Abstract
In five separate fecal collections spanning three years, group A rotaviruses were detected by enzyme-linked immunosorbent assay in 35 (25%) of 142 specimens obtained from nondiarrheic, hospitalized neonates in Blantyre, Malawi. Molecular characterization of each strain identified, for the first time in neonates, a short electropherotype, genotype P[6], G8 strain type, similar to the dominant, cocirculating community strain detected in symptomatic infants in Blantyre. Partial sequence analysis of the VP4 and NSP4 genes of neonatal and community strains failed to identify changes which could explain the differences in clinical outcome. Neonatal serotype G8 rotaviruses should be considered as potential rotavirus vaccine candidates for use in Malawi.
Human rotavirus (HRV) is the leading cause of severe gastroenteritis in infants and young children (16). HRV also infects neonates within some hospital nurseries, where infection is nosocomially acquired and is characteristically asymptomatic (5, 17). Despite the lack of associated symptoms, neonatal rotavirus infections have gained considerable attention because they protect against severe rotavirus diarrhea in later infancy (2, 3). A number of rotaviruses that have been recovered from neonates (so-called nursery strains) have been tested as rotavirus vaccine candidates (1, 24).
The rotavirus genome comprises 11 segments of double-stranded RNA (dsRNA), contained within the core of the mature, triple-layered particle. The 11 dsRNA segments can be separated by using electrophoresis, and two major strain types (short and long electropherotypes) are differentiated by differences in the relative migration patterns of segments 10 and 11 (16). Each dsRNA segment encodes a viral protein, which include six structural proteins and five nonstructural proteins. The two surface proteins, VP7 (encoded by segment 7, 8, or 9) and VP4 (encoded by segment 4), independently induce neutralizing antibodies and form the basis of a dual serotyping scheme. Thus, 14 VP7 (G) serotypes and 11 VP4 (P) serotypes have been described (15). Molecular analyses have defined 14 VP7 genotypes (absolutely in agreement with serotyping studies), but have identified at least 20 VP4 genotypes (not each genotype has been assigned a serotype). VP4 is also implicated in rotavirus pathogenicity, and amino acid 469 has been identified as a possible pathogenicity determinant (4). Of the nonstructural proteins, NSP4 (the product of segment 10), as well as having a critical and unique role in viral assembly, is now known to function as a viral enterotoxin, and the region spanning amino acids 114 to 135 has been identified as the putative “toxic peptide” (10).
Several explanations of the generally benign outcome of HRV infection in neonates have been proposed, including both host factors (presence of neutralizing maternal antibody and immature gastrointestinal tract) and sequence changes in proposed virulence proteins (e.g., VP4 and NSP4). Early studies indicated that HRV strains circulating in neonatal nurseries were distinct from those that circulate in the community (23). In particular, many neonatal strains possessed the M37-like P[6] VP4 gene allele, which was considered an uncommon community strain (14). Examination of the VP4 genes of such strains by hybridization and nucleic acid sequencing demonstrated conserved differences from strains recovered from infants with diarrhea (11, 14). Variability in the VP4 gene of M37-like rotaviruses was therefore proposed as a molecular basis for their apparent attenuation, although subsequent sequencing studies failed to support this hypothesis (22). In view of the recently identified enterotoxigenic properties of NSP4, investigators have undertaken sequencing studies of this glycoprotein in an effort to explain why HRV infections of neonates rarely result in diarrhea, with conflicting findings (18-20).
Since 1997, we have conducted a program of rotavirus research at the Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi. As part of this work, we characterized by electropherotype and G and P type rotaviruses detected in children with acute gastroenteritis who attended the QECH. A total of 10 different strain types were identified between 1997 and 1999, and a novel, short electropherotype, subgroup I, P2A[6], G8 strain (prototype strain MW23) was the second-most-common isolate identified (6, 7). The objectives of the present study were to (i) determine whether HRVs could be detected in neonates treated in the neonatal nursery at the QECH; (ii) compare the G and P types of neonatal rotaviruses with community strains detected in children with acute gastroenteritis; and (iii) compare the VP4 and NSP4 sequences of neonatal strains with corresponding sequences of community strains.
The study was based in the Chatinkha Nursery at the QECH, Blantyre. This is a 50-cot nursery that provides nonventilatory support (warmth, feeding, antibiotics, intravenous fluids) for newborn infants, most of whom are born at the QECH. Approximately half the infants are admitted to the nursery with complications of prematurity (e.g., respiratory distress syndrome or sepsis), and approximately half are term infants with a variety of complications (e.g., low birth weight, asphyxia, sepsis, or congenital malformations). Between November 1997 and November 2000, we conducted five surveys in the Chatinkha Nursery. Each survey lasted 24 h, during which time a fecal sample was collected from each child. Fecal samples were frozen at −80°C within 3 h of collection. Group A rotavirus was detected by the Rotaclone EIA kit (Meridian Diagnostics, Cincinnati, Ohio). Rotavirus dsRNA was extracted using a guanidine and silica method (12). For electropherotyping, rotavirus dsRNA segments were separated by electrophoresis on a 10% polyacrylamide gel and stained with silver nitrate. Rotavirus G and P typing utilized nested, reverse transcription-PCR (RT-PCR) with consensus and type-specific primers as previously described (7).
Two neonatal rotaviruses (NEO7 and NEO25) and three com-munity P[6], G8 rotaviruses (MW63, MW131, and MW467) were culture adapted in MA104 cells before subgrouping, G-serotyping, and genogrouping as previously described for the prototype P[6], G8 Malawi strain MW23 (6). Prior to nucleotide sequencing, the VP8* fragment of VP4 (containing the serotype-specific neutralization sites) was amplified by using primers con2 and con3 to generate an 877-bp product (12). A 739-bp fragment of NSP4 gene 10, including the whole open reading frame, was amplified by using primers described previously (8). For both VP4 and NSP4, the corresponding primers were used to sequence the RT-PCR products directly. The NSP4 and VP4 nucleotide sequences for MW23 have been previously published (6).
In fecal collections from November 1997 (35 stools), August 1998 (26 stools), March 1999 (22 stools), May 2000 (28 stools), and November 2000 (31 stools), rotavirus was detected by enzyme-linked immunosorbent assay in 26, 27, 14, 14, and 39% of samples, respectively. None of the children had diarrhea. Of the 35 rotaviruses detected, 30 samples had sufficient fecal material remaining to enable characterization of the strains to be undertaken. Each strain possessed a short electropherotype and the P[6], G8 genotype (Fig. 1). The G serotype of the neonatal strains was selectively confirmed by neutralization with the G8-specific monoclonal antibody, B37, and their subgroup was confirmed as subgroup I (data not shown). RNA-RNA hybridization of NEO7 and NEO25 to the prototypic strains Wa (Wa genogroup) and DS-1 (DS-1 genogroup) and to the prototype Malawi community P[6], G8 strain MW23 identified a DS-1 genogroup origin for the neonatal strains and a very close genetic relationship to MW23 (6).
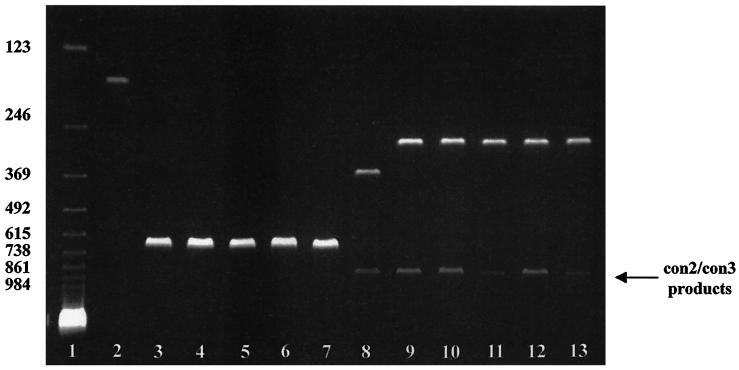
FIG. 1.
Multiplex RT-PCR typing of five representative Malawian neonatal rotavirus strains. RT-PCR type-specific products were resolved on a 2% agarose gel and stained with ethidium bromide. Lane 1, 123-bp DNA ladder (GIBCO-BRL, Long Island, N.Y.). Molecular weights are indicated to the left. Lanes 2 to 7, G typing. Second-amplification RT-PCR products of known serotype G1 control strain (lane 2) and Malawian neonatal strains (serotype G8, lanes 3 to 7) are shown. Lanes 8 to 13, P-typing. Second-amplification RT-PCR products of known genotype P[8] control strain (lane 8) and Malawian neonatal strains (genotype P[6], lanes 9 to 13) are shown. The first-amplification con2/con3 products are indicated (arrow).
Sequence analysis of con2/con3 VP4 gene fragments of NEO7 and NEO25 demonstrated >99% amino acid identity to the community P[6], G8 strains (MW23, MW63, MW131, and MW467). Comparison of deduced VP4 amino acid sequences of NEO7 and NEO25 with the community P[6], G8 strains identified a single amino acid residue at position 73 that was conserved in the two neonatal strains (His) but distinct from the four community strains (Asn) (data not shown). For NSP4, the 175-amino-acid NSP4 protein of the neonatal strains shared >93% identity with the community strains and 98.3% identity with MW23. A single-amino-acid residue at position 56 was conserved in the two neonatal strains (Met) but distinct from the four community strains (Ile) (data not shown).
This study has demonstrated the circulation, over 3 years, of rotavirus strains with the P[6], G8 genotype in a neonatal nursery in Blantyre. This is the first time that serotype G8 rotaviruses have been recovered from neonates. Previously identified neonatal rotaviruses include strains with VP4 genotype P[6] and VP7 serotype G1, G2, G3, G4, or G9; P[11] strains with G3, G9 or G10 VP7 specificity; and P[4], G2 strains (13). In contrast to most previous studies, we found that strains with the same genotype (P[6], G8) were simultaneously circulating in the community (7). The presence in neonates of passively acquired, neutralizing maternal antibody to rotavirus strains commonly circulating in the community, but not to uncommon rotavirus serotypes, is one factor that has been proposed to explain why neonates may become infected with noncommunity strains (21). In Malawi, maternal-infant transfer of antibody may be reduced by a variety of factors including prematurity, malnutrition, malaria, and human immunodeficiency virus (9). While none of these factors was specifically investigated in this study, the neonates for these reasons may have lacked sufficient neutralizing maternal antibody to protect against infection with P[6], G8 strains while having sufficient antibody to prevent the development of symptoms (25).
Sequence analysis of the VP4 and NSP4 genes of two neonatal strains identified a close relationship to the corresponding genes of community P[6], G8 strains, including the prototype community P[6], G8 strain MW23. Furthermore, amino acid alignments of the VP4 and NSP4 proteins of the neonatal and community strains identified a single, conserved amino acid substitution in VP4 (not at a known neutralization site) and in NSP4 (outside of the toxic peptide region) in the neonatal strains. While possible pathogenicity sites in the VP8* fragment of VP4 have yet to be established, these data do not support the hypothesis that variation in NSP4 sequence can explain the differences in outcome of neonatal (asymptomatic) and community (symptomatic) rotavirus P[6], G8 infections.
In five small studies spanning 3 years, we have found P[6], G8 rotavirus strains to be endemic in this hospital nursery for newborn infants in Blantyre. Given the ability of neonatal rotavirus strains to protect against symptomatic rotavirus diarrhea in later infancy, these strains warrant consideration as potential rotavirus vaccine candidates, especially since serotype G8 is the dominant community strain in Malawi (7).
Nucleotide sequence accession number.
The nucleotide sequences of the strains described in this study, including the neonatal strains NEO7 and NEO25 and community strains MW63, MW131, and MW467, have been submitted to the EMBL Nucleotide Sequence Database. Accession numbers for the NSP4 and VP4 nucleotide sequences, respectively, for each strain are as follows: for NEO7, AJ427313 and AJ427318; for NEO25, AJ427314 and AJ427319; for MW63, AJ427315 and AJ427320; for MW131, AJ427317 and AJ427322; and for MW467, AJ427316 and AJ427321.
Acknowledgments
We thank the staff of the Chatinkha Nursery, QECH, for their cooperation with this study.
This work received financial support from the Wellcome Trust of Great Britain (grant number 049485/Z/96) and forms part of the Malawi-Liverpool-Wellcome Trust Programme of Research in Clinical Tropical Medicine.
REFERENCES
- 1.Barnes, G. L., J. S. Lund, L. Adams, A. Mora, S. V. Mitchell, A. Caples, and R. F. Bishop. 1997. Phase 1 trial of a candidate rotavirus vaccine (RV3) derived from a human neonate. J. Paediatr. Child Health 33:300-304. [DOI] [PubMed] [Google Scholar]
- 2.Bhan, M. K., J. F. Lew, S. Sazawal, B. K. Das, J. R. Gentsch, and R. I. Glass. 1993. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J. Infect. Dis. 168:282-287. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, R. F., G. L. Barnes, E. Cipriani, and J. S. Lund. 1983. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. New Engl. J. Med. 309:72-76. [DOI] [PubMed] [Google Scholar]
- 4.Burke, B., and U. Desselberger. 1996. Rotavirus pathogenicity. Virology 218:299-305. [DOI] [PubMed] [Google Scholar]
- 5.Cicirello, H. G., B. K. Das, A. Gupta, M. K. Bhan, J. R. Gentsch, R. Kumar, and R. I. Glass. 1994. High prevalence of rotavirus infection among neonates born at hospitals in Delhi, India: predisposition of newborns for infection with unusual rotavirus. Pediatr. Infect. Dis. J. 13:720-724. [DOI] [PubMed] [Google Scholar]
- 6.Cunliffe, N. A., J. R. Gentsch, C. D. Kirkwood, J. S. Gondwe, W. Dove, O. Nakagomi, T. Nakagomi, Y. Hoshino, J. S. Bresee, R. I. Glass, M. E. Molyneux, and C. A. Hart. 2000. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology 274:309-320. [DOI] [PubMed] [Google Scholar]
- 7.Cunliffe, N. A., J. S. Gondwe, S. M. Graham, B. D. M. Thindwa, W. Dove, R. L. Broadhead, M. E. Molyneux, and C. A. Hart. 2001. Rotavirus strain diversity in Blantyre, Malawi, from 1997 to 1999. J. Clin. Microbiol. 39:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunliffe, N. A., P. A. Woods, J. P. G. Leite, B. K. Das, M. Ramachandran, M. K. Bhan, C. A. Hart, R. I. Glass, and J. R. Gentsch. 1997. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J. Med. Virol. 53:41-50. [PubMed] [Google Scholar]
- 9.De Moraes-Pinto, M. I., F. Verhoeff, L. Chimsuku, P. J. M. Milligan, L. Wesumperuma, R. L. Broadhead, B. J. Brabin, P. M. Johnson, and C. A. Hart. 1998. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch. Dis. Child. Fetal Neonatal ed. 79:F202-F205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes, M. K., and A. P. Morris. 1999. A viral enterotoxin. A new mechanism of virus-induced pathogenesis. Adv. Exp. Med. Biol. 473:73-82. [PubMed] [Google Scholar]
- 11.Flores, J., K. Midthun, Y. Hoshino, K. Green, M. Gorziglia, A. Z. Kapikian, and R. M. Chanock. 1986. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J. Virol. 60:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentsch, J. R., P. A. Woods, M. Ramachandran, B. K. Das, J. P. Leite, A. Alfieri, R. Kumar, M. K. Bhan, and R. I. Glass. 1996. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J. Infect. Dis. 174:S30-S36. [DOI] [PubMed] [Google Scholar]
- 14.Gorziglia, M., K. Green, K. Nishikawa, K. Taniguchi, R. Jones, A. Z. Kapikian, and R. M. Chanock. 1988. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J. Virol. 62:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino, Y., and A. Z. Kapikian. 2000. Rotavirus serotypes: classification and importance in epidemiology, immunity, and vaccine development. J. Health Popul. Nutr. 18:5-14. [PubMed] [Google Scholar]
- 16.Kapikian, A. Z., and R. M. Chanock. 1996. Rotaviruses, p.1657-1708. In B. N. Fields, D. M. Knipe, and P. M. Howley, (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Press, Philadelphia, Pa.
- 17.Kilgore, P. E., L. E. Unicomb, J. R. Gentsch, M. J. Albert, C. A. McElroy, and R. I. Glass. 1996. Neonatal rotavirus infection in Bangladesh: strain characterization and risk factors for nosocomial infection. Pediatr. Infect. Dis. J. 15:672-677. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood, C. D., B. S. Coulson, and R. F. Bishop. 1996. G3P2 rotaviruses causing diarrhoeal disease in neonates differ in VP4, VP7, and NSP4 sequence from G3P2 strains causing asymptomatic neonatal infection. Arch. Virol. 141:1661-1676. [DOI] [PubMed] [Google Scholar]
- 19.Lee, C. N., Y. L. Wang, C. L. Kao, C. L. Zao, C. Y. Lee, and H. N. Chen. 2000. NSP4 gene analysis of rotaviruses recovered from infected children with and without diarrhea. J. Clin. Microbiol. 38:4471-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pager, C. T., J. J. Alexander, and A. D. Steele. 2000. South African G4P[6] asymptomatic and symptomatic neonatal rotavirus strains differ in their NSP4, VP8* and VP7 genes. J. Med. Virol. 62:208-216. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran, M., A. Vij, R. Kumar, B. K. Das, J. R. Gentsch, M. K. Bhan,and R. I. Glass. 1998. Lack of maternal antibodies to P-serotypes may predispose neonates to infections with unusual rotavirus strains. Clin. Diagn. Lab. Immunol. 5:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos, N., V. Gouvea, M. C. Timenetsky, H. F. Clark, M. Riepenhoff-Talty, and A. Garbarg-Chenon. 1994. Comparative analysis of VP8* sequences from rotaviruses possessing M37-like VP4 recovered from children with and without diarrhoea. J. Gen. Virol. 75:1775-1780. [DOI] [PubMed] [Google Scholar]
- 23.Tam, J. S. L., B. J. Zheng, S. K. Lo, C. Y. Yeung, M. Lo, and M. H. Ng. 1990. Distinct populations of rotaviruses circulating among neonates and older infants. J. Clin. Microbiol. 28:1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vesikari, T., T. Ruuska, H. P. Koivu, K. Y. Green, J. Flores, and A. Z. Kapikian. 1991. Evaluation of the M37 human rotavirus vaccine in 2- to 6-month-old infants. Pediatr. Infect. Dis. J. 10:912-917. [DOI] [PubMed] [Google Scholar]
- 25.Widdowson, M. A., G. J. J. van Doornum, W. H. M. van der Poel, A. S. de Boer, U. Mahdi, and M. Koopmans. 2000. Emerging group-A rotavirus and a nosocomial outbreak of diarrhoea. Lancet 356:1161-1162. [DOI] [PubMed] [Google Scholar]