Abstract
Escherichia coli O157:H7 (O157) strains are commonly typed by pulsed-field gel electrophoresis (PFGE) following digestion of genomic DNA with the restriction enzyme XbaI. We have shown that O157 strains differ from each other by a series of discrete insertions or deletions, some of which contain recognition sites for XbaI, suggesting that these insertions and deletions are responsible for the differences in PFGE patterns. We have devised a new O157 strain typing protocol, polymorphic amplified typing sequences (PATS), based on this information. We designed PCR primer pairs to amplify genomic DNA flanking each of 40 individual XbaI sites in the genomes of two O157 reference strains. These primer pairs were tested with 44 O157 isolates, 2 each from 22 different outbreaks of infection. Thirty-two primer pairs amplified identical fragments from all 44 isolates, while eight primer pairs amplified regions that were polymorphic between isolates. The isolates could be differentiated solely on the basis of which of the eight polymorphic amplicons was detected. PATS correctly identified 21 of 22 outbreak pairs as being identical or highly related, whereas PFGE correctly identified 14 of the 22 outbreak pairs as being identical or highly related; PATS was also able to type isolates from three outbreaks that were untypeable by PFGE. However, PATS was less sensitive than PFGE in discriminating between outbreaks. These data suggest that typing by PATS may provide a simple procedure for strain typing of O157 and other bacteria and that further evaluation of the utility of this method for epidemiologic investigations is warranted.
Comparison of two or more isolates of a given bacterial species to determine if they are the same or different is a key step in many epidemiologic, phylogenetic, and population studies. Strains of a particular bacterial species may diverge from each other by acquisition or loss of mobile genetic elements, by point mutation, or by insertions, deletions, or inversions (1). A number of phenotypic and genotypic approaches have been used to examine the relatedness of different isolates within a given bacterial species or serotype, both for epidemiologic purposes and for the gathering of insights into the mechanisms of microbial evolution. However, most of these systems for strain typing are limited because of a lack of typeability, reproducibility, discriminatory power, ease of interpretation, or ease of performance (1).
Of the phenotypic methods, multilocus enzyme electrophoresis, based on variations in the electrophoretic mobilities of enzymes encoded by housekeeping genes, is a discriminating phenotypic method that has been used to study the population genetics of bacterial species with reproducible results (15, 16, 18). Multilocus enzyme electrophoresis, however, is labor intensive and expensive and may fail to distinguish alleles encoding distinct enzymes with the same mobilities. Genotypic methods for strain typing have increasingly been used in recent years. The molecular biology-based technique considered the most reliable and applicable for strain typing of several bacterial species is pulsed-field gel electrophoresis (PFGE) (12, 17). By this procedure, genomic DNA is digested with a restriction endonuclease and PFGE is used to separate the resulting fragments. The distinctive profiles generated enable differentiation of strains in a reproducible manner. Not all strains are typeable by PFGE, however, because of methylation of restriction sites, degradation of DNA in agarose plugs, or other technical problems (7, 8, 11). The most important drawback of PFGE, however, is that the comparison of results for isolates analyzed at different locations or times (and hence on different gels) requires sophisticated pattern-recognition computer software (12).
Many outbreaks of infection, particularly those that are food borne, affect patients nearly simultaneously in different states or countries. Rapid detection of these widespread outbreaks may limit spread of disease by allowing identification and withdrawal of the common source of infection from the marketplace. Development of a rapid, reproducible, and easily comparable strain typing system for closely related bacterial strains such as enterohemorrhagic Escherichia coli O157:H7 (O157) has been a particular challenge. PFGE is used to type O157 strains following XbaI digestion of genomic DNA (3, 4, 7).
We have found that O157 strains differ from each other by a series of discrete insertions or deletions of DNA, some of which contain recognition sites for restriction enzymes (9a). Hence, detection of the presence or absence of these sequences may give information comparable to that provided by PFGE. In this study, we explored the potential of directly detecting these polymorphic sequences with a new strain typing system termed polymorphic amplified typing sequences (PATS).
MATERIALS AND METHODS
Bacteria used in this study.
Two reference strains of O157 were used in the standardization of PATS, strain 86-24 and strain EDL933 (Table 1). O157 strain EDL933 was sequenced at the University of Wisconsin (14). In addition, 44 isolates of O157, 2 each from 22 different outbreaks, were obtained from the Centers for Disease Control and Prevention (CDC) (Table 1).
TABLE 1.
Summary of E. coli O157:H7 isolates used in this study
| Isolate(s) | Description or source | Outbreak no. | Outbreak location | Yr of isolation |
|---|---|---|---|---|
| 86-24 | Human; Smr strain; A. D. O'Brien, personal communication | NAa | NA | NA |
| EDL933 | Human; ATCC 43895 | NA | NA | NA |
| CDC isolate pairs | ||||
| G5320, G5327 | Human | 1 | Michigan | 1982 |
| G5323, G5326 | Human | 2 | Oregon | 1982 |
| G5321, G5322 | Human | 3 | Nebraska | 1984 |
| G5324, G5325 | Human | 4 | North Carolina | 1984 |
| G5283, G5284 | Human | 5 | North Carolina | 1986 |
| G5285, G5286 | Human | 6 | Washington | 1986 |
| G5287, G5288 | Human | 7 | Washington | 1986 |
| G5289, G5290 | Human | 8 | Washington | 1986 |
| G5291, G5292 | Human | 9 | Utah | 1987 |
| G5293, G5294 | Human | 10 | Wisconsin | 1988 |
| G5295, G5296 | Human | 11 | Minnesota | 1988 |
| G5297, G5298 | Human | 12 | Minnesota | 1988 |
| G5317, G5318 | Human | 13 | Missouri | 1990 |
| G5299, G5300 | Human | 14 | Idaho | 1990 |
| G5301, G5302 | Human | 15 | Montana | 1991 |
| G5303, G5304 | Human | 16 | Massachusetts | 1991 |
| G5305, G5306 | Human | 17 | Nevada | 1992 |
| G5307, G5308 | Human, garden | 18 | Maine | 1992 |
| G5309, G5310 | Human, meat | 19 | Washington | 1993 |
| G5311, G5312 | Human | 20 | Oregon | 1993 |
| G5313, G5314 | Human | 21 | Oregon | 1993 |
| G5315, G5316 | Human | 22 | Oregon | 1993 |
NA, not applicable.
DNA extraction, sequencing, and probe labeling.
Genomic DNA was prepared with an Invitrogen Easy-DNA Isolation kit (Invitrogen Corporation, Carlsbad, Calif.), according to the instructions of the manufacturer. Plasmid DNA was extracted with plasmid purification kits (Qiagen Inc., Valencia, Calif.). DNA sequencing was done at the DNA Sequencing Core Facility, Department of Molecular Biology, Massachusetts General Hospital. All DNA probes were labeled with an ECL direct nucleic acid labeling and detection system (Amersham Pharmacia Biotech, Inc., Piscataway, NJ.).
Design of PATS primer pairs amplifying DNA around O157 XbaI sites and virulence gene primer pairs.
A total of 40 PATS primer pairs that flank XbaI restriction sites in genomic DNAs from two O157 reference strains, strains 86-24 and EDL933, were used in this study. These primer pairs have been described previously (9a).
We also designed primer pairs to amplify specific virulence genes found in O157 strains. These primer pairs were similar to those designed by Paton and Paton (13). The four primer pairs included stx1F (5′-ATAAATCGCCATTCGTTGACTAC-3′) and stx1R (5′-GAACGCCCACTGAGATCAT C-3′), stx2F (5′-GGCACTGTCTGAAACTGCTCC-3′) and stx2R (5′-TCGCCAGTTATCTGACAT TCTG-3′), eaeF (5′-GACCCGGCACAAGCATA AGC-3′) and eaeR (5′-CCACCTGCAGCAA CAAGAGG-3′), and hlyAF (5′-GCATCATCAAGCGT ACGTTCC-3′) and hlyAR (5′-AATGAGC CAAGCTGGTTAAGCT-3′).
Typing by PATS.
We used the PATS primers and touchdown, hot start PCR to assay for the presence or absence of DNA segments containing individual XbaI sites in different isolates of O157, as described previously (5, 6, 9a). Each reaction was done in triplicate. The amplicons obtained by PCR were purified with a Qiaquick PCR purification kit (Qiagen Inc.) and were digested with XbaI to confirm the presence of an XbaI site within the amplicon. Undigested and digested DNA fragments were resolved on a 4% agarose gel prepared with a combination of 3% Nusieve GTG agarose (FMC BioProducts, Rockland, Maine) and 1% agarose (Shelton Scientific Inc., Shelton, Conn.), and the gels were stained with ethidium bromide.
PFGE.
PFGE was done in a reference laboratory at CDC by standard procedures described previously (2, 3), with the following modifications. Briefly, the genomic DNA of each isolate was embedded in separate agarose plugs and digested at 37°C for 2 h with 30 U of XbaI (Gibco BRL, Grand Island, N.Y.) per plug. The plugs were loaded onto a 1% agarose-Tris buffer gel (SeaKem Gold Agarose; BioWhittaker Molecular Applications, Rockland, Maine), and PFGE was performed with a CHEF Mapper XA apparatus (Bio-Rad Laboratories, Hercules, Calif.). DNA was electrophoresed for 18 h at a constant voltage of 200 V (6 V/cm), with a pulse time of 2.2 to 54.2 s, an electric field angle of 120o, and a temperature of 14°C, before being stained with ethidium bromide.
DNA dot blots.
Primer pairs IK8A-IK8B, IK25A-IK25B, IK114A-IK114B, IK118A-IK118B, IK123A-IK123B, IK127A-IK127B, IKB3A-IKB3B, and IKB5A-IKB5B were used for the DNA dot blot assay. Amplicons were first obtained from O157 reference strain 86-24 or EDL933 with each primer pair in a separate reaction mixture. Following purification, 2.5 μl of each amplicon was spotted onto Hybond N+ membrane (Amersham Pharmacia) strips and UV cross-linked; these constituted the target amplicons. Ten O157 isolates from five different outbreaks were selected for analysis by dot blotting. For each of these isolates, amplicons were derived by using seven of the eight primer pairs in a multiplex PCR; each primer in primer pairs IK25A-IK25B, IK114A-IK114B, IK123A-IK123B, and IK127A-IK127B was used at a concentration of 200 pmol; and each primer in primer pairs IK8A-IK8B, IK118A-IK118B, and IKB3A-IKB3B was used at a concentration of 100 pmol. In a separate PCR, each primer in the IKB5A-IKB5B primer pair was used at a concentration of 200 pmol. These amplicons were purified, labeled by use of the ECL kit, and pooled; these constituted the probe amplicons. Each membrane strip containing the target amplicons was hybridized with the pool of purified probe amplicons generated from a single isolate, and autoradiographs were prepared by exposure of the processed blots to X-OMAT AR film (Eastman Kodak Company) to test for the presence or absence of hybridizing amplicons in the isolates being analyzed.
RESULTS
PATS primer pairs amplify sequences in the O157 genome containing XbaI restriction sites, some of which are polymorphic between strains.
Thirty-six of the 40 PATS primer pairs amplified XbaI-containing DNA fragments of identical sizes from both reference strains. However, PATS primer pairs IK114A-IK114B and IKB3A-IKB3B, derived from strain EDL933, failed to yield an amplicon when strain 86-24 was used as the template, and PATS primer pairs IK8A-IK8B and IK25A-IK25B, derived from strain 86-24, did not yield an amplicon when strain EDL933 DNA was used as the template. Thus, the PATS primer pairs were able to establish a discriminating profile between these two strains, based on the presence or absence of amplicons.
The PATS primers provide a novel strain typing system for O157.
The ability of the 40 PATS primer pairs to discriminate O157 isolates in a reproducible manner was assessed. To enhance the profile for each isolate being typed, primer pairs derived from four virulence genes (stx1, stx2, eae, and hlyA) were also included in the typing system. Forty-four isolates, two each from 22 different outbreaks investigated by CDC (Table 1), were analyzed with this typing system. The presence or absence of an amplicon, as well as the presence or absence of an XbaI site within each amplicon, was assessed by agarose gel electrophoresis. Representative agarose gel electrophoresis patterns of undigested and XbaI-digested amplicons obtained from some of the isolates are shown in Fig. 1. We recorded electrophoresis results as 0, 1, or 2, indicating the absence of an amplicon, the presence of an amplicon without an XbaI site, and the presence of an amplicon with an XbaI site, respectively. Surprisingly, all amplicons derived with the PATS primer pairs had a score of 0 or 2; i.e., all isolates that had an amplicon with a given primer pair always had an internal XbaI site in the amplicon, as seen originally in the reference strain used to design the PATS primers. Amplicons obtained with the virulence gene primer pairs had a score of 0 or 1. On the basis of the score assigned to each amplicon obtained from every isolate-primer pair combination tested, the 44 O157 isolates were differentiated into 14 PATS types, arbitrarily designated PATS types A through N (Table 2). The reproducibility of this typing system was demonstrated by the consistency of the profiles obtained in three separate analyses of the 44 outbreak isolates.
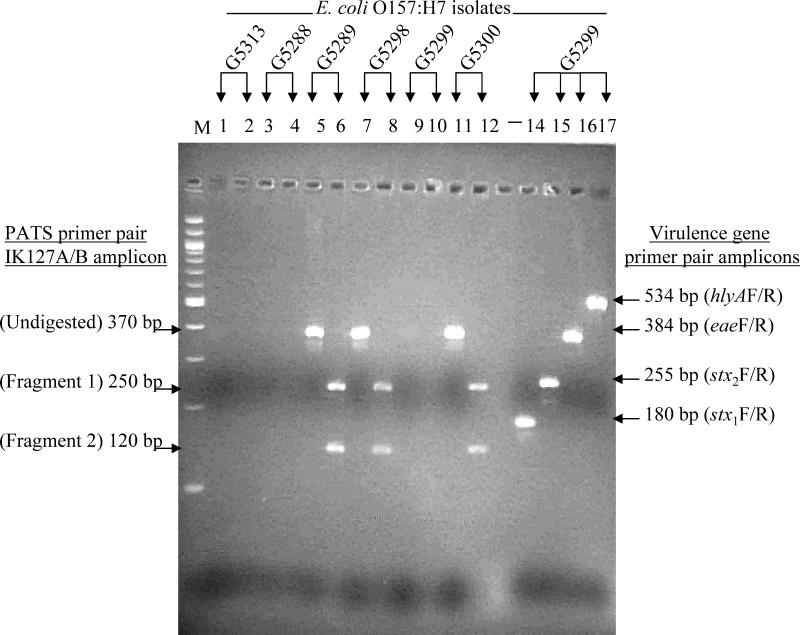
FIG. 1.
Representative agarose gel electrophoresis patterns of amplicons generated from O157 isolates with PATS and virulence gene primer pairs. The presence or absence of amplicons was isolate specific. Lanes 1 to 12, PCR results for six isolates obtained with PATS primer pair IK127A-IK127B; the odd-numbered lanes are before XbaI digestion and the even-numbered lanes are after XbaI digestion. Amplicons, when present, were always digested with restriction enzyme XbaI into two fragments. Lanes 14 to 17, PCR results for a single isolate (G5299) obtained with virulence gene primer pairs, stx1F- stx1R, stx2F- stx2R, eaeF-eaeR, and hlyAF-hlyAR. These amplicons lacked an XbaI restriction site and were not digested with this enzyme (data not shown). Lane M, molecular size marker (100-bp DNA ladder; New England Biolabs, Inc., Beverly, Mass.).
TABLE 2.
PATS profiles of O157 isolates
| PATS typea | PCR amplification and XbaI restriction digestion scores for amplicons obtained with 40 PATS and 4 virulence gene primer pairsb
|
Isolate(s)c | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IK1 | IK2 | IK8 | IK10 | IK12 | IK18 | IK23 | IK25 | IK38 | IK39 | IK51 | IK56 | IK111 | IK114 | IK116 | IK117 | IK118 | IK123 | IK127 | IK131 | IK142 | IK148 | IKB1 | IKB3 | IKB4 | IKB5 | IKB6 | IKB7 | IKB8 | IKB9 | IKB10 | IKB13 | IKB14 | IKB15 | IKB16 | IKB17 | IKB18 | IKB19 | IKB20 | IKB21 | stx1 | stx2 | eae | hlyA | ||
| Control | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | E. coli O157:H7 strain 86-24 |
| Control | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | E. coli O157:H7 strain EDL933 |
| A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | G5289, G5290, G5311, G5312 |
| B | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5320, G5327 |
| C | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5317, G5324, G5325 |
| D | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | G5283, G5284, G5307, G5308 |
| E | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5285, G5286, G5287, G5293, G5294, G5300, G5315, G5321, G5322, G5326 |
| F | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5288, G5299 |
| G | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5291, G5292, G5297, G5298, G5301, G5302, G5309, G5310, G5316 |
| H | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | G5295, G5296 |
| I | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5303 |
| J | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5304 |
| K | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5305, G5306 |
| L | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5313, G5314 |
| M | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | G5318 |
| N | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | G5323 |
PATS types are designated arbitrarily with different letters.
The prefixes of each PATS A-B primer pair and virulence gene F-R primer pair are indicated. 0, no amplicon; 1, amplicon without XbaI site; 2, amplicon with XbaI site. PATS primer pairs that amplified regions that were polymorphic between strains are shown in boldface. Boldface numbers indicate primer pairs that yielded no amplicons from some of the isolates tested.
Isolates of E. coli O157:H7 from various outbreaks (see Table 1) that fell within a given PATS type.
XbaI sites that differ between different O157 strains are located on inserted or deleted O157-specific sequences.
The only differences in the PATS profiles between strains occurred with 8 of the 40 PATS primer pairs, which amplified regions of the O157 genome that were polymorphic between strains; that is, these 8 primer pairs failed to yield an amplification product for some of the strains tested. These eight PATS primer pairs were IK8A-IK8B, IK25A-IK25B, IK114A-IK114B, IK118A-IK118B, IK123A-IK123B, IK127A-IK127B, IKB3A-IKB3B, and IKB5A-IKB5B (Table 2). The regions amplified by the remaining 32 PATS primer pairs were conserved across all strains tested; that is, for each of these 32 primer pairs, all strains tested had identically sized PCR products with a conserved XbaI site. The eight polymorphic XbaI sites were located on inserted or deleted O157-specific sequences (Kudva et al., submitted).
Phylogenetic analysis of PATS profiles suggests a clonal lineage for O157 isolates.
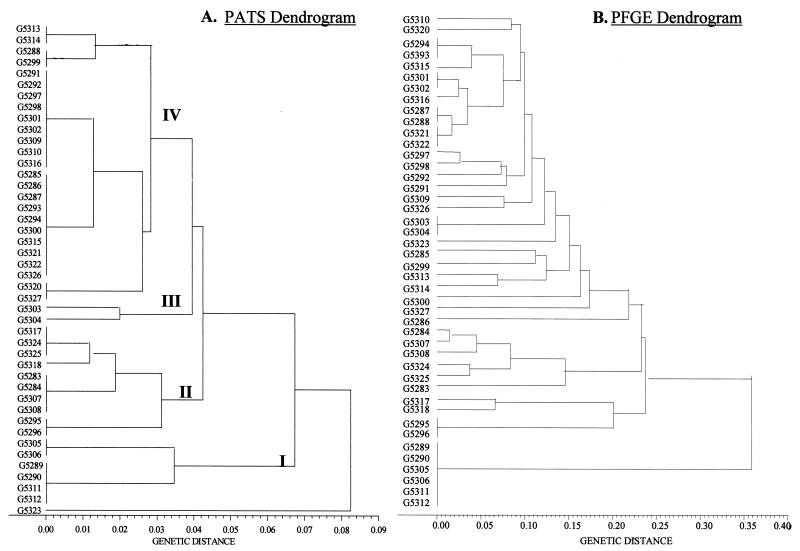
On the basis of the PATS profiles, the 44 O157 isolates could be grouped into four major phylogenetic clusters (Fig. 2A). A genetic distance of <0.1 between each cluster was suggestive of clonal relatedness. A closer analysis of the paired isolates from each outbreak was carried out. The PATS profile types were identical for the two isolates from 16 of the 22 outbreaks (Tables 1 and 2 and Fig. 2A). Paired isolates from five additional outbreaks had highly related PATS types, with only one polymorphism between each of the pairs. The remaining two isolates, G5323 and G5326, were different due to multiple polymorphisms; these isolates also had substantially different PFGE patterns (Fig. 3) and may not, in fact, be related isolates. Overall, the PATS typing system was able to correctly relate pairs of isolates from 21 of the 22 outbreaks (95%) tested. Some isolates from different outbreaks shared a common PATS type, leading to the larger clusters seen in the dendrogram (Fig. 2A).
FIG. 2.
A phylogenetic analysis of O157 isolates with PATS and PFGE data. Dendrograms were constructed by the unweighted pair-group method with arithmetic means. PFGE gels were analyzed with Molecular Analyst Fingerprinting Plus software (Bio-Rad), and the data were exported as a band-matching table so that the two sets of data could be analyzed by the same method. (A) PATS dendrogram. The PATS profiles resolved the isolates into four major clusters. The genetic distance is indicated in increments of 0.01 below the dendrogram. (B) PFGE dendrogram. The PFGE profiles resolved the isolates into smaller clusters and showed greater genetic distances between the isolates.
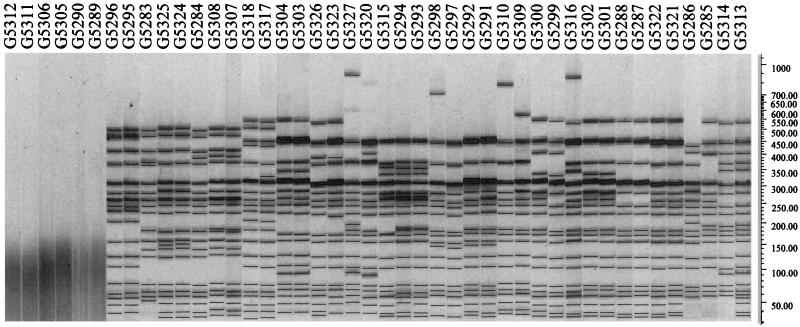
FIG. 3.
PFGE patterns of the 44 O157 isolates from 22 outbreaks. Isolate numbers are indicated above the gel. Note that isolates G5312, G5311, G5306, G5305, G5290, and G5289 could not be typed by PFGE (and are grouped together at the bottom of Fig. ). The bacteriophage lambda DNA ladder standard for PFGE applications (Bio-Rad) was used. Molecular sizes (in kilobase pairs) are shown to the right.
PFGE was also used to categorize the 44 isolates from CDC. The PATS dendrogram for the isolates was compared with the PFGE dendrogram for the isolates in order to compare the potential of these two techniques to relate or discriminate outbreak-associated O157 isolates (Fig. 2). Phylogenetic analysis based on the PFGE profiles resolved the 44 CDC isolates into smaller clusters with greater genetic distances between them than the results from PATS (Fig. 2B). PFGE identified pairs of isolates from six outbreaks as indistinguishable. Sixteen isolates from eight outbreaks had patterns sufficiently similar (differences of one to three bands) to be considered closely related in the context of epidemiologic information that suggested that they had a common source (17). Ten isolates from five outbreaks were more distantly related (differences of more than three bands). Six isolates from three outbreaks were untypeable by PFGE by standard methods (Fig. 3). These six isolates were all typeable by PATS and fell into a distinctive cluster (cluster I in Fig. 2A).
PFGE was more discriminatory than PATS, with no overlaps in patterns between isolates from different outbreaks detected. However, PFGE matched fewer O157 isolates within outbreaks (pairs of isolates from 14 of 22 outbreaks were classified as identical or closely related) and was unable to type six isolates. In contrast, PATS typed all 44 isolates and matched 21 of 22 pairs of isolates from outbreaks as identical or closely related.
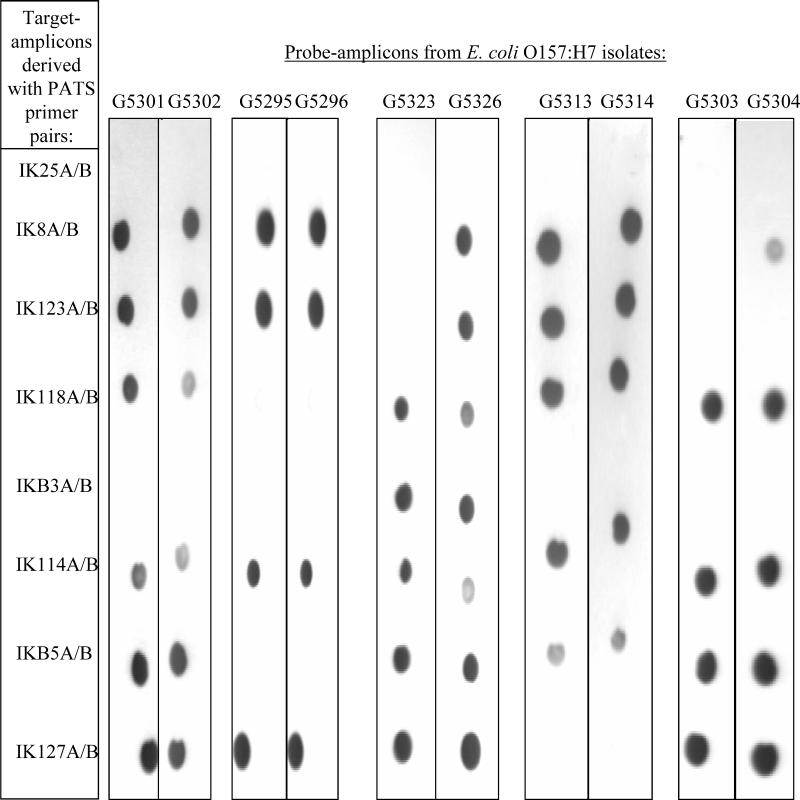
DNA dot blots can effectively detect PATS amplicons.
A dot blot assay for the detection of PATS amplicons was developed to assess the feasibility of automating the PATS typing system. Eight PATS primer pairs that amplified polymorphic regions in the O157 genome were selected for the assay, as these were critical to the discriminatory power of PATS (Table 2). By using these primer pairs, target amplicons were derived from O157 strain 86-24 or EDL933 and were spotted on nylon filters. Multiplex PCR was used to synthesize the probe amplicons to further expedite the assay. Dot blots of the target amplicons were hybridized with the probe amplicons tagged with a chemiluminescent label. The resulting hybridization patterns correlated precisely with the PATS profiles for the respective isolates (Fig. 4; Table 2).
FIG. 4.
Multiplex PCR and DNA dot blot assays to detect PATS polymorphisms between strains. Target amplicons were derived from O157 reference strains 86-24 and EDL933 by using each of the eight indicated PATS primer pairs individually. Probe amplicons were obtained from each of a total of 10 isolates by using seven of the eight PATS primer pairs in a multiplex PCR and a separate PCR with primer pair IKB5A-IKB5B. These probe amplicons were hybridized to nylon membrane strips containing 2.5 μl of each purified target amplicon. The hybridization patterns seen on the dot blot autoradiographs matched the corresponding PATS profiles (Table 2).
DISCUSSION
This study describes a novel O157 typing system that uses a technique termed PATS, which is based on the presence or absence of discrete segments of genomic DNA. The technique is simple, is highly reproducible, and allows accurate and objective interpretation of results. PFGE is considered the “gold standard” for strain typing, as it generates distinctive profiles that distinguish strains in several serotypes and species, including O157 (3, 4, 12). Since the XbaI restriction enzyme site occurs infrequently in the O157 genome, it is frequently used with PFGE for typing of this organism (3, 4, 7). Although PFGE has successfully been used to support outbreak investigations, it may be impossible to fully resolve all bands on a gel under a single set of conditions, making interpretation and comparisons difficult (7, 8, 10).
We sought to use the underlying molecular basis of PFGE to develop a more rapid and simple technique for the typing of O157. We discovered that XbaI polymorphisms in O157 are not due to point mutations in XbaI restriction sites but, rather, are due to the presence or absence of discrete DNA segments containing the individual XbaI sites themselves (9a). We therefore developed a new approach for typing termed PATS, in which we directly tested for the presence or absence of the DNA segments containing each individual polymorphic XbaI site. We also incorporated four virulence gene-specific primer pairs into our typing system. The pathogenicity of O157 is linked to these four genes, and their identification would help detect strains with the potential for virulence in humans (9, 13).
PATS typed every O157 isolate tested, matching 21 of 22 outbreak pairs as identical or closely related and 1 pair as different. PFGE matched fewer O157 outbreak pairs (pairs from 14 of 22 outbreaks were classified as identical or closely related) and was unable to type six isolates. Since the outbreak strains tested here were collected between 1982 and 1993, it is possible that some of the isolates were misclassified as being outbreak related since subtyping was not available at the time. Since it is not possible to obtain any further information regarding potential patient exposures, we have chosen to accept the reported epidemiologic association as the gold standard for method comparison.
Unlike PFGE, methylation of XbaI sites does not interfere with typing by PATS as PATS is a PCR-based procedure (5). One drawback of PATS is that it is less discriminatory than PFGE. While PATS tests for the presence or absence of sequences containing XbaI sites, PFGE is also sensitive to insertions or deletions that may occur between XbaI sites and that may change the size of the intervening fragment without altering the XbaI sites themselves. Also, two of the XbaI sites used in the PATS procedure are located in DNA segments duplicated elsewhere in the genome (data not shown). While PATS is not sensitive to this duplication (it cannot distinguish between one or two copies of identical DNA segments in a genome), such duplications can affect the PFGE pattern. Although PATS was less discriminatory than PFGE in our study, the precision of the PATS procedure could, in principle, be enhanced by identifying additional insertions or deletions in O157 isolates and designing corresponding PATS primer pairs. Studies are ongoing in our laboratory to identify these additional PATS primer pairs. We are also further assessing the use of PATS as an epidemiologic tool by examining additional O157 isolates of both human and animal origin.
Automation could further enhance the applicability of PATS for routine typing of bacterial isolates. The concordance of the results obtained by the DNA dot blot assay with the results obtained by agarose gel electrophoresis suggests that a variety of techniques, including techniques that use DNA microarrays, might be useful for automation of PATS.
Acknowledgments
We thank A. D. O'Brien for providing O157 strain 86-24. We acknowledge the technical assistance provided by Francis O'Neill.
I.T.K. is the recipient of a training grant from the National Institute of Allergy and Infectious Diseases (grant T32 AI07061). P.S.E. was supported by the CDC-APHL Emerging Infectious Diseases Fellowship Program.
REFERENCES
- 1.Arbeit, R. D. 1995. Laboratory procedures for the epidemiologic analysis of microorganisms, p. 190-208. In P. J. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1993. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Barrett, T. J., H. Lior, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohm, H., and H. Karch. 1992. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 30:2169-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieffenbach, C. W., and G. S. Dveksler. 1995. PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Don, R. H., P. T. Cox, B. J. Wainwright, K. Baker, and J. S. Mattick. 1991. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19:4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harsono, K. D., C. W. Kaspar, and J. B. Luchansky. 1993. Comparison and genomic sizing of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 59:3141-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, J. M., S. D. Weagant, K. C. Jinneman, and J. L. Bryant. 1995. Use of pulsed-field gel electrophoresis for epidemiological study of Escherichia coli O157:H7 during a food-borne outbreak. Appl. Environ. Microbiol. 61:2806-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaper, J. B., and A. D. O'Brien. 1998. Escherichia coli O157:H7 and other shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 9a.Kudva, I. T., P. S. Evans, N. T. Perna, T. J. Barrett, F. M. Ausubel, F. R. Blattner, and S. B. Calderwood. J. Bacteriol., in press.
- 10.Meng, J., S. Zhao, T. Zhao, and M. P. Doyle. 1995. Molecular characterization of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis and plasmid DNA analysis. J. Med. Microbiol. 42:258-263. [DOI] [PubMed] [Google Scholar]
- 11.Murase, T., S. Yamai, and H. Watanabe. 1999. Changes in pulsed-field gel electrophoresis patterns in clinical isolates of enterohemorrhagic Escherichia coli O157:H7 associated with loss of Shiga toxin genes. Curr. Microbiol. 38:48-50. [DOI] [PubMed] [Google Scholar]
- 12.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. Dimalanta, K. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 15.Pupo, G. M., D. K. Karaolis, R. Lan, and P. R. Reeves. 1997. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect. Immun. 65:2685-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selander, R. K., D. A. Caugant, H. Ochman, J. M. Musser, M. N. Gilmour, and T. S. Whittam. 1986. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environ. Microbiol. 51:873-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, G., T. S. Whittam, C. M. Berg, and D. E. Berg. 1993. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 21:5930-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]