Abstract
Borrelial protein BBK32 was evaluated as an antigen in the serodiagnosis of early and disseminated Lyme borreliosis (LB). bbk32 was cloned and sequenced from eight isolates of the three pathogenic Borrelia species. The identities between the amino acid sequences of the BBK32 proteins from Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii isolates were 71 to 100%. By immunoglobulin G (IgG) Western blotting (WB) or enzyme-linked immunosorbent assay (ELISA), up to 74 and 100% of acute- and convalescent-phase samples, respectively, from 23 patients with erythema migrans (EM) were positive for recombinant BBK32 protein from B. afzelii. In the serology of disseminated LB, the three variant BBK32 antigens cross-reacted. In total, 14 of 14 samples from patients with neuroborreliosis and 15 of 15 samples from patients with Lyme arthritis were positive. The specificities of the IgG ELISA with the variant BBK32 antigens for EM and disseminated borreliosis were 81 to 92% and 89 to 95%, respectively. Our findings indicate that the BBK32 proteins are promising serodiagnostic antigens for the detection of early and disseminated LB but that variant BBK32 proteins may be needed either in parallel or in combination with an immunoassay for LB to cover all the relevant borrelial species that cause the disease.
Lyme borreliosis (LB) is a tick-transmitted spirochetal infectious disease, caused by Borrelia burgdorferi, which is characterized by multistage skin, joint, neurologic, and cardiac manifestations (29). The diagnosis of LB is based on clinical evaluation of the patients, but serologic assays, most frequently, enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB), are often used to provide supporting evidence of infection with B. burgdorferi. The antigens predominantly used in the present routine LB serodiagnostic tests are borrelial flagellin protein or whole-cell lysate (WCL) of the microbes cultured in vitro. In different laboratories, the performances of serologic assays with these antigens in terms of their specificities and sensitivities are highly variable (26). Especially in LB patients with early manifestations, e.g., erythema migrans (EM) and facial palsy, antibody responses to the antigens used at present may be weak or delayed (22). In Europe, evaluation of the serologic responses is further complicated by the existence of three species of B. burgdorferi sensu lato as causes of human LB: B. burgdorferi sensu stricto, B. afzelii, and B. garinii (6). One of the main reasons for these problems is the antigenic diversity due to variations in the sequences and expression of immunogenic proteins in these different borrelial species (26, 27).
The expression of borrelial proteins also varies at different stages in the life cycle of Borrelia in ticks and in the mammalian hosts. Several genes, e.g., bbk32, bbk50, vls, and ospE and ospF homologs (4, 8, 10, 12, 14, 25, 30-33, 36), have been shown to be selectively expressed in vivo. Moreover, bbk32 expression is detectable in spirochetes during tick feeding even before transmission to the host but not in unfed ticks (15). Thus, it can be hypothesized that if BBK32 were immunogenic, it might be useful as an antigen for the serodiagnosis of early LB. In two previous studies, antibodies to BBK32 were observed in the sera of B. burgdorferi sensu stricto-infected mice and human patients with LB (3, 12).
So far, the immunogenic properties of the BBK32 proteins in B. burgdorferi sensu lato isolates are poorly known. The purpose of the present study was to evaluate BBK32 as an antigen in the serodiagnosis of LB. In order to cover all pathogenic borrelial species that cause human LB, we sequenced and cloned the bbk32 genes from the three pathogenic borrelial species, B. burgdorferi sensu stricto, B. afzelii, and B. garinii. The respective variant BBK32 recombinant proteins were tested for use in LB serology by using serum samples from patients with early- and late-stage LB.
MATERIALS AND METHODS
Bacterial strains.
Finnish borrelial strains were received from the National Public Health Institute, Turku, Finland. B. burgdorferi sensu stricto strain ia was isolated from the cerebrospinal fluid of a Finnish patient with neuroborreliosis (NB). Of the B. afzelii strains tested, strains A91 and 1082 were isolated from skin biopsy samples of Finnish patients with EM and strains 570 and 600 were isolated from ticks. B. garinii strains 40, 46, and 50 were isolated from skin biopsy samples of Finnish patients with EM. The genotypes of culture-positive borreliae were confirmed by sequencing a fragment of the flagellin gene (19). Borreliae were cultivated in Barbour-Stoenner-Kelly medium (Sigma, St. Louis, Mo.) at 33°C in 5% CO2. B. afzelii strain SK1 was used in our in-house ELISA for the detection of antibodies against borrelial WCL. Escherichia coli host cells for cloning and for expression of recombinant proteins were INFαF (Invitrogen, Leek, The Netherlands) and BL21 (Amersham Pharmacia Biotech, Uppsala, Sweden), respectively.
DNA purification.
Borrelial genomic DNA was purified with a Dneasy Tissue Kit (Qiagen, Hilden, Germany). Purified DNA was used in the PCR and cloning experiments. Plasmid DNA was purified with a QIAprep-spin plasmid kit (Qiagen, Hilden, Germany).
PCR and DNA sequencing.
A PCR-based approach was used to amplify and sequence the bbk32 alleles from eight different isolates of B. burgdorferi sensu lato. Primers for bbk32 sequencing were designed on the basis of published bbk32 sequences (Table 1). Several primer pairs were designed and tested to ensure that the entire coding sequence of the bbk32 gene was obtained. To eliminate possible errors caused by Taq polymerase, the two strands for each bbk32 gene were sequenced independently at least twice. For each strain, sequences specific for the regions encoding the mature portion of the BBK32 protein after the cysteine at the site of posttranslational acylation were chosen from the sequences analyzed for use as expression primers. For each borrelial strain, the bbk32 sequences were generated by PCR amplification of B. burgdorferi genomic DNA. Approximately 1 ng of template DNA was used under standard PCR conditions: 30 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min and 30 s with AmpliTaqGold DNA polymerase (Perkin-Elmer, Norwalk, Conn.). The PCR-amplified full-length or partial bbk32 genes were cloned into the pCR 2.1-TOPO plasmid vector (Invitrogen, Groningen, The Netherlands) for sequencing. DNA sequencing was performed at the Core Facility of the Haartman Institute, University of Helsinki, with a DyePrimer (primers T7 and M13Rev) cycle sequencing kit (Applied Biosystems Inc., Foster City, Calif.). Sequencing reactions were run and analyzed with an automated sequencing apparatus (model 373A; Applied Biosystems Inc.). DNA and protein sequences were analyzed with Lasergene software (DNASTAR, Madison, Wis.).
TABLE 1.
Primers used for PCR amplification of the bbk32 genes
| Primer no. a | Species | Primer sequence (5′−3′)b | Location | Source (GenBank accession no.) |
|---|---|---|---|---|
| 1 | B. burgdorferi sensu stricto | CAC CCT CTT GAT AGC ACT TA | −203-−184 | B31(AF000788) |
| 2 | CTT TAA AGG AGA GAA AGC ATG | −18-3 | B. burgdorferi sensu stricto ia (AF472532) | |
| 3 | CCG GAT CCG ATT TAT TCA TAA GAT ATG AAA T | 60-82 | B. burgdorferi sensu stricto ia | |
| 4 | GCA ATC TGA GAC TAG AAA AG | 329-348 | B. burgdorferi sensu stricto ia | |
| 5 | TGC AGT CTT TAC ACT TAC TT | 879-860 | B. burgdorferi sensu stricto ia | |
| 6 | CCC TCG AGA TTA GTA CCA AAC GCC ATT | 1084-1065 | B. burgdorferi sensu stricto ia | |
| 7 | ACA TAT TAT GTA GCC TGT TTT A | 1122-1101 | B31 | |
| 2 | B. garinii | CTT TAA AGG AGA GAA AGC ATG | −18-3 | B. burgdorferi sensu stricto ia |
| 3 | CCG GAT CCG ATT TAT TCA TAA GAT ATG AAA T | 60-82 | B. garinii 40 (AF472529) | |
| 4 | GCA ATC TGA GAC TAG AAA AG | 329-348 | B. garinii 40 | |
| 5 | TGC AGT CTT TAC ACT TAC TT | 879-860 | B. garinii 40 | |
| 8 | CCC TCG AGA GTA CCA AAT GCC ATT CT | 1084-1064 | B. garinii 40 | |
| 7 | ACA TAT TAT GTA GCC TGT TTT A | 1122-1101 | B31 | |
| 2 | B. afzelii | CTT TAA AGG AGA GAA AGC ATG | −18-3 | B. burgdorferi sensu stricto ia (AF472532) |
| 9 | CCG GAT CCG ATT TAT TCA TAA GAG ATG AAA T | 57-79 | B. afzelii A91 (AF472525) | |
| 10 | TGA GCA TAA AAG GAT GCT TC | 369-387 | B. afzelii A91 | |
| 11 | GCA GTC CTT GCA CTC ACT | 855-838 | B. afzelii A91 | |
| 12 | CCC TCG AGC AAA GAT TAG TAC CAA ACA C | 1065-1046 | B. afzelii A91 | |
| 7 | ACA TAT TAT GTA GCC TGT TTT A | 1122-1101 | B31 |
Primers 2 and 7 were used for all strains. Primers 3, 4, and 5 were used for both B. burgdorferi sensu stricto and B. garinii PCR amplifications.
Restriction enzyme sites for BamHI and XhoI in the expression primers are underlined.
Cloning and expression of recombinant BBK32.
Glutathione S-transferase (GST) fusion protein constructs were generated for expression of recombinant BBK32 (rBBK32). The PCR-amplified DNA encoding the mature portion of BBK32 was cloned into the pCR 2.1-TOPO plasmid (Invitrogen). The recombinant plasmid was purified and digested with restriction enzymes BamHI and XhoI. The cleaved bbk32 was then ligated to a similarly digested pGEX-4T-1 expression plasmid (Amersham Pharmacia Biotech) and was transformed into E. coli BL21 host cells. The GST-rBBK32 fusion protein was expressed according to the instructions of the manufacturer (Amersham Pharmacia Biotech). The expression and purity of the GST-rBBK32 fusion protein were confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
WB.
GST-rBBK32 fusion proteins originating from B. burgdorferi sensu stricto ia, B. afzelii A91, and B. garinii 40 (referred to here as rBBK32Bbia, rBBK32BaA91, and rBBK32Bg40, respectively) were fractionated by SDS-PAGE in 10% polyacrylamide gels and transferred to a nitrocellulose membrane (pore size, 0.2 μm; Bio-Rad Laboratories, Richmond, Calif.) by semidry transfer with 40 mM glycine-50 mM Tris (pH 9.0)-0.375% (wt/vol) SDS-20% (vol/vol) methanol buffer. Equal amounts of each GST-rBBK32 fusion protein were used on one 7-cm-wide nitrocellulose membrane. Two-millimeter strips of the nitrocellulose membranes were soaked in 0.1% Tween 20-0.9% NaCl. Serum samples were diluted in 0.1% Tween 20-0.9% NaCl-0.1 g of fat-free bovine milk powder (Valio, Helsinki, Finland) per liter. Samples were incubated at 1:100 dilutions for 2 h. After four rinses with buffer, the Western blots were incubated with alkaline phosphatase-conjugated rabbit anti-human immunoglobulin G (IgG; Jackson Immuno Research Laboratories Inc., West Grove, Pa.) at a dilution of 1:5,000 for 2 h. After washing of the blots, the bands were visualized with 5-bromo-4-chloro-3-indolylphosphate and Nitro Blue Tetrazolium (Sigma Chemical Co.). The reaction was terminated 10 to 15 min later by washing with distilled water. The WB strips were scanned with an Agfa Arcus II scanner and Adobe Photoshop software (Adobe Systems Inc., San Jose, Calif.) and were then analyzed with MacBAS 2.5 (Fuji) software. The cutoff for a positive IgG WB result was defined as the mean plus 3 standard deviations (SDs) of the values for healthy blood donors. For detection of GST, monoclonal anti-GST antibodies (Sigma Chemical Co.) were used.
ELISA.
ELISAs for antiflagellin antibodies were done as described earlier (28). Briefly, IgG antibodies against B. burgdorferi were measured with a commercial flagellin-based ELISA kit (Dako, Glostrup, Denmark) modified by titrating the antibodies. Sera were serially diluted threefold for the test and applied to the plates for overnight incubation. The bound antibodies were detected with biotin-labeled goat anti-human IgG (Zymed, Los Angeles, Calif.). The optical density (OD) of the endpoint titer was obtained with a cutoff control provided with the kit. The limit for a positive IgG antibody titer was 500. The cutoff value obtained with the control material was the mean plus 3 SDs, which conformed to the level for the reference population living in central Finland, an area with a low prevalence of LB (28).
For ELISAs that measured anti-BBK32 antibody titers, the wells in a microtiter plate were coated with 100 μl (2 μg/ml) of variant rBBK32 proteins overnight. After the plates were washed, 100 μl of each of the diluted serum samples was added to the wells and the plates were incubated overnight. Serum samples were diluted 1:10 (for sera from patients with EM) or 1:100 (for sera from patients with NB and Lyme arthritis [LA]) in 5 mg of bovine serum albumin (BSA) per ml in 0.155 M NaCl-0.04% Tween 20 buffer (BSA-NaCl-Tween). After the plates were washed, alkaline phosphatase-conjugated rabbit anti-human IgG or IgM (Jackson Immuno Research Laboratories Inc.) diluted 1:5,000 in BSA-NaCl-Tween was added to the wells and the plates were incubated for 2 h. The reactions were visualized with 4-nitrophenylphosphate (1 mg/ml; Boehringer Mannheim GmbH, Mannheim, Germany) in diethanolamine buffer (pH 10.0). The OD measurements were made after 10 to 20 min at a wavelength of 405 nm with a Multiscan photometer (Thermo Labsystems, Helsinki, Finland). The cutoff for a positive ELISA result was defined as the mean values for healthy blood donors plus 3 SDs.
Patient samples.
For serological analyses, human serum samples were collected from patients with culture- or PCR-positive EM, NB, or LA. Samples were collected from EM patients at the time of diagnosis (acute phase) and 1 to 3 months after treatment (convalescent phase). Genotyping of the organisms from 23 patients with EM by PCR analysis (19) showed that B. afzelii was present in 17 of the skin biopsy specimens and that B. garinii was present in 4 of the skin biopsy specimens. Genotyping of the organism in two biopsy specimens was not feasible. In the patients with disseminated LB, the clinical manifestations agreed with the criteria of the Centers for Disease Control and Prevention for LB (35). The clinical diagnosis was confirmed by ELISA by the demonstration in serum of antibodies against flagellin and B. burgdorferi WCL and by the demonstration of antiflagellin antibodies in the cerebrospinal fluid of NB patients. Serum samples from patients with syphilis, Epstein-Barr virus (EBV) infection, systemic lupus erythematosus, rheumatoid factor (RF) positivity, antistreptolysin positivity, and healthy blood donors were used as controls.
Statistical analyses.
Excel 2000 software (Microsoft, Redmond, Wash.) was used for calculations of standard statistics.
Nucleotide sequence accession numbers.
The nucleotide sequences of the bbk32 genes were submitted to GenBank under accession numbers AF472525 for B. afzelii A91, AF472527 for B. afzelii 1082, AF472526 for B. afzelii 570, AF472528 for B. afzelii 600, AF472529 for B. garinii 40, AF472530 for B. garinii 46, AF472531 for B. garinii 50, and AF472532 for B. burgdorferi sensu stricto ia.
RESULTS
Sequence analysis of bbk32 in Finnish borrelial isolates.
The open reading frames of the bbk32 sequences analyzed from the presumed start codon (ATG) consisted of 1,059 to 1,083 nucleotides. At the nucleotide level, the identities of the bbk32 sequences between borrelial subspecies were 82 to 100%. The sequences of B. burgdorferi sensu stricto and B. garinii were 96 to 100% identical. The bbk32 sequences of four B. afzelii isolates all had eight codon deletions. Seventy to 76% of the polymorphisms between subspecies putatively affect amino acid sequences.
Analysis of deduced amino acid sequences of BBK32 in the Finnish borrelial isolates.
The deduced amino acid sequences of the BBK32 proteins from B. afzelii A91, B. afzelii 1082, B. afzelii 570, B. afzelii 600, B. garinii 40, B. garinii 46, B. garinii 50, and B. burgdorferi sensu stricto ia contained 352 to 360 residues. The sequences of all the BBK32 proteins revealed a hydrophobic leader sequence of 18 to 19 residues and a phenylalanine-isoleucine-serine-cysteine motif, consistent with the signal peptidase site commonly seen in prokaryotic lipoproteins. Characteristically for borrelial lipoproteins, the greater part of the mature portion of the BBK32 protein was hydrophilic (data not shown). The BBK32 leader sequences in the B. garinii strains and the B. burgdorferi sensu stricto strain were identical but differed by 3 amino acids from the leader sequences in the identical B. afzelii strains. The interspecies identities of the deduced amino acid sequences of the BBK32 proteins ranged from 71 to 95% (Table 2). The differences in the amino acid sequences were distributed evenly along the sequence. The identities of the BBK32 amino acid sequences within the borrelial subspecies ranged from 94 to 100%. The calculated molecular sizes of the mature BBK32 proteins (without putative lipid acylation) ranged from 38.7 to 39.5 kDa.
TABLE 2.
Identities of deduced amino acid sequences of BBK32 among the Finnish isolates of B. burgdorferi sensu stricto; B. garinii 40, 46, and 50; and B. afzelii A91, 1082, 570, and 600
| Strain | % Identitya
|
||||||
|---|---|---|---|---|---|---|---|
| B. garinii 40 | B. garinii 46 | B. garinii 50 | B. afzelii A91 | B. afzelii 1082 | B. afzelii 570 | B. afzelii 600 | |
| B. burgdorferi sensu stricto ia | 95.3 | 92.5 | 95.3 | 73.1 | 73.1 | 72.8 | 73.1 |
| B. garinii 40 | 93.9 | 100 | 71.7 | 71.7 | 71.4 | 71.7 | |
| B. garinii 46 | 93.9 | 72.2 | 72.2 | 72.5 | 72.8 | ||
| B. garinii 50 | 71.7 | 71.7 | 71.4 | 71.7 | |||
| B. afzelii A91 | 100 | 99.2 | 99.4 | ||||
| B. afzelii 1082 | 99.2 | 99.4 | |||||
| B. afzelii 570 | 99.7 | ||||||
The identities were calculated from the sequences of the entire proteins including the leader peptides by multiple-sequence alignment by the Jotun Hein method with Lasergene software.
Sequence analysis of BBK32 in B. afzelii strains.
We sequenced the bbk32 genes from two human isolates (B. afzelii A91 and 1082) and two tick isolates (B. afzelii 570 and 600). The identities of the deduced amino acid sequences were from 99 to 100%. The BBK32 sequences of the two human B. afzelii isolates were identical. In a search of the sequences in GenBank, one B. afzelii bbk32 sequence was found. The BBK32 sequence of the ACA1 strain (GenBank accession number AF213179) is only partial and corresponds to the sequence between amino acid positions 34 and 336 of the deduced sequence of the BBK32 protein of B. afzelii A91. The identities of the matching regions of the four BBK32 sequences from B. afzelii studied and the partial sequence of BBK32 from strain ACA1 ranged from 99 to 100%.
Sequence analysis of BBK32 proteins from B. garinii strains.
The deduced amino acid sequences of the BBK32 proteins from B. garinii 40 and 50 were identical, and the sequence of the BBK32 protein from B. garinii 46 was 94% identical to sequences from strains 40 and 50. In a search of the sequences in GenBank, one partial bbk32 sequence from B. garinii strain Ip90 (AF213178) was found. This sequence matched the sequence from B. garinii 40 between amino acid residues 35 and 343, but in the Ip90 sequence, a 6-amino-acid deletion was observed from positions 201 to 206. The sequence identities of the corresponding regions of the BBK32 sequences from the B. garinii strains evaluated in this study and the partial sequence of B. garinii Ip90 ranged from 92 to 93%.
Sequence analysis of BBK32 in B. burgdorferi sensu stricto strains.
The bbk32 sequence of local strain B. burgdorferi sensu stricto ia was compared with two sequences published in GenBank. The bbk32 sequence of the B31 strain (GenBank accession number AE000788) was complete, and that of strain N40 (GenBank accession number U82107) was a partial sequence, as it lacked the first 86 amino acids. The BBK32 amino acid sequences from B. burgdorferi sensu stricto ia and B31 were 96% identical. The identities in the corresponding regions of the BBK32 sequences of B. burgdorferi sensu stricto ia, B31, and N40 ranged from 91 to 94%. In the B31 BBK32 sequence there was a 6-amino-acid deletion at positions 201 to 206 of the B. burgdorferi sensu stricto ia BBK32 sequence. In the same region, three tyrosine residues were deleted from the N40 BBK32 sequence.
WB.
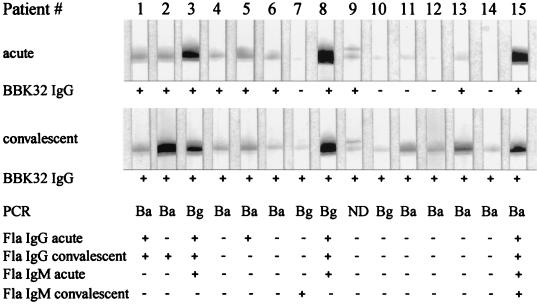
WB assays with rBBK32 proteins as the antigens were done as preliminary serologic analyses. In IgG Western blots with rBBK32 from B. afzelii A91 as the antigen with serum samples from patients with culture- or PCR-positive EM, 10 of 15 (67%) samples from patients in the acute phase and 15 of 15 (100%) samples from patients in the convalescent phase were positive (Fig. 1). When rBBK32Bg40 was used as the antigen, 4 of 15 and 8 of 15 samples from patients in the acute and convalescent phases were positive, respectively; and when rBBK32Bbia was used as the antigen, 7 of 15 and 3 of 15 samples from patients in the acute and convalescent phases were positive, respectively. Of the 10 control patients (5 patients with syphilis and 5 healthy blood donors), 1 patient with syphilis had a weak antibody response to rBBK32BaA91 (data not shown). For comparative purposes, the same serum samples were tested by a commercial ELISA for antiflagellin antibodies (Dako). Six of the 15 (40%) patients in the acute or convalescent phase had IgG or IgM antibodies (Fig. 1). One patient had IgM antibodies by our in-house ELISA (with WCL as the antigen).
FIG. 1.
Evaluation of sensitivity of BBK32 detection by IgG WB for serodiagnosis of early LB. Serum samples were collected from culture- or PCR-positive patients with EM at the time of diagnosis (acute phase) and 1 to 3 months after antibiotic treatment (convalescent phase). Immunoreactivity was assessed by densitometric measurements with an Agfa Arcus II scanner and MacBas (version 2.5) software. The cutoff value for a positive WB result was defined as the mean value for healthy blood donors plus 3 SDs. In the IgM and IgG antiflagellin (Fla) ELISA (Dako), the cutoff value was based on the mean OD for healthy controls plus 3 SDs. Ba, B. afzelii; Bg, B. garinii; ND, not performed; + and −, positive and negative WB or ELISA results, respectively.
In another WB experiment, serum samples from 10 patients with NB and 10 patients with LA were analyzed for the presence of anti-BBK32 IgG antibodies. All 20 patients with disseminated LB reacted positively with the three rBBK32 proteins, as determined with the MacBAS program, by using the mean for the blood donors plus 3 SDs as the cutoff value (Table 3). Minor differences in the immunoreactivities of the serum samples against different rBBK32 proteins were observed. Specifically, for four serum samples, weaker reactivities against rBBK32BaA91 than against the other rBBK32 proteins were observed (data not shown). A few of the control samples had low-positive values (Table 3). None of the patient or control serum samples recognized pure GST (data not shown).
TABLE 3.
IgG WB reactivity against recombinant BBK32 proteins from B. afzelii A91, B. garinii 40, and B. burgdorferi sensu stricto ia from LB patients and controlsa
| Patient or control group | No. of positive samples/no. of samples tested with rBBK32 from:
|
||
|---|---|---|---|
| B. afzelii A91 | recombinant BBK32 B. garinii 40 | B. burgdorferi sensu stricto ia | |
| NB patients | 10/10 | 10/10 | 10/10 |
| LA patients | 10/10 | 10/10 | 10/10 |
| Syphilis patients | 1/5 | 0/5 | 2/5 |
| Patients positive for RF | 0/5 | 0/5 | 1/5 |
| Healthy blood donors | 0/5 | 0/5 | 0/5 |
The intensities of the WB bands were analyzed with MacBas 2.5 software.
ELISA.
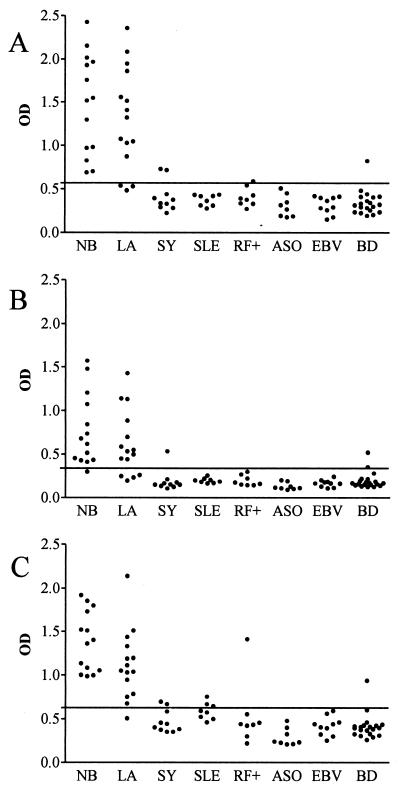
Serum samples from LB patients and controls were tested by an IgG ELISA with each of the three rBBK32 proteins as the antigens. In patients with NB, 14 of 14, 13 of 14, and 14 of 14 samples were positive when rBBK32BaA91, rBBK32Bg40, and rBBK32Bbia were used as the antigens, respectively. Among the serum samples from patients with LA, the proportions were 12 of 15, 11 of 15, and 14 of 15 serum samples, respectively. In total, 14 of 14 (100%) samples from patients with NB and 15 of 15 (100%) samples from patients with LA were positive for one or more rBBK32 proteins (Fig. 2). The ELISA OD values for NB patients correlated well between assays with variant rBBK32 proteins as the antigens, with the correlation coefficients being 0.91, 0.78, and 0.83 between assays with rBBK32 proteins from B. afzelii A91 and B. garinii 40, B. afzelii A91 and B. burgdorferi sensu stricto ia, and B. garinii 40 and B. burgdorferi sensu stricto ia, respectively. In LA patients, the respective correlation coefficients were 0.89, 0.90, and 0.93. Among the control patients, 4 of 64 (6%), 3 of 64 (5%), and 7 of 64 (11%) were positive when rBBK32BaA91, rBBK32Bg40, and rBBK32Bbia were used as the antigens, respectively (Fig. 2). Accordingly, the calculated specificities were 94, 95, and 89%, respectively.
FIG. 2.
OD values obtained by IgG ELISA with rBBK32 proteins from B. afzelii A91 (A), B. garinii 40 (B), and B. burgdorferi sensu stricto ia (C) as the antigens and serum samples from patients with NB or LA. Control samples were from patients with syphilis (SY), systemic lupus erythematosus (SLE), EBV infection, positivity for rheumatoid factor (RF+), positivity for antistreptolysin (ASO), and from healthy blood donors (BD). The cutoff level (mean for samples from healthy blood donors plus 3 SDs) is indicated with lines.
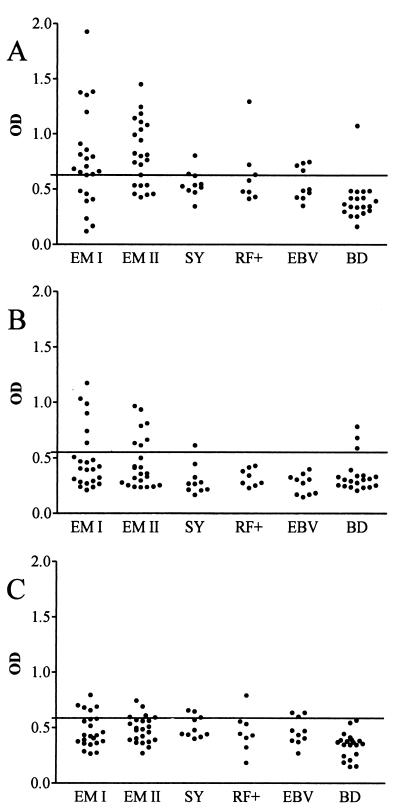
Serum samples from patients with EM were analyzed by both IgG and IgM ELISAs. In the IgG ELISA with rBBK32BaA91 as the antigen, 17 of 23 (74%) samples obtained at the acute phase and 15 of 23 (65%) samples obtained at the convalescent phase were positive (Fig. 3). On the other hand, when rBBK32Bg40 and rBBK32Bbia were used as the antigens, 6 of 23 (26%) and 5 of 23 (22%) of the acute-phase samples and 7 of 23 (30%) and 4 of 23 (17%) of the convalescent samples were positive, respectively. Of the control samples, 2 of 10 samples from patients with syphilis, 2 of 8 samples from patients with RF positivity, 4 of 10 samples from patients positive for EBV, and 3 of 20 samples from healthy blood donors samples showed low-positive OD values (Fig. 3). At the acute phase of EM, the calculated specificities of the IgG ELISAs with rBBK32BaA91, rBBK32Bg40, and rBBK32Bbia as the antigens were 81, 92, and 88%, respectively. In the IgM ELISA with samples from EM patients, 4 to 13% of the acute- or convalescent-phase samples were positive, depending on the rBBK32 antigen used.
FIG. 3.
OD values obtained by IgG ELISA with rBBK32 proteins from B. afzelii A91 (A), B. garinii 40 (B), and B. burgdorferi sensu stricto ia (C) as the antigens and serum samples from EM patients at the acute phase (EM I) and the convalescent phase (EM II). Control samples were from patients with syphilis (SY), EBV infection, positivity for rheumatoid factor (RF+), and healthy blood donors (BD). The cutoff level (mean for samples from healthy blood donors plus 3 SDs) is indicated with lines.
In the ELISAs for antiflagellin antibodies, 6 of 23 (26%) patients with EM had IgG antibodies in either acute- or convalescent-phase samples. In the IgM ELISAs for antiflagellin antibodies, 4 of 23 (17%) were positive. All serum samples positive for antiflagellin antibodies were positive by at least one of the antibody tests for rBBK32.
DISCUSSION
Serologic confirmation of early LB has been problematic because of the sensitivity problems of the current serodiagnostic assays. Compared to those assays, in the present study antibodies to BBK32, an immunogenic borrelial protein, were detected in an unprecedentedly high proportion of patients with early EM. Furthermore, by the IgG ELISA, up to 100% of patients with disseminated LB were positive for BBK32 antigens. The present findings confirm the previously published results on the antigenicity of BBK32 of B. burgdorferi sensu stricto (3, 12). The BBK32 protein has potential as a target for the serodiagnosis of both early and late LB.
Antibodies to the BBK32 protein seem to arise very early during human LB. In up to 74% of the patients with acute-phase EM, IgG antibodies to BBK32 were detectable by ELISA and/or WB. A recent study reported cloning of bbk32 from an American B. burgdorferi sensu stricto strain and provided preliminary results on the antibody responses to the rBBK32 protein during experimental murine borreliosis and in patients with LB (12). Reverse transcriptase PCR studies have also demonstrated bbk32 expression in the EM lesions of three patients, indicating that, during human LB, bbk32 is expressed early (13). However, antibody responses to BBK32 in patients with early local EM have not previously been studied in detail.
With the current LB serology, based mainly on flagellin or WCL as the antigen, IgM and IgG antibodies are not detectable by ELISA or immunoblot assays until 2 to 4 or 6 to 8 weeks after the onset of the disease (9, 22). During early local LB, the sensitivity of the IgM ELISA seldom exceeds 50% (1, 11, 16, 22). A study with patients with culture-confirmed EM showed that positive serology at presentation and the rate of seroconversion correlated directly with disease duration (2). If the EM lesion emerged less than 7 days prior to sampling, only 10% of the patients showed antibodies by ELISA; on the other hand, among the patients whose EM lesions occurred 7 to 14 days earlier, 58% had detectable antibodies. In the present series, the time of occurrence of the EM lesion could not be accurately assessed. However, given the low proportion of seropositivity for flagellin at the time of presentation with EM and the broad awareness of LB among the general population in regions in Finland where LB is endemic, it can be presumed that, in most cases, the EM lesions represented early disease. In an early exposure to borreliae, the expected IgM isotype antibodies could be detected only infrequently. The reason for this is unknown, but the low sensitivity of IgM serology has also been associated with most of the other new recombinant borrelial antigens tested (17, 20, 21, 24). Hence, the present results imply that assessments for IgG antibodies, although not IgM antibodies, to BBK32 proteins may afford a major improvement in the serodiagnosis of early LB. The antibody response to rBBK32 also seems to precede the humoral response to microbes (WCLs) grown in vitro, which do not necessarily synthesize this protein expressed in vivo (12, 25). There is ample evidence that the borrelial proteins expressed in vivo (4, 8, 10, 12, 14, 25, 30-33, 36) may be involved in the pathogenesis of LB. Our results suggest that application of such proteins to LB serology might also be beneficial for improving the sensitivity of the assay.
Only a few studies have evaluated the immunogenic properties of the BBK32 protein. In those studies, the BBK32 proteins originated from American B. burgdorferi sensu stricto strains (3, 12, 13). Our study expands the knowledge of BBK32 proteins by using variant recombinant proteins from the three pathogenic borrelial species, B. burgdorferi sensu stricto, B. afzelii, and B. garinii, in the serodiagnosis of LB. Recombinant BBK32 originating from a local B. afzelii isolate appeared to be superior to the other rBBK32 proteins for the diagnosis of EM. This finding agrees with the PCR results obtained with the EM skin biopsy specimens, in which the majority of the infecting species proved to be B. afzelii. These observations are also in accord with two recent European studies, in which over 90% of the Borrelia isolates from EM lesions were B. afzelii, less than 10% were B. garinii, and none were B. burgdorferi sensu stricto (5, 23).
Although the immunoreactivities of sera from patients with EM to variant BBK32 proteins diverged, the three rBBK32 antigens cross-reacted in the serologic analysis of disseminated LB. Furthermore, the intensities of the serologic responses against variant BBK32 proteins, as measured by determination of OD values by ELISA, correlated well. Therefore, our results indicate that variant BBK32 proteins may have both individual and common antigenic epitopes. In European epidemiological studies, the most prevalent Borrelia species have been B. afzelii and B. garinii (34), with B. burgdorferi sensu stricto occurring infrequently, especially in Scandinavia (19, 23). The species predominantly isolated from cerebrospinal fluid samples from European patients with NB has been B. garinii (7, 18). The hypothesis that epitope specificity varies in early and late LB is in line with an analysis of the BBK32 sequences of eight Finnish isolates of B. burgdorferi sensu lato, which showed that the sequences of B. burgdorferi sensu stricto and B. garinii have over 90% identity. In contrast, the identity between the BBK32 sequences of B. afzelii strains and those of other species was approximately 70%.
In the serodiagnosis of disseminated LB, the BBK32 antigen has so far been evaluated with only a limited number of patient samples (3, 12). Fikrig et al. (12) reported high titers of IgG antibodies to BBK32 in three of three patients with NB and three of seven patients with LA. Akin et al. (3) showed IgG responses to BBK32 in 83 to 92% of 25 patients with LA. In that study, they also reported IgG BBK32 antibodies in 84% of patients with EM. However, in those patients, the EM lesion had been present for from 2 weeks to 3 months after onset of the disease, suggesting dissemination of LB. In patients with disseminated borreliosis, our approach, in which we used the three variant BBK32 proteins as the antigens in parallel, improved the sensitivity of the ELISA to 100%, with specificities of 89 to 95%. In the serodiagnosis of NB, all but one case would have been detected, irrespective of the origin of the BBK32 antigen. Instead, especially in an ELISA for LA, use of a single BBK32 antigen would have left the sensitivity at 73 to 93%. The occasional discrepancies between the results of WB and ELISA may be due to differences in the orientations of the antigenic epitopes and/or antigen-antibody complex formation. Optimization of the antigen-antibody conditions or use of the three variant BBK32 proteins in combination may ensure that serological assays have nearly 100% sensitivity without compromising their specificities.
In summary, we have demonstrated that the BBK32 proteins are potential antigens for both early and late LB serology. Future studies are aimed at epitope mapping and characterization of the specificities of antigenic epitopes. At present, it is evident that variant BBK32 proteins should be used either in parallel or in combination in an immunoassay for LB to cover all the relevant borrelial species, whose prevalences differ regionally in Europe.
Acknowledgments
This study was supported by the Foundation for Pediatric Research, Helsinki, Finland, the National Technology Agency (TEKES), Helsinki, Finland, and the Helsinki Central Hospital Research Funds, Helsinki, Finland.
We thank Matti Viljanen, the National Public Health Institute, Turku, Finland, for donating the B. burgdorferi strains used in this study. The English language was checked by Jean Margaret Perttunen.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Nowakowski, D. F. McKenna, C. A. Carbonaro, and G. P. Wormser. 1993. Serodiagnosis in early Lyme disease. J. Clin. Microbiol. 31:3090-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol. Microbiol. 18:507-520. [DOI] [PubMed] [Google Scholar]
- 5.Arnez, M., E. Ruzic-Sabljic, J. Ahcan, A. Radsel-Medvescek, D. Pleterski-Rigler, and F. Strle. 2001. Isolation of Borrelia burgdorferi sensu lato from blood of children with solitary erythema migrans. Pediatr. Infect. Dis. J. 20:251-255. [DOI] [PubMed] [Google Scholar]
- 6.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 7.Busch, U., C. Hizo-Teufel, R. Boehmer, V. Fingerle, H. Nitschko, B. Wilske, and V. Preac-Mursic. 1996. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulse-field gel electrophoresis and PCR. J. Clin. Microbiol. 34:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion, C. I., D. R. Blanco, J. T. Skare, D. A. Haake, M. Giladi, D. Foley, J. N. Miller, and M. A. Lovett. 1994. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect. Immun. 62:2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craft, J. E., R. L. Grodzicki, and A. C. Steere. 1984. Antibody response in Lyme disease: evaluation of diagnostic tests. J. Infect. Dis. 149:789-795. [DOI] [PubMed] [Google Scholar]
- 10.Das, S., S. W. Barthold, S. Stocker Giles, R. R. Montgomery, S. R. Telford III, and E. Fikrig. 1997. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J. Clin. Investig. 99:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford III, and R. A. Flavell. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 13.Fikrig, E., W. Feng, J. Aversa, R. T. Schoen, and R. A. Flavell. 1998. Differential expression of Borrelia burgdorferi genes during erythema migrans and Lyme arthritis. J. Infect. Dis. 178:1198-1201. [DOI] [PubMed] [Google Scholar]
- 14.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 15.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 16.Grodzicki, R. L., and A. C. Steere. 1988. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J. Infect. Dis. 157:790-797. [DOI] [PubMed] [Google Scholar]
- 17.Heikkilä, T., I. Seppälä, H. Saxen, J. Panelius, H. Yrjänäinen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis between Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii with decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubalek, Z., and J. Halouzka. 1997. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 13:951-957. [DOI] [PubMed] [Google Scholar]
- 19.Junttila, J., M. Peltomaa, H. Soini, M. Marjamäki, and M. K. Viljanen. 1999. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J. Clin. Microbiol. 37:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnarelli, L. A., E. Fikrig, S. J. Padula, J. F. Anderson, and R. A. Flavell. 1996. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J. Clin. Microbiol. 34:237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell, P. D., K. D. Reed, T. L. Aspeslet, M. F. Vandermause, and J. W. Melski. 1994. Comparison of four immunoserologic assays for detection of antibodies to Borrelia burgdorferi in patients with culture-positive erythema migrans. J. Clin. Microbiol. 32:1958-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ornstein, K., J. Berglund, I. Nilsson, R. Norrby, and S. Bergström. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J. Clin. Microbiol. 39:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panelius, J., P. Lahdenne, H. Saxen, T. Heikkilä, and I. Seppälä. 2001. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 39:4013-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 26.Robertson, J., E. Guy, N. Andrews, B. Wilske, P. Anda, M. Granström, U. Hauser, Y. Moosmann, V. Sambri, J. Schellenkens, G. Stanek, and J. Gray. 2000. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 38:2097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roessler, D., U. Hauser, and B. Wilske. 1997. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 35:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seppälä, I. J. T., R. Kroneld, K. Schauman, K. O. Forsen, and R. Lassenius. 1994. Diagnosis of Lyme borreliosis: non-specific serological reactions with Borrelia burgdorferi sonicate antigen caused by IgG2 antibodies. J. Med. Microbiol. 40:293-302. [DOI] [PubMed] [Google Scholar]
- 29.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson, B., J. L. Bono, T. G. Schwan, and P. A. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallich, R., C. Brenner, M. D. Kramer, and M. M. Simon. 1995. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect. Immun. 63:3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiologic, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wharton, M., T. L. Chorba, R. L. Vogt, D. L. Morse, and J. W. Buehler. 1990. Case definitions for public health surveillance. Morb. Mortal. Wkly. Rep. 39:19-21. [PubMed] [Google Scholar]
- 36.Zhang, J. R., J. M. Hardman, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombinant VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]