. coli expression of the Be82 gene products.
Two gene products with molecular sizes of 145 kDa (gene 10-Be82) and 136 kDa (GST-Be82) were obtained by using the expression vectors pGEMEX-2 and pGEX-4T, respectively (Fig. 2). Anti-B. equi horse serum recognized these proteins in Western blots (data not shown). Since these products contained 35-kDa gene 10 and 26-kDa GST tags, the molecular size of the Be82 protein was estimated at approximately 110 kDa after cleavage of the fusion protein tags. The size of the Be82 gene product, however, was still larger than the size estimated by computer-aided analysis (69.7 kDa).
FIG. 2.
Expression of the Be82 gene products in E. coli cells stained with Coomassie blue. (A) Lysates of cells with the pGEMEX-2 (lane a) and pGEMEX-2/Be82 (lane b) expression vectors; (B) lysates of cells with the pGEX-4T (lane a) and pGEX-4T/Be82 (lane b) expression vectors. Molecular size markers (in kilodaltons) are shown to the left and right of the lanes.
Detection of the native B. equi protein with mouse immune serum against the Be82 gene product.
Mouse immune serum was prepared against the gene 10-Be82 protein to examine the specificity of Be82 gene products by IFAT. The immune serum bound to B. equi parasites but not to uninfected horse erythrocytes (Fig. 3). Mouse immune serum was also used to examine the molecular size of native B. equi Be82 protein by Western blot analysis. Mouse serum recognized both the 82- and 52-kDa proteins in the lysate of B. equi-infected erythrocytes but not in the lysates of B. caballi-infected and uninfected erythrocytes (Fig. 4). The cDNA clone encoding an 82-kDa antigen of B. equi was estimated by computer analysis to be approximately 2.3 kbp in length.
FIG. 3.

IFAT analysis of anti-B. equi antibody produced in mice immunized with Be82 expressed in E. coli. Mouse antiserum reacted with B. equi-infected horse erythrocytes (a) but not with uninfected horse erythrocytes (b).
FIG. 4.
Western blot analysis of the lysates of B. equi (lane a)- and B. caballi (lane b)-infected and uninfected (lane c) horse erythrocytes with the anti-Be82 gene product mouse serum. The 82- and 52-kDa proteins are seen only in the lysate of B. equi-infected erythrocytes.
Detection of anti-Be82 protein antibodies from B. equi-infected horse sera by ELISA.
To evaluate the efficacy of the Be82 gene product as a diagnostic antigen in the ELISA, the GST-Be82 protein or the GST protein was expressed in transformed E. coli cells and purified by using glutathione-Sepharose 4B beads (Fig. 5). All of the sera were confirmed to be nonreactive to the GST control antigen (data not shown). Specific antibodies were detected in 8 out of 10 B. equi-infected horse sera at optical densities at 415 nm (OD415s) higher than 0.4 (Fig. 6). Cross-reactivity of the GST-Be82 protein was observed with three anti-B. caballi horse sera with OD415s below 0.4. Our findings demonstrate that the GST-Be82 gene product used in the ELISA was able to specifically recognize the sera of B. equi-infected horses but not those of either B. caballi-infected or uninfected horses at OD415s of 0.4 and higher.
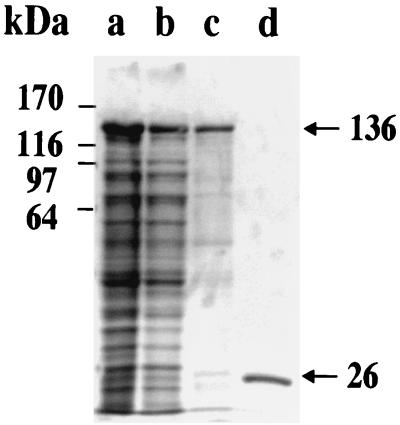
FIG. 5.
Purification of GST-Be82 with glutathione-Sepharose 4B beads. Shown are results with whole GST-Be82 (lane a), the supernatant of GST-Be82 (lane b), and purified GST-Be82 and GST proteins (lanes c and d, respectively).
FIG. 6.
ELISA results showing reactivity of the Be82 gene product to horse sera. Dots represent OD415s of B. equi-infected sera (column 1), B. caballi-infected sera (column 2), and uninfected sera (column 3).
We used sequential sera previously obtained from horses experimentally infected with B. equi and B. caballi that had shown specific antibodies to B. equi and B. caballi as determined by CFT (7, 26). The reactivities of the B. equi- and B. caballi-infected horse sera collected at intervals of several days postinfection with the Be82 antigen were examined (Fig. 7). Sera from the two B. equi-infected horses recognized the Be82 gene product as early as days 12 and 18 after infection. Sera from two B. caballi-infected horses failed to recognize this same antigen.
FIG. 7.
Antibody response to Be82 protein in an ELISA. Titers of serum samples from B. equi (E3 and E4)- and B. caballi (C3 and C4)-infected horses are shown.
DISCUSSION
In the present study, we isolated a cDNA clone of 1,953 bp by immunoscreening with B. equi-infected horse serum. This clone had a 1,907-bp truncated open reading frame lacking the 5′-terminal sequence. Mouse immune serum against expressed recombinant protein recognized an 82-kDa native antigen of B. equi. This clone was designated Be82, and the cDNA of the Be82 gene was estimated to be approximately 2.3 kbp in length. Therefore, the cloned Be82 gene was smaller than the predicted complete Be82 gene by about 300 bp from the 5′ end. These data suggest that the Be82 gene we obtained represented just a fragment of the entire length of the cDNA. Work to elucidate the complete Be82 gene by using another cDNA library is ongoing in our laboratory. We have not found any proteins registered with the NCBI database whose amino acid compositions show homology with the amino acid composition that we have deduced from the Be82 gene. Thus, the Be82 gene that we have partially elucidated and presented in this paper could be considered a novel gene of B. equi.
The gene 10-Be82 and GST-Be82 products were found to be about 40 kDa larger than their expected sizes. This discrepancy in molecular sizes may be attributed to the presence of highly charged amino acid residues and to the low pI of the predicted protein, which may have disrupted the binding of SDS to the protein. Similar findings have been reported for the pf322 gene of Plasmodium falciparum and the 200-kDa protein of Babesia bigemina (16, 23). Despite the discrepancy in molecular sizes, anti-B. equi horse serum recognized these gene products, suggesting that the Be82 gene encodes an immunogenic component of the B. equi parasite that induces humoral immunity. This suggestion was confirmed by the specific binding of anti-gene 10-Be82 mouse serum only to B. equi-infected erythrocytes and not to uninfected erythrocytes, as determined by IFAT. As shown by Western blot analysis, both the 82- and 52-kDa proteins of the lysate of B. equi-infected erythrocytes were detected by anti-Be82 mouse immune serum. Further analysis using Northern and Southern blotting is necessary to determine if the Be82 gene is located in two regions in the B. equi parasite genome and whether the 52-kDa protein is a precursor of the 82-kDa protein. Since similar proteins were not detected in the lysate of B. caballi-infected erythrocytes by Western blot analysis, the 82- and 52-kDa proteins can be considered specific antigens of the B. equi parasite. The Be82 gene was a partial-length cDNA, and it was deduced that Be82 could be lacking about 330 bp from the 5′ end.
ELISAs for babesiosis that use recombinant equi merozoite antigens 1 and 2 produced by E. coli (13) or the baculovirus-insect cell system (22, 26) have been developed. In the present study, an ELISA we developed that uses the GST-Be82 protein proved to be highly specific to B. equi antibodies when an OD415 of 0.4 was used as the cutoff titer. Three B. caballi-infected horse sera cross-reacted with the GST-Be82 protein at an OD415 of about 0.2. Although the number of serum samples was not enough to examine whether cross-reactivity of the GST-Be82 protein with B. caballi-infected sera was authentic, there is a possibility that B. caballi parasites may share a region bearing antigenicity similar to that of the large-molecular-size recombinant Be82 product, because the gene 10-Be82 and GST-Be82 products were found to be about 40 kDa larger than their expected sizes and the nonspecific cross-reactivity may possibly reside in this shared region. Therefore, more anti-B. caballi and anti-B. equi horse sera may be necessary to establish the authenticity of the cross-reaction as well as to determine the appropriate cutoff titers for the practical diagnosis of B. equi and B. caballi in the ELISA (10, 11, 19).
In conclusion, we have provided convincing data demonstrating the specificity of the GST-Be82 gene product in the detection of B. equi infection. While this highly specific recombinant protein shows promise for its utilization in the diagnosis of B. equi infection by ELISA, additional work needs to be carried out to elucidate the complete Be82 gene sequence, to further clarify cross-reactivity with B. caballi, and to determine precisely the cutoff titers for B. equi and B. caballi infections by using more horse sera.
Acknowledgments
We thank T. Kanemaru of the Equine Research Institute of the Japan Racing Association for providing horse sera and F. Claveria for critical reading of the manuscript.
This study was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science and a Gakujutsu-Frontier Cooperative Research grant from Rakuno-gakuen University.
REFERENCES
- 1.Avarzed, A., D. T. de Waal, I. Igarashi, A. Saito, T. Oyamada, Y. Toyoda, and N. Suzuki. 1997. Prevalence of equine piroplasmosis in central Mongolia. Onderstepoort J. Vet. Res. 64:141-145. [PubMed] [Google Scholar]
- 2.Avarzed, A., I. Igarashi, T. Kanemaru, K. Hirumi, Y. Omata, A. Saito, T. Oyamada, H. Nagasawa, Y. Toyoda, and N. Suzuki. 1997. Improved in vitro cultivation of Babesia caballi. J. Vet. Med. Sci. 59:479-481. [DOI] [PubMed] [Google Scholar]
- 3.Böse, R., W. K. Jorgensen, R. J. Dalgliesh, K. T. Friedhof, and A. J. de Vos. 1995. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 57:61-74. [DOI] [PubMed] [Google Scholar]
- 4.Brüning, R. 1996. Equine piroplasmosis: an update on diagnosis, treatment, and prevention. Br. Vet. J. 152:139-151. [DOI] [PubMed] [Google Scholar]
- 5.Friedhoff, K. T. 1982. The piroplasms of equidae. Significance for the international equine trade. Berl. Muench. Tieraerztl. Wochenschr. 95:368-374. [PubMed] [Google Scholar]
- 6.Halbrook, A. A. 1969. Biology of equine piroplasmosis. Am. J. Vet. Med. Assoc. 155:453-454. [PubMed] [Google Scholar]
- 7.Ikadai, H., C. R. Osorio, X. Xuan, I. Igarashi, T. Kanemaru, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Detection of Babesia caballi infection by enzyme-linked immunosorbent assay using recombinant 48-kDa merozoite rhoptry protein. Int. J. Parasitol. 30:633-635. [DOI] [PubMed] [Google Scholar]
- 8.Ikadai, H., S. Kabamoto, X. Xuan, I. Igarashi, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Protein analysis of Babesia caballi merozoites from in vitro culture by two-dimensional polyacrylamide gel electrophoresis and Western blotting. J. Vet. Med. Sci. 62:323-327. [DOI] [PubMed] [Google Scholar]
- 9.Ikadai, H., Y. Tamaki, X. Xuan, I. Igarashi, S. Kawai, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Mikami. 1999. Inhibitory effect of monoclonal antibodies on the growth of Babesia caballi. Int. J. Parasitol. 29: 1785-1791. [DOI] [PubMed] [Google Scholar]
- 10.Ikadai, H., X. Xuan, I. Igarashi, T. Kanemaru, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 1999. Cloning and expression of a 48-kilodalton Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz, J., R. Dewald, and N. Judy. 2000. Procedurally similar competitive immunoassay systems for the serodiagnosis of Babesia equi, Babesia caballi, Trypanosoma equiperdum, and Burkholderia mallei infection in horses. J. Vet. Diagn. Investig. 12:46-50. [DOI] [PubMed] [Google Scholar]
- 12.Knowles, D. P. 1996. Control of Babesia equi parasitemia. Parasitol. Today 12:195-198. [DOI] [PubMed] [Google Scholar]
- 13.Knowles, D. P., L. S. Kappmeyer, D. Stiller, S. G. Hennager, and L. E. Perryman. 1992. Antibody to a recombinant merozoite protein epitope identified horses infected with B. equi. J. Clin. Microbiol. 30:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado, R. Z., C. A. A. Valadão, R. W. Melo, and A. C. Alessi. 1994. Isolation of Babesia bigemina and Babesia bovis merozoites by ammonium chloride lysis of infected erythrocytes. Braz. J. Med. Biol. Res. 27:2591-2598. [PubMed] [Google Scholar]
- 15.Martin, W. J., J. Finerty, and A. Rosenthal. 1971. Isolation of Plasmodium berghei (malaria) parasites by ammonium chloride lysis of infected erythrocytes. Nat. New Biol. 233:260-261. [DOI] [PubMed] [Google Scholar]
- 16.Mattei, D., and A. Scherf. 1992. The Pf 332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene 110:71-79. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Schein, E. 1988. Equine babesiosis, p. 197-208. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 19.Shimizu, S., S. Shimura, S. Ito, and T. Minami. 1992. Babesia ovata: isolation from erythrocytes and development of an enzyme-linked immunosorbent assay for detection of antibodies. Parasitol. Res. 78:684-688. [DOI] [PubMed] [Google Scholar]
- 20.Short, J. M., J. A. Sorge, and W. D. Huse. 1988. λZAP: a bacteriophage with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, D. B., and K. S. Johonson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka, T., X. Xuan, H. Ikadai, I. Igarashi, H. Nagasawa, K. Fujisaki, T. Mikami, and N. Suzuki. 1999. Expression of Babesia equi merozoite antigen-2 by recombinant baculovirus and its use in the ELISA. Int. J. Parasitol. 29:1803-1808. [DOI] [PubMed] [Google Scholar]
- 23.Tebele, N., A. R. Skilton, J. Katende, W. C. Wells, V. Nene, T. Mcelwain, P. S. Morzaria, and J. A. Musoke. 2000. Cloning, characterization, and expression of a 200-kilodalton diagnostic antigen of Babesia bigemina. J. Clin. Microbiol. 38:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenter, A. M., and K. T. Friedhoff. 1986. Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Vet. Parasitol. 20:49-61. [DOI] [PubMed] [Google Scholar]
- 25.Weiland, G. 1986. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test, immunofluorescence, and enzyme-linked immunosorbent assay. Vet. Parasitol. 20:43-48. [DOI] [PubMed] [Google Scholar]
- 26.Xuan, X., A. Larsen, H. Ikadai, T. Tanaka, I. Igarashi, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Mikami. 2001. Expression of Babesia equi merozoite antigen 1 in insect cells by recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan, X., K. Maeda, T. Mikami, and H. Otsuka. 1996. Characterization of canine herpesvirus glycoprotein C expressed in insect cells. Virus Res. 46:57-64. [DOI] [PubMed] [Google Scholar]