Abstract
Recent studies have demonstrated that a large number of organisms carry linear mitochondrial DNA molecules possessing specialized telomeric structures at their ends. Based on this specific structural feature of linear mitochondrial genomes, we have developed an approach for identification of the opportunistic yeast pathogen Candida parapsilosis. The strategy for identification of C. parapsilosis strains is based on PCR amplification of specific DNA sequences derived from the mitochondrial telomere region. This assay is complemented by immunodetection of a protein component of mitochondrial telomeres. The results demonstrate that mitochondrial telomeres represent specific molecular markers with potential applications in yeast diagnostics and taxonomy.
Several yeast species are associated with opportunistic infections of humans and other mammals. Among them, candidoses are of the greatest clinical importance. These mycoses manifest themselves as localized, invasive or systemic infections that are frequently associated with immune deficiencies, AIDS, immunosuppressive therapy, anticancer treatments, organ transplantations, and various invasive medical procedures. They are caused mainly by Candida albicans, but many recent clinical surveys have illustrated the rising significance of non-C. albicans infections. As a result, there has been an increased interest in the biology and taxonomy of Candida species originally believed to be nonpathogenic (9, 14, 36).
C. parapsilosis is a widespread pathogen, accounting for up to 30% of nosocomial fungemias. It is also associated with septic arthritis, peritonitis, vaginitis, and nail and skin infections. An increasing prevalence of C. parapsilosis has also been observed in cases of endocarditis, either indicating a selective affinity of this yeast for endocardial tissues or reflecting its propensity to colonize damaged skin and gain ingress along intravascular lines (5, 10, 14, 36).
Due to differences in susceptibility of non-C. albicans species to antifungal drugs, rapid and accurate species identification is essential for the implementation of appropriate therapy. Methods for identification and classification of clinically important Candida species based on phenotypic and/or morphologic characteristics do not always lead to unambiguous results, and identification of a pathogen is sometimes difficult. The recent development of various molecular techniques has brought significant improvements in yeast diagnostics and strain typing (33). Molecular typing approaches for C. parapsilosis include restriction fragment length polymorphism analysis, DNA fingerprinting, protein and tRNA profiling, PCR, and electrophoretic karyotype analysis (4, 6, 12, 27, 29, 30, 35).
Although mitochondrial DNA (mtDNA) is typically portrayed as a circular molecule, the mitochondrial genomes of many organisms are linear double-stranded DNA molecules (23). Recent analyses of mtDNA in various yeasts revealed that closely related species differ in the form of the mtDNA. A relatively high occurrence of the linear form of the mitochondrial genome was found in species of the genera Pichia, Williopsis and Candida (8, 21). Among the special molecular features of linear mitochondrial genomes are telomeres, the structures present at the ends of a linear DNA molecule. Inspection of linear mitochondrial genomes revealed several distinct types of mitochondrial telomeres. In the yeast C. parapsilosis, mitochondrial telomeres consist of long arrays of tandem repeats of a 738-bp unit. Detailed analysis revealed that the mtDNA molecules terminate with an incomplete repeat unit possessing a 5′ single-stranded extension. The extreme end of the molecule is specifically recognized by the mitochondrial telomere-binding protein (mtTBP) that protects the single-stranded overhang from enzymatic degradation (21, 24, 34).
Molecular diagnostics in clinical microbiology require rapid and highly selective methods for identification of pathogenic microorganisms. In general, species-specific procedures take advantage of the unique traits of a pathogenic microorganism. Since mitochondrial telomeres represent a unique feature of the linear form of mtDNA, we propose that they may represent specific molecular markers suitable for identification of organisms harboring linear mitochondrial genomes. Here we tested the pathogenic yeast C. parapsilosis, whose close relatives (e.g., C. albicans and C. tropicalis [32, 37]) possess circular genomes in their mitochondria. Our results demonstrate that the nucleotide sequence of mitochondrial telomeres and the antigenic properties of the protein specifically binding to this sequence have great potential for facilitating the molecular identification of C. parapsilosis.
MATERIALS AND METHODS
Yeast strains.
Yeasts were obtained from the Centraalbureau voor Schimmelcultures (CBS), Delft, The Netherlands. C. parapsilosis SR23 (CBS 7157) and Saccharomyces cerevisiae W303-1A are laboratory strains from the collection of the Department of Biochemistry, Comenius University, Bratislava, Slovakia. C. parapsilosis strains designated MCO and PL were kindly provided by P. F. Lehmann (Medical College of Ohio, Toledo) and S. A. Meyer (Georgia State University, Atlanta), respectively. Yeast cells were grown on YPD plates (1% [wt/vol] yeast extract [Difco], 1% [wt/vol] Bacto Peptone [Difco], 2% [wt/vol] glucose, 2% [wt/vol] agar) at 28°C.
Amplification by PCR and gel electrophoresis.
Yeast cells (approximately 104) from a single colony grown overnight on a fresh YPD plate were picked with a yellow tip (Gilson pipette) and resuspended in 20 μl of 50 mM KCl-10 mM Tris-HCl (pH 9.0)-0.1% (wt/vol) Triton X-100. The suspension was heated at 95 to 100°C for 5 to 10 min and then centrifuged briefly (10,000 × g for 5 s) to suppress condensation. Alternatively, cells were resuspended in 20 mM NaOH (20 μl) and incubated for 5 to 10 min at room temperature. PCRs (20-μl final volume) were performed with 50 mM KCl-10 mM Tris-HCl (pH 9.0)-0.1% (wt/vol) Triton X-100-1.25 mM MgCl2-0.2 mM each deoxynucleoside triphosphate-0.5 μM each primer-2 μl of cell lysate-Taq DNA polymerase (0.5 to 2 U per reaction mixture). PCR primers (5′-CTTGTGCTGGCGATGGTTCA-3′, 5′-GCTCTCAATCTGTCAATCCT-3′, 5′-TAAATTTATGTATATGTTTGCATATATCTTA-3′, and 5′-TAGGGATTGATTATTTACCTATATATTATCA-3′) were designed with the Vector NTI 4.0 software package (InforMax Inc.) and synthesized by Genset. Reactions were prepared on ice by combining 18 μl of premixed reaction components (master mix) and 2 μl of cell lysate (see above). Amplifications were started in a preheated DNA Thermal Cycler 480 (Perkin-Elmer Cetus) with the following standard three-step program: 3 min at 95°C, followed by 25 cycles of 45 s at 94°C, 1 min at 49°C, and 30 s at 72°C and then 5 min at 72°C. The samples were separated by agarose gel electrophoresis (1.5% [wt/vol] containing 0.5 μg of ethidium bromide per ml) at 5 to 10 V/cm for 45 to 60 min in 90 mM Tris-borate buffer.
Immunoblotting.
Yeast cells were grown until the late logarithmic phase in YPD medium (1% [wt/vol] yeast extract, 1% [wt/vol] peptone, 2% [wt/vol] glucose), and cells (0.1 ml of the culture) were washed with double-distilled water and lysed for 5 min at 95°C in 0.1 ml of 1× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 5% [vol/vol] 2-mercaptoethanol, 10% [vol/vol] glycerol) as described by Horvath and Riezman (13). Proteins were separated by SDS-13% (wt/vol) polyacrylamide gel electrophoresis (16). Resolved proteins were transferred to nitrocellulose filters in transfer buffer (25 mM Tris, 192 mM glycine, 20% [vol/vol] methanol [pH 8.3]) with a semidry electroblotter system (Panther HEP-1; Owl Scientific, Portsmouth, N.H.) for 60 min at 200 mA. Filters were blocked for 2 h at room temperature with blocking solution (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2% [wt/vol] skim milk [Difco]) and then incubated overnight at 4°C in blocking solution containing anti-mtTBP polyclonal rabbit antibody SE1785 at a 1:200 dilution (24). Membranes were washed four times with rinsing buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% [vol/vol] Tween 20) and once with rinsing buffer lacking Tween 20 and then incubated with the blocking solution containing a 1:3,000 dilution of a goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma) for 2 h at room temperature. Blots were washed as described above and developed by the addition of 0.3 mg of p-nitroblue tetrazolium chloride (Sigma) per ml and 0.15 mg of 5-bromo-4-chloro-3-indolylphosphate toluidine salt (Sigma) per ml in alkaline phosphatase buffer (100 mM NaHCO3, 1 mM MgCl2 [pH 9.8]) for 5 to 20 min at room temperature.
Miscellaneous.
mtDNA was prepared as described by Defontaine et al. (7), digested with the restriction endonucleases BglII, HindIII, PvuII, and EcoRV (New England Biolabs) in accordance with the manufacturer's instructions, and separated by agarose gel electrophoresis. Total cellular DNA was isolated from 5-ml yeast culture samples as described by Phillippsen et al. (26). DNA samples for pulsed-field gel electrophoresis were prepared as described previously (22) and separated on a 0.8% (wt/vol) agarose gel in 45 mM Tris-borate-1 mM EDTA buffer in a Pulsaphor apparatus (LKB) in contour-clamped homogeneous electric field configuration. The pulse switching program for chromosomal DNA separation involved two steps of linear interpolation, i.e., 60 to 65 s for 2.5 h, followed by 65 to 600 s for 69.5 h, at 100 V and 9°C throughout.
Reproducibility of data.
All PCR and immunoblot analyses were repeated at least twice with the same results.
RESULTS AND DISCUSSION
Mitochondrial telomeres as molecular markers.
The mitochondrial genomes of many organisms were found to be represented by linear DNA molecules possessing telomeres (23). The structure and nucleotide sequence of mitochondrial telomeres appear to be species specific and thus may represent useful molecular markers applicable in molecular diagnostics.
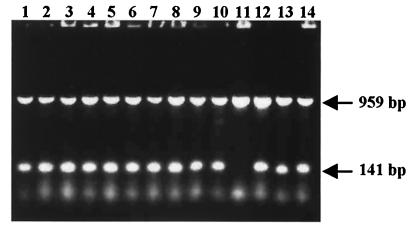
To develop an approach for identification of C. parapsilosis, we designed two pairs of oligonucleotide primers for PCR amplification (see Materials and Methods). The oligonucleotides were derived from nucleotide sequences of C. parapsilosis mitochondrial telomeres (21) (EMBL data library accession numbers X76196 and X76197) and the conserved region of the nuclear 18S rRNA gene (accession number M60307) to serve as C. parapsilosis-specific and yeast-specific primers, respectively. The sensitivity of this system is based on a high redundancy of both molecular markers in C. parapsilosis cells. The redundancy of mitochondrial telomeres is due to the repeated nature of telomeric sequences and the presence of multiple copies of the mtDNA. Similarly, the redundancy of the 18S rRNA gene is due to the presence of 100 to 200 copies within the nuclear genome. When cell lysates of C. parapsilosis (e.g., type strain CBS 604) were used as the source of template DNA, PCR resulted in amplification of two products of 141 and 959 bp derived from the mitochondrial telomere and the gene encoding the 18S rRNA, respectively (Fig. 1).
FIG. 1.
Identification of C. parapsilosis strains with mitochondrial telomere-derived (C. parapsilosis-specific) and 18S rRNA gene-derived (yeast-specific) primers. PCR amplification and gel electrophoresis were performed as described in Materials and Methods. Lanes 1 to 14 contained samples of strains CBS 7157, CBS 604T, CBS 1954, CBS 2152, CBS 2193, CBS 2195, CBS 2197, CBS 2211, CBS 2215, CBS 2916, CBS 5301, CBS 6318, CBS 8050, and CBS 8181. Arrows indicate the positions of the mitochondrial telomere-specific PCR product (141 bp) and the 18S rRNA-specific PCR product (959 bp).
Analysis of non-C. parapsilosis species.
Having an optimized protocol in hand, we were interested in the specificity of this approach. We selected 114 yeast strains belonging to 83 different species (Table 1). This collection contained mainly Candida species that are considered to be phylogenetically related to C. parapsilosis, including C. albicans, C. dubliniensis, C. maltosa, C. tropicalis, and C. sojae (1, 18, 19). In addition, yeast species known to harbor a linear mitochondrial genome (such as Williopsis saturnus, Pichia pijperii, P. jadinii, P. philodendra, C. utilis, C. salmanticensis, and C. vartiovaarae) were added to the list. Finally, the panel also included 10 strains of Lodderomyces elongisporus, as this species was found to be the most closely related to C. parapsilosis phylogenetically (15, 19) and previously was even considered to be its teleomorphic form (11, 20). The results of PCR analysis showed that the C. parapsilosis-specific product was not amplified in any of these species (Table 1). In 18 strains belonging to 12 different species, neither the mitochondrial telomere-specific nor the 18S rRNA-derived PCR product was amplified. A closer inspection of these cases revealed that these results were due to the presence of an inhibitor of Taq DNA polymerase originating from the cell lysates, since the amplifications performed on purified DNA samples yielded the 18S rRNA PCR product (e.g., C. apicola, C. magnoliae, C. salmanticensis, and C. shehatae). Alternatively, a divergence in the nucleotide sequence of the 18S rRNA (e.g., Yarrowia lipolytica) may be responsible for the lack of a PCR product.
TABLE 1.
List of yeast species/strains tested for the presence of the C. parapsilosis mitochondrial telomere-specific PCR product
| Species | Straina | 18S rRNA product | Mitochondrial telomere product | Species | Straina | 18S rRNA product | Mitochondrial telomere product | |
|---|---|---|---|---|---|---|---|---|
| Candida akabanensis | CBS 7878T | + | − | |||||
| Candida albicans | CBS 562NT | + | − | |||||
| Candida albicans | CBS 1949 | + | − | |||||
| Candida albicans | CBS 2716 | + | − | |||||
| Candida albicans | CBS 5983 | + | − | |||||
| Candida albicans | CBS 6431 | + | − | |||||
| Candida apicola | CBS 7444 | − | − | |||||
| Candida berthetii | CBS 6113 | + | − | |||||
| Candida boidinii | CBS 7447 | + | − | |||||
| Candida butyri | CBS 6421T | + | − | |||||
| Candida cantarellii | CBS 4878T | + | − | |||||
| Candida caseinolytica | CBS 7881 | + | − | |||||
| Candida catenulata | CBS 565T | − | − | |||||
| Candida catenulata | CBS 2014 | − | − | |||||
| Candida catenulata | CBS 6174 | − | − | |||||
| Candida cellulolytica | CBS 7920T | + | − | |||||
| Candida diddensiae | CBS 2214T | + | − | |||||
| Candida dubliniensis | CBS 7987T | + | − | |||||
| Candida entomaea | CBS 6306T | + | − | |||||
| Candida ergatensis | CBS 6248T | + | − | |||||
| Candida ernobii | CBS 1737T | + | − | |||||
| Candida ethanolica | CBS 8041T | + | − | |||||
| Candida fabianii | CBS 5481T | + | − | |||||
| Candida fermentati | CBS 8302 | + | − | |||||
| Candida floricola | CBS 7289T | + | − | |||||
| Candida fluviatilis | CBS 6776T | + | − | |||||
| Candida friedrichii | CBS 4114T | + | − | |||||
| Candida fukuyamaensis | CBS 7921T | + | − | |||||
| Candida glabrata | CBS 138T | + | − | |||||
| Candida homilentoma | CBS 6312T | + | − | |||||
| Candida inconspicua | CBS 180T | − | − | |||||
| Candida insectamans | CBS 6033T | + | − | |||||
| Candida intermedia | CBS 572T | + | − | |||||
| Candida ishiwadae | CBS 7401 | + | − | |||||
| Candida krissii | CBS 6519T | + | − | |||||
| Candida magnoliae | CBS 166T | − | − | |||||
| Candida magnoliae | CBS 2677 | − | − | |||||
| Candida magnoliae | CBS 3086 | − | − | |||||
| Candida magnoliae | CBS 6201 | − | − | |||||
| Candida maltosa | CBS 5611T | + | − | |||||
| Candida melibiosica | CBS 5814T | + | − | |||||
| Candida membranifaciens | CBS 6060 | + | − | |||||
| Candida nitratophila | CBS 2027T | + | − | |||||
| Candida norvegica | CBS 2874 | + | − | |||||
| Candida oleophila | CBS 8269 | + | − | |||||
| Candida ooitensis | CBS 7299T | + | − | |||||
| Candida oregonensis | CBS 5036T | + | − | |||||
| Candida ovalis | CBS 7298T | + | − | |||||
| Candida paludigena | CBS 8005T | + | − | |||||
| Candida pararugosa | CBS 1010T | + | − | |||||
| Candida pignaliae | CBS 6071T | + | − | |||||
| Candida pini | CBS 970T | + | − | |||||
| Candida pseudolambica | CBS 2063T | + | − | |||||
| Candida pseudotropicalis | CBS 607T | + | − | |||||
| Candida quercuum | CBS 6422T | + | − | |||||
| Candida rhagii | CBS 4237T | + | − | |||||
| Candida santjacobensis | CBS 8183T | + | − | |||||
| Candida sake | CBS 159T | + | − | |||||
| Candida sake | CBS 5093 | + | − | |||||
| Candida salmanticensis | CBS 5121T | − | − | |||||
| Candida savonica | CBS 6563T | − | − | |||||
| Candida sequanensis | CBS 8118T | − | − | |||||
| Candida shehatae | CBS 5813T | − | − | |||||
| Candida schatavii | CBS 6452T | − | − | |||||
| Candida silvae | CBS 5498T | − | − | |||||
| Candida silvanorum | CBS 6274T | − | − | |||||
| Candida sojae | CBS 7871T | − | − | |||||
| Candida sonorensis | CBS 6792T | − | − | |||||
| Candida sorbophila | CBS 7922 | − | − | |||||
| Candida sp. | CBS 5927 | − | − | |||||
| Candida sp. | CBS 8262 | − | − | |||||
| Candida stellimalicola | CBS 7853T | − | − | |||||
| Candida succiphila | CBS 7297 | − | − | |||||
| Candida tenuis | CBS 615T | − | − | |||||
| Candida tenuis | CBS 2309 | − | − | |||||
| Candida tropicalis | CBS 94T | − | − | |||||
| Candida tropicalis | CBS 643 | − | − | |||||
| Candida tropicalis | CBS 2321 | − | − | |||||
| Candida tropicalis | CBS 2323 | − | − | |||||
| Candida tropicalis | CBS 6719 | − | − | |||||
| Candida tropicalis | CBS 6948 | − | − | |||||
| Candida tropicalis | CBS 5701 | − | − | |||||
| Candida tropicalis | CBS 7923 | − | − | |||||
| Candida utilis | CBS 621T | − | − | |||||
| Candida vaccinii | CBS 7318T | − | − | |||||
| Candida vartiovaarae | CBS 4289T | − | − | |||||
| Candida versatilis | CBS 1752T | − | − | |||||
| Candida vini | CBS 639 | − | − | |||||
| Candida vini | CBS 2122 | − | − | |||||
| Candida zeylanoides | CBS 619NT | − | − | |||||
| Clavispora lusitaniae | CBS 6936T | − | − | |||||
| Kluyveromyces lactis | CBS 2359 | − | − | |||||
| Lodderomyces elongisporus | CBS 1946 | − | − | |||||
| Lodderomyces elongisporus | CBS 2605T | − | − | |||||
| Lodderomyces elongisporus | CBS 2606 | − | − | |||||
| Lodderomyces elongisporus | CBS 5912 | − | − | |||||
| Lodderomyces elongisporus | CBS 6120 | − | − | |||||
| Lodderomyces elongisporus | CBS 6180 | − | − | |||||
| Lodderomyces elongisporus | CBS 6181 | − | − | |||||
| Lodderomyces elongisporus | CBS 6182 | − | − | |||||
| Lodderomyces elongisporus | CBS 6298 | − | − | |||||
| Lodderomyces elongisporus | CBS 7803 | − | − | |||||
| Pichia canadensis | CBS 1992T | − | − | |||||
| Pichia jadinii | CBS 1600T | − | − | |||||
| Pichia kluyveri | CBS 7907 | − | − | |||||
| Pichia pastoris | CBS 704T | − | − | |||||
| Pichia philodendri | CBS 6075T | − | − | |||||
| Pichia pijperi | CBS 2887T | − | − | |||||
| Saccharomyces cerevisiae | W303-1A | − | − | |||||
| Williopsis saturnus var. mrakii | CBS 1707T | − | − | |||||
| Williopsis saturnus var. saturnus | CBS 5761T | − | − | |||||
| Williopsis saturnus var. suaveolens | CBS 255T | − | − | |||||
| Williopsis saturnus var. suaveolens | CBS 1670 | − | − | |||||
| Yarrowia lipolytica | CBS 599T | − | − |
A superscript capital T or NT indicates the type or neotype strain of the species, respectively.
These results demonstrate the specificity of a mitochondrial telomere-derived marker since the corresponding PCR products could not be generated in samples from non-C. parapsilosis species. The high selectivity of this approach is also illustrated by the ability to discriminate between L. elongisporus and C. parapsilosis.
Survey of C. parapsilosis strains.
Genetic heterogeneity has been reported in C. parapsilosis (2, 4, 5, 18, 25, 30). Recently, it has been demonstrated that C. parapsilosis isolates can be divided into three distinct genotype groups (17, 28). The differences between these groups are profound, and it was suggested they may represent distinct species (5, 6, 17, 28). Due to the genetic heterogeneity mentioned above, it was of interest to determine whether a molecular marker based on mitochondrial telomeres could allow discrimination among these groups (Table 2).
TABLE 2.
List of C. parapsilosis strains tested for the presence of mitochondrial telomere-specific PCR product.
| Species | Straina | 18S rRNA product | Mitochondrial telomere product |
|---|---|---|---|
| Candida parapsilosis | CBS 604T (I) | + | + |
| Candida parapsilosis | CBS 1954 | + | + |
| Candida parapsilosis | CBS 2152 | + | + |
| Candida parapsilosis | CBS 2193 | + | + |
| Candida parapsilosis | CBS 2194 | + | + |
| Candida parapsilosis | CBS 2195 | + | + |
| Candida parapsilosis | CBS 2197 | + | + |
| Candida parapsilosis | CBS 2211 | + | + |
| Candida parapsilosis | CBS 2215 (I) | + | + |
| Candida parapsilosis | CBS 2916 | + | + |
| Candida parapsilosis | CBS 6318 | + | + |
| Candida parapsilosis | CBS 7157 (SR23) | + | + |
| Candida parapsilosis | CBS 8050 (I) | + | + |
| Candida parapsilosis | CBS 8181 (I) | + | + |
| Candida parapsilosis | CBS 5301 | + | − |
| Candida parapsilosis | MCO 433 (I) | + | + |
| Candida parapsilosis | MCO 441 (I) | + | + |
| Candida parapsilosis | MCO 448 (III) | + | + |
| Candida parapsilosis | MCO 456 (II) | + | − |
| Candida parapsilosis | MCO 457 (II) | + | − |
| Candida parapsilosis | MCO 462 (II) | + | − |
| Candida parapsilosis | MCO 471 (II) | + | + |
| Candida parapsilosis | MCO 478 (I) | + | + |
| Candida parapsilosis | PL 429 (III) | + | + |
| Candida parapsilosis | PL 448 (III) | + | − |
| Candida parapsilosis | PL 452 (II) | + | − |
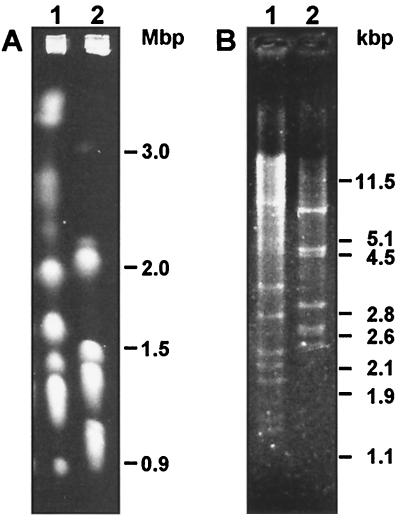
First, we examined 15 different C. parapsilosis strains obtained from the CBS yeast collection. All of these strains, except CBS 5301, reproducibly yielded both of the PCR products described above, indicating positive identification as C. parapsilosis (Fig. 1). To test the hypothesis that the result obtained with CBS 5301 was caused by its genetic difference from other C. parapsilosis strains, we analyzed this case in more detail. To exclude the possibility that the cells used for analysis contained incidental contamination, we analyzed a new sample of this strain from the CBS collection and obtained the same results. Based on physiologic and morphologic criteria, E. Slavikova (Czechoslovak Culture of Yeasts, Chemical Institute of Slovak Academy of Sciences, Bratislava, Slovakia) recognized this strain as non-l-arabinose-utilizing form II of C. parapsilosis. However, identification by the API 20C kit (Biomerieux, Marcy l'Etoile, France) revealed that the biotype of CBS 5301 (i.e., 6176171) differs from that typical of C. parapsilosis strains (i.e., 6756171) due to the absence of l-arabinose assimilation and weak utilization of d-xylitol. Also, comparison of the electrophoretic karyotypes and mtDNA restriction enzyme digestion patterns of CBS 5301 and CBS 604 revealed remarkable differences in nuclear and mitochondrial genome organization (Fig. 2) corresponding to the absence of mitochondrial telomere-derived PCR products in CBS 5301 cells. Thus, according to the molecular criteria, strain CBS 5301 seems to be substantially different from C. parapsilosis type strain CBS 604 and may represent a distinct species.
FIG. 2.
Comparison of electrophoretic karyotypes (A) and BglII restriction enzyme digestion patterns of mtDNA (B) of strains CBS 5301 (lane 1) and CBS 604 (lane 2) (see Materials and Methods).
Next, we analyzed clinical isolates belonging to three different genotype groups of C. parapsilosis as defined by Lin et al. (17) and Roy and Meyer (28). PCR amplification on lysates from all group I strains reproducibly yielded both products. However, tests of four strains belonging to group II (MCO456, MCO457, MCO462, and PL452) and one group III strain (PL448) did not display PCR products derived from mitochondrial telomeres. This discrepancy may be attributed to the variability of C. parapsilosis mentioned above and requires further examination of the genetic relatedness of strains belonging to different groups by alternative approaches.
The reproducible results of the PCR analysis of the group I strains (type strain CBS 604 group) that predominate among clinical isolates (17) correspond well to the stability of restriction enzyme digestion patterns of mtDNA previously observed by Camougrand et al. (3) and further strengthen the idea that mtDNA-derived markers can be used for appropriate identification of this yeast in clinical samples.
Immunoblotting approach.
The use of the mitochondrial telomere sequences for C. parapsilosis identification may be complemented by detection of proteins that specifically interact with the terminal structures of mtDNA. We recently purified the first mtTBP from C. parapsilosis and subsequently cloned the corresponding gene (24, 34). Rabbit antisera raised against the recombinant form of mtTBP were then examined for suitability for C. parapsilosis identifications. Western blot analysis of 57 strains belonging to 24 yeast species demonstrated the presence of a 15-kDa protein corresponding to mtTBP only in C. parapsilosis strains (Table 3), illustrating the specificity of this approach. The immunoblot analyses of strains belonging to C. parapsilosis groups I, II, and III always demonstrated the presence of the 15-kDa marker, indicating a close relationship among these strains. On the other hand, the differences between the PCR and immunoblot approaches observed in some isolates from groups II and III of C. parapsilosis have to be further investigated in terms of genetic polymorphism. Analogous to the results mentioned above (PCR approach, electrophoretic karyotype, mtDNA restriction enzyme pattern, API 20C) CBS 5301 did not display the 15-kDa antigen. Other yeast species (including the most closely related species, L. elongisporus) tested by immunoblot analysis yielded either no or only minor cross-reacting bands with higher molecular weights (Table 3).
TABLE 3.
Detection of the 15 kDa band in the lysates of various yeast species with anti-mtTBP antibody SE1785
| Species | Straina | Presence of 15-kDa proteinb | Cross-reacting band molecular mass(es) (kDa) |
|---|---|---|---|
| Candida albicans | CBS 562NT | − | |
| Candida caseinolytica | CBS 7881 | − | |
| Candida catenulata | CBS 565T | − | 90 |
| Candida glabrata | CBS 138T | − | 45 |
| Candida maltosa | CBS 5611T | − | |
| Candida parapsilosis | CBS 604T | + | |
| Candida parapsilosis | CBS 1954 | + | |
| Candida parapsilosis | CBS 2152 | + | |
| Candida parapsilosis | CBS 2193 | + | |
| Candida parapsilosis | CBS 2194 | + | |
| Candida parapsilosis | CBS 2195 | + | |
| Candida parapsilosis | CBS 2197 | + | |
| Candida parapsilosis | CBS 2211 | + | |
| Candida parapsilosis | CBS 2215 | + | |
| Candida parapsilosis | CBS 2916 | + | |
| Candida parapsilosis | CBS 6318 | + | |
| Candida parapsilosis | CBS 7157 | + | |
| Candida parapsilosis | CBS 8050 | + | |
| Candida parapsilosis | CBS 8181 | + | |
| Candida parapsilosis | CBS 5301 | − | |
| Candida parapsilosis | MCO 433 (I) | + | |
| Candida parapsilosis | MCO 441 (I) | + | |
| Candida parapsilosis | MCO 448 (III) | + | |
| Candida parapsilosis | MCO 456 (II) | + | |
| Candida parapsilosis | MCO 457 (II) | + | |
| Candida parapsilosis | MCO 462 (II) | + | |
| Candida parapsilosis | MCO 471 (II) | + | |
| Candida parapsilosis | MCO 478 (I) | + | |
| Candida parapsilosis | PL 429 (III) | + | |
| Candida parapsilosis | PL 448 (III) | + | |
| Candida parapsilosis | PL 452 (II) | + | |
| Candida pararugosa | CBS 1010T | − | |
| Candida pseudotropicalis | CBS 607T | − | |
| Candida rhagii | CBS 4237T | − | 35, 40, 45, 50 |
| Candida sake | CBS 159T | − | |
| Candida salmanticensis | CBS 5121T | − | 25, 30, 33, 43, 50, 60, 70 |
| Candida shehatae | CBS 5813T | − | |
| Candida tropicalis | CBS 94T | − | |
| Candida utilis | CBS 621T | − | 27, 35, 40, 45 |
| Clavispora lusitaniae | CBS 6936T | − | 30, 40, 45, 50 |
| Kluyveromyces lactis | CBS 2359 | − | 43 |
| Lodderomyces elongisporus | CBS 2605T | − | 55 |
| Lodderomyces elongisporus | CBS 2606 | − | 55 |
| Lodderomyces elongisporus | CBS 5912 | − | 55 |
| Lodderomyces elongisporus | CBS 6120 | − | 55 |
| Lodderomyces elongisporus | CBS 6181 | − | 55 |
| Lodderomyces elongisporus | CBS 6182 | − | 55 |
| Pichia canadensis | CBS 1992T | − | 43 |
| Pichia jadinii | CBS 1600T | − | 27, 35, 40, 45 |
| Pichia pastoris | CBS 704T | − | 25, 45 |
| Pichia philodendri | CBS 6075T | − | 43, 70, 90 |
| Pichia pijperi | CBS 2887T | − | 29, 37, 45 |
| Williopsis saturnus var. mrakii | CBS 1707T | − | |
| Williopsis saturnus var. saturnus | CBS 5761T | − | 43 |
| Williopsis saturnus var. suaveolens | CBS 255T | − | 43 |
| Williopsis saturnus var. suaveolens | CBS 1670 | − | 43 |
| Yarrowia lipolytica | CBS 599T | − |
A superscript capital T or NT indicates the type or neotype strain of the species. I, II, or III is the group as defined in reference 17 and 28.
Plus and minus signs indicate the presence and absence, respectively, of the 15-kDa protein recognized by the antibody. Molecular masses are those of minor cross-reacting proteins present in lysates of the corresponding yeast strains.
Taken together, our results illustrate that mitochondrial telomeres are suitable targets for identification of C. parapsilosis strains. Moreover, the utilization of mitochondrial telomere-derived molecular markers is not limited to C. parapsilosis but, in principle, may be applied to the identification of other microorganisms associated with human or animal infections, provided they possess linear mitochondrial genomes (e.g., C. utilis [8] Theileria annulata, and T. parva [31]). The specificity of mitochondrial telomere-derived molecular markers, together with the sensitivity and versatility of the PCR and immunoblot assays, would enable the adaptation and optimization of this approach for the direct detection of C. parapsilosis in clinical samples or mixed fungal populations without prior cultivation and/or purification of yeasts. In addition to PCR and immunoblot analyses, dot blot or DNA microarray methods might become alternative tools for employing mitochondrial telomere-derived molecular markers in molecular diagnostics.
Acknowledgments
We thank L. Kováč (Comenius University, Bratislava, Slovakia) for continuous support, helpful discussions, and comments; P. F. Lehmann (Medical College of Ohio, Toledo) and S. A. Meyer (Georgia State University, Atlanta) for providing C. parapsilosis MCO and PL isolates; A. Vaughan-Martini (University of Perugia, Perugia, Italy) for the gift of the type strain of L. elongisporus; and D. Subramanian, R. Stansel, and T. Cesare (University of North Carolina, Chapel Hill) for reading the manuscript and editorial advice.
This work was supported in part by grants from the European Union (BIO4-CT96-0003), the Howard Hughes Medical Institute (55000327), the Slovak grant agency VEGA (1/6168/99 and 1/7248/00), and a Fogarty International Research Collaboration Award (1-R03-TW05654-01). J.N. was supported by a postdoctoral fellowship from the Ministry of Education of the French government.
REFERENCES
- 1.Barns, S., D. J. Lane, M. L. Sogin, C. Bibeau, and W. G. Weisburg. 1991. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 173:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branchini, M. L., M. A. Pfaller, J. Rhine-Chalberg, T. Frempong, and H. D. Isenberg. 1994. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J. Clin. Microbiol. 32:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camougrand, N., B. Mila, G. Velours, J. Lazowska, and M. Guerin. 1988. Discrimination between different groups of Candida parapsilosis by mitochondrial DNA restriction analysis. Curr. Genet. 13:445-449. [DOI] [PubMed] [Google Scholar]
- 4.Carruba, G., E. Pontieri, F. De Bernardis, P. Martino, and A. Cassone. 1991. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J. Clin. Microbiol. 29:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassone, A., F. De Bernardis, E. Pontieri, G. Carruba, C. Girmenia, P. Martino, M. Fernandez-Rodriguez, G. Quindos, and J. Ponton. 1995. Biotype diversity of Candida parapsilosis and its relationship to the clinical source and experimental pathogenicity. J. Infect. Dis. 171:967-975. [DOI] [PubMed] [Google Scholar]
- 6.De Bernardis, F., F. Mondello, R. San Millan, J. Ponton, and A. Cassone. 1999. Biotyping and virulence properties of skin isolates of Candida parapsilosis. J. Clin. Microbiol. 37:3481-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defontaine, A., F. M. Lecocq, and J. N. Hallet. 1991. A rapid miniprep method for the preparation of yeast mitochondrial DNA. Nucleic Acids Res. 19:185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuhara, H., F. Sor, R. Drissi, N. Dinouel, I. Miyakawa, S. Rousset, and A. M. Viola. 1993. Linear mitochondrial DNAs of yeasts: frequency of occurrence and general features. Mol. Cell. Biol. 13:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber, G. 2001. An overview of fungal infections. Drugs 61:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Girmenia, C., P. Martino, F. De Bernardis, G. Gentile, M. Boccanera, M. Monaco, G. Antonucci, and A. Cassone. 1996. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin. Infect. Dis. 23:506-514. [DOI] [PubMed] [Google Scholar]
- 11.Hamajima, K., A. Nishikawa, T. Shinoda, and Y. Fukazawa. 1987. Deoxyribonucleic acid base composition and its homology between two forms of Candida parapsilosis and Lodderomyces elongisporus. J. Gen. Appl. Microbiol. 33:299-302. [Google Scholar]
- 12.Haynes, K. A., and T. J. Westerneng. 1996. Rapid identification of Candida albicans, C. glabrata, C. parapsilosis and C. krusei by species-specific PCR of large subunit ribosomal DNA. J. Med. Microbiol. 44:390-396. [DOI] [PubMed] [Google Scholar]
- 13.Horvath, A., and H. Riezman. 1994. Rapid protein extraction from Saccharomyces cerevisiae. Yeast 10:1305-1310. [DOI] [PubMed] [Google Scholar]
- 14.Hurley, R., J. de Louvois, and A. Mulhall. 1987. Yeast as human and animal pathogens, p. 207-281. In A. H. Rose and J. S. Harrison (ed.), The yeasts, vol. 1. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 15.James, S. A., M. D. Collins, and I. N. Roberts. 1994. The genetic relationships of Lodderomyces elongisporus to other ascomycete yeast species as revealed by small-subunit rRNA gene sequences. Lett. Appl. Microbiol. 19:308-311. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lin, D., L.-C. Wu, M. G. Rinaldi, and P. F. Lehmann. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 33:1815-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lott, T. J., R. J. Kuykendall, S. F. Welbel, A. Pramanik, and B. A. Lasker. 1993. Genomic heterogeneity in the yeast Candida parapsilosis. Curr. Genet. 23:463-467. [DOI] [PubMed] [Google Scholar]
- 19.Mannarelli, B. M., and C. P. Kurtzman. 1998. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J. Clin. Microbiol. 36:1634-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakase, T., K. Komagata, and Y. Fukazawa. 1979. A comparative taxonomic study on two forms of Candida parapsilosis. J. Gen. Appl. Microbiol. 25:375-386. [Google Scholar]
- 21.Nosek, J., N. Dinouel, L. Kovac, and H. Fukuhara. 1995. Linear mitochondrial DNAs from yeasts: telomeres with large tandem repetitions. Mol. Gen. Genet. 247:61-72. [DOI] [PubMed] [Google Scholar]
- 22.Nosek, J., and H. Fukuhara. 1994. NADH dehydrogenase subunit genes in the mitochondrial DNA of yeasts. J. Bacteriol. 176:5622-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosek, J., L. Tomaska, H. Fukuhara, Y. Suyama, and L. Kovac. 1998. Linear mitochondrial genomes: 30 years down the line. Trends Genet. 14:184-188. [DOI] [PubMed] [Google Scholar]
- 24.Nosek, J., L. Tomaska, B. Pagacova, and H. Fukuhara. 1999. Mitochondrial telomere-binding protein from Candida parapsilosis suggests an evolutionary adaptation of a nonspecific single-stranded DNA-binding protein. J. Biol. Chem. 274:8850-8857. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1995. Variations in DNA subtype, antifungal susceptibility, and slime production among clinical isolates of Candida parapsilosis. Diagn. Microbiol. Infect. Dis. 21:9-14. [DOI] [PubMed] [Google Scholar]
- 26.Phillippsen, P., A. Stotz, and C. Scherf. 1991. DNA isolation of Saccharomyces cerevisiae. Methods Enzymol. 194:169-182. [DOI] [PubMed] [Google Scholar]
- 27.Pontieri, E., L. Gregori, M. Gennarelli, T. Ceddia, G. Novelli, B. Dallapiccola, F. De Bernardis, and G. Carruba. 1996. Correlation of SfiI macrorestriction endonuclease fingerprint analysis of Candida parapsilosis isolates with source of isolation. J. Med. Microbiol. 45:173-178. [DOI] [PubMed] [Google Scholar]
- 28.Roy, B., and S. A. Meyer. 1998. Confirmation of the distinct genotype groups within the form species Candida parapsilosis. J. Clin. Microbiol. 36:216-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos, M. A. S., C. El-Adlouni, A. D. Cox, J. M. Luz, G. Keith, and M. F. Tuite. 1994. Transfer RNA profiling: a new method for the identification of pathogenic Candida species. Yeast 10:625-636. [DOI] [PubMed] [Google Scholar]
- 30.Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 25:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla, G. C., and V. Nene. 1998. Telomeric features of Theileria parva mitochondrial DNA derived from cycle sequence data of total genomic DNA. Mol. Biochem. Parasitol. 95:159-163. [DOI] [PubMed] [Google Scholar]
- 32.Su, C. S., and S. A. Meyer. 1989. Restriction endonuclease analysis of mitochondrial DNA from Candida parapsilosis and other Candida species. Yeast 5:S355-S360. [PubMed] [Google Scholar]
- 33.Sullivan, D. J., M. C. Henman, G. P. Moran, L. C. O'Neil, D. E. Bennett, D. B. Shanley, and D. C. Coleman. 1996. Molecular genetic approaches to identification, epidemiology and taxonomy of non-albicans Candida species. J. Med. Microbiol. 44:399-408. [DOI] [PubMed] [Google Scholar]
- 34.Tomaska, L., J. Nosek, and H. Fukuhara. 1997. Identification of a putative mitochondrial telomere-binding protein of the yeast Candida parapsilosis. J. Biol. Chem. 272:3049-3056. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez, J. A., A. Beckley, S. Donabedian, J. D. Sobel, and M. J. Zervos. 1993. Comparison of restriction enzyme analysis versus pulsed-field gradient gel electrophoresis as a typing system for Torulopsis glabrata and Candida species other than C. albicans. J. Clin. Microbiol. 31:2021-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weems, J. J. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 37.Wills, J. W., W. B. Troutman, and W. S. Riggsby. 1985. Circular mitochondrial genome of Candida albicans contains a large inverted duplication. J. Bacteriol. 164:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]