Abstract
We developed a TaqMan-based real-time PCR assay for quantifying Mycoplasma genitalium. This assay is able to specifically quantify concentrations of the M. genitalium 16S rRNA gene ranging from 107 to 10 copies/reaction. Using the TaqMan assay, we quantified the M. genitalium 16S rRNA gene in first-pass urine of men with urethritis and asymptomatic men who were positive for M. genitalium by PCR- and phylogeny-based assay. Of 130 men with gonococcal urethritis (GU), five were positive for M. genitalium. The mycoplasma load for each specimen was <5 × 10 copies/ml. Of 84 men with chlamydial non-GU (CNGU), seven were positive for M. genitalium. One man had an M. genitalium load of <5 × 10 copies/ml, and six men had loads ranging from 1.1 × 107 to 2.7 × 102 copies/ml. Of 86 men with nonchlamydial NGU (NCNGU), 17 were positive for M. genitalium. The mycoplasma loads for these men ranged from 3.3 × 106 to 2.3 × 102 copies/ml. Of 76 asymptomatic men, only two were positive for M. genitalium. For these men, the loads were 2 × 102 and <5 × 10 copies/ml. The patients with NGU had significantly higher concentrations of M. genitalium in their first-pass urine than did men with GU (P < 0.01) or asymptomatic men (P < 0.05). In addition, M. genitalium loads were significantly higher in men with NCNGU than those in asymptomatic men (P < 0.05). The quantitative assessment of M. genitalium loads by the TaqMan assay will provide useful information for understanding the pathogenicity of this mycoplasma in the urogenital tract.
Mycoplasma genitalium was isolated in urethral cultures from two men with nongonococcal urethritis (NGU) in 1981 (21). Although M. genitalium had been proposed as a cause of human NGU (22), the precise role of the mycoplasma in the etiology of NGU had not been established because of the immense difficulty in isolating it from clinical samples. Since PCR-based assays facilitated the detection of M. genitalium in clinical specimens (10, 17), a significant association between M. genitalium and NGU has been demonstrated (7, 9, 13, 20). So far, however, any studies to investigate the potential association of M. genitalium loads with the pathogenicity of the mycoplasma in the urogenital tract have not been performed, because isolation of M. genitalium in culture is still difficult and because conventional PCR-based assays are lacking in quantitative assessment of the mycoplasma in clinical specimens. For this study, therefore, we developed a TaqMan-based real-time PCR assay for quantifying M. genitalium. Using this assay, we quantified M. genitalium DNA in first-pass urine of men with urethritis and asymptomatic men and assessed whether the bacterial load of M. genitalium might be associated with the pathogenicity of the mycoplasma in the urogenital tract.
MATERIALS AND METHODS
Bacterial strains.
Strains of 15 species of human mycoplasmas and ureaplasmas, including Mycoplasma buccale, Mycoplasma faucium, Mycoplasma fermentans, M. genitalium, Mycoplasma hominis, Mycoplasma lipophilum, Mycoplasma orale, Mycoplasma penetrans, Mycoplasma pirum, Mycoplasma pneumoniae, Mycoplasma primatum, Mycoplasma salivarium, Mycoplasma spermatophilum, Ureaplasma parvum, and Ureaplasma urealyticum, were obtained from the National Institute of Infectious Diseases, Tokyo, Japan, or from the American Type Culture Collection, Manassas, Va. These strains were directly used for DNA extraction without further propagation.
First-pass urine specimens.
Three hundred male patients with urethritis and 76 asymptomatic men who attended the Department of Urology, Toyota Memorial Hospital, Toyota, Japan, between July 1999 and May 2001 were enrolled. They provided informed consent for their participation in this study. All 300 patients had symptoms and signs compatible with acute urethritis. In the gram-stained urethral smears for these patients, five or more polymorphonuclear leukocytes per high-power (×1,000) microscopic field were observed at least on three fields. All 76 asymptomatic men, who had no symptoms and signs of urethritis, visited the clinic for evaluation of sexually transmitted diseases. For these men, no polymorphonuclear leukocytes were observed on the urethral smears. Approximately 20 ml of first-pass urine was obtained from each man. All urine specimens were subjected to the AMPLICOR STD-1 assay (Roche Diagnostics Systems, Indianapolis, Ind.) for detecting Neisseria gonorrhoeae and Chlamydia trachomatis and to a PCR- and phylogeny-based assay for detecting mycoplasmas and ureaplasmas. The AMPLICOR STD-1 assay was performed as described in the manufacturer's instructions. The phylogeny-based assay was performed as described in our previous study (25). All urine specimens that were positive for M. genitalium by the phylogeny-based assay were subjected to the TaqMan assay to quantify M. genitalium DNA.
Preparation of bacterial DNA for the TaqMan assay.
Bacterial DNA was extracted from 15 species of mycoplasmas and ureaplasmas. After lysis by proteinase K, DNA was purified by a classic phenol-chloroform procedure followed by ethanol precipitation. Primers (My-1S and My-2A) were used to amplify a 771-bp DNA fragment of the 16S rRNA gene from M. genitalium genomic DNA. The sequence of My-1S was 5′-GAATAGCCACAATGGGACTGAGA-3′ (nucleotides 293 to 315 in the sequence with GenBank accession number X77334), and that of My-2A was 5′-TCACGACACGAGCTGACGACAAC-3′ (nucleotides 1041 to 1063 in the sequence with GenBank accession number X77334). The insertion of the amplified 771-bp fragment into pT7Blue T-Vector (Novagen, Madison, Wis.) yielded the plasmid pMyg16S (25). The plasmid pMyg16S was introduced into recipient cells and replicated in them. Reproduced pMyg16S was purified with a QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany) and dissolved in TE buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA). The amount of DNA in the solution was quantified by measuring the optical density at 260 nm, and the copy number of the partial fragment of the 16S rRNA gene was calculated. The solution was adjusted to contain 1010 copies/ml; thereafter, single-stock solutions of serial 10-fold dilutions from 109 to 103 copies/ml were prepared.
Preparation of urine samples for the TaqMan assay.
A precipitate from 1 ml of the first-pass urine that was positive for M. genitalium by the phylogeny-based assay was harvested by centrifugation at 15,000 × g for 30 min and washed with 0.9 ml of phosphate-buffered saline (pH 7.4). The precipitate was treated with proteinase K (700 μg/ml) at 55°C for 2 h in 500 μl of digestion buffer (10 mM Tris-HCl, pH 8.0; 50 mM KCl; 1.5 mM MgCl2; 0.01% gelatin; 0.45% NP-40; 0.45% Tween20; 0.5% sodium dodecyl sulfate), and the DNA was extracted by a phenol-chloroform method. After ethanol precipitation, DNA was collected by centrifugation and was then dissolved in 50 μl of TE buffer.
TaqMan assay.
The principle of the TaqMan real-time PCR is based on DNA amplification and cleavage of an internal probe that is hybridized to the amplified DNA by the 5′-3′ exonuclease activity of the Taq DNA polymerase during PCR cycles (4). The sequence of the forward primer (My-ins) was 5′-GTAATACATAGGTCGCAAGCGTTATC-3′ (nucleotides 520 to 545 in the sequence with GenBank accession number X77334), and that of the reverse primer (MGSO-2) was 5′-CACCACCTGTCACTCGGTTAACCTC-3′ (nucleotides 1012 to 1036 in the sequence with GenBank accession number X77334). The sequence of the probe (Mgen-P1) was 5′-FAM-CTGTCGGAGCGATCCCTTCGGTA-3′-TAMRA (nucleotides 819 to 841 in the sequence with GenBank accession number X77334) with a 3′ phosphate block used to prevent elongation of the probe (where FAM is the reporter dye 6-carboxyfluorescein, and TAMRA is the quencher dye 6-carboxytetramethylrhodamine [3]). A PCR mixture contained 1× TaqMan buffer A (Applied Biosystems, Foster City, Calif.), 5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 200 nM concentration of each primer, 100 nM probe, 1.25 U of AmpliTaq Gold DNA polymerase (Applied Biosystems), 0.5 U of AmpErase (uracil N-glycosylase [UNG]) (Applied Biosystems), and 10 μl of the template DNA solution in a total volume of 50 μl. After activation of UNG for 2 min at 50°C followed by deactivation of UNG and activation of the AmpliTaq Gold DNA polymerase for 10 min at 95°C, PCR was performed for 60 cycles of 15 s of denaturation at 95°C and 1 min of annealing and extension at 66°C. All standard dilutions, controls, and clinical specimens were run simultaneously. Amplification, data acquisition, and analyses were performed with the ABI Prism 7700 Sequence Detection System (Applied Biosystems). From cycle to cycle, ΔRn is calculated by subtraction of the baseline fluorescence from the reporter fluorescence that is normalized by an internal reference. The threshold cycle (Ct) is defined as the cycle number at which the reporter fluorescence exceeds the threshold value, a parameter defined as 10 standard deviations above baseline fluorescence. The log10 of number of targets initially present is proportional to the Ct and can be measured with a standard curve (3).
Statistical analyses.
The Wilcoxon rank-sum test was used for statistical analyses. All statistical analyses were two-tailed and were performed with the significance set at a P of <0.05.
RESULTS
Assay specificity.
The genomic DNA solutions (105 copies/ml) from mycoplasmas and ureaplasmas were tested by the TaqMan assay. Quantification of PCR products was observed only with M. genitalium DNA. No PCR products were detected with the DNA samples of other mycoplasmas and ureaplasmas.
Assay sensitivity and range of detection.
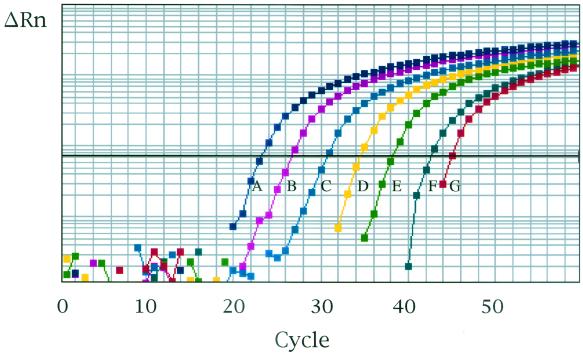
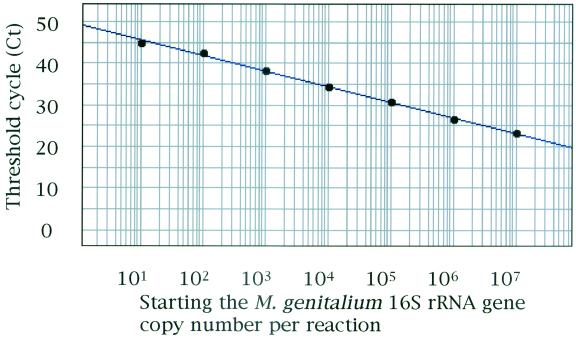
To determine the sensitivity of the TaqMan assay, a series of 10-fold dilutions of the plasmid pMyg16S, ranging from 109 to 103 copies of the partial fragment of the M. genitalium 16S rRNA gene per ml, were tested. Ten microliters of each solution was subjected to the TaqMan assay, so the M. genitalium 16S rRNA gene, ranging from 107 to 10 copies per reaction, was tested. A representative amplification plot of the TaqMan assay provided by the ABI Prism 7700 instrument is shown in Fig. 1. PCR amplification was observed for all dilutions of pMyg16S. When Cts were plotted against the log10 of the copy number of the M. genitalium 16S rRNA gene fragment per reaction, linearity was observed over the range from 107 to 10 copies/reaction (Fig. 2). A significant coefficient of correlation was repeatedly found for the Cts and the copy numbers (r > 0.990). The endpoint detection limit of the TaqMan assay was 10 copies/reaction. In quantification of M. genitalium in first-pass urine specimens, one-fifth of the DNA isolated from 1 ml of urine was used for the TaqMan assay. For first-pass urine specimens, therefore, the working range of the TaqMan assay was from 5 × 107 to 5 × 10 copies of the M. genitalium 16S rRNA gene per ml of urine.
FIG. 1.
PCR amplification of serial 10-fold dilutions of the M. genitalium 16S rRNA gene containing 107 (A), 106 (B), 105 (C), 104 (D), 103 (E), 102 (F), and 101 (G) copies/reaction. ΔRn is calculated by subtraction of the baseline fluorescence from the reporter fluorescence that is normalized by an internal reference.
FIG. 2.
Correlation between Cts and the log10 of the M. genitalium 16S rRNA gene copy number per reaction. Linearity is observed over the range from 107 to 101 copies per reaction.
Quantification of M. genitalium
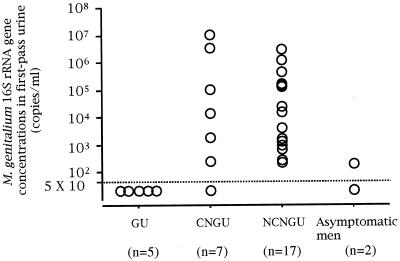
in first-pass urine specimens. N. gonorrhoeae was detected in specimens from 130 of 300 men with urethritis. Of men with gonococcal urethritis (GU), five were positive for M. genitalium by the PCR- and phylogeny-based assay. The first-pass urine specimens from these patients were examined by the TaqMan assay for quantification of the M. genitalium 16S rRNA gene. The M. genitalium loads for all the specimens were <5 × 10 copies/ml (Fig. 3). Of 170 men with NGU, 84 were positive for C. trachomatis. Of these men with chlamydial NGU (CNGU), seven were positive for M. genitalium. The M. genitalium load detected by the TaqMan assay was <5 × 10 copies/ml in the specimen from one man, and the loads ranged from 1.1 × 107 to 2.7 × 102 copies/ml in specimens from the remaining six men. Of 86 men with nonchlamydial NGU (NCNGU), 17 were positive for M. genitalium. M. genitalium loads in the first-pass urine specimens from these patients ranged from 3.3 × 106 to 2.3 × 102 copies/ml. Of 76 asymptomatic men, only 2 were positive for M. genitalium. For these men, the M. genitalium loads were 2.0 × 102 and <5 × 10 copies/ml.
FIG. 3.
Quantitative detection of the M. genitalium 16S rRNA gene in first-pass urine of men with GU, CNGU, and NCNGU and asymptomatic men.
There were no significant differences in M. genitalium loads between men with GU and asymptomatic men. However, first-pass urine specimens from patients with NGU, including both men with CNGU and those with NCNGU, had significantly higher concentrations of the M. genitalium 16S rRNA gene than did those from men with GU (P < 0.01) or asymptomatic men (P < 0.05). The M. genitalium loads in specimens from men with NCNGU were also significantly higher than those in specimens from asymptomatic men (P < 0.05).
DISCUSSION
M. genitalium was initially isolated from two urethral specimens in culture (21). Despite repeated attempts to isolate M. genitalium from the urogenital tract, however, no other strains have been reported. In 1996, Jensen et al. (8) developed a new technique to isolate M. genitalium from clinical specimens. This technique involves initial propagation of M. genitalium in Vero cell cultures and PCR monitoring of mycoplasmal growth. Using this technique, they succeeded in isolating four strains of M. genitalium from urethral specimens of patients with urethritis. Although this method can be applied to the isolation of M. genitalium clinical strains from the urogenital tract and other sites, a large number of clinical specimens cannot be processed, and quantification of the mycoplasma in the specimens cannot be carried out. No other methods of quantitative detection of M. genitalium, including culture or nonculture procedures, are available. Therefore, we developed the TaqMan assay to quantify M. genitalium. In the present study, we show that the TaqMan assay was able to specifically quantify concentrations of the M. genitalium 16S rRNA gene ranging from 107 to 10 copies/reaction. Using the TaqMan assay, we were able to quantify M. genitalium in first-pass urine from men with urethritis and from asymptomatic men.
In men with GU, the prevalence of M. genitalium has been reported to be low and significantly less than that of C. trachomatis (13). In the present study, all five patients with GU had low M. genitalium loads of <5 × 10 copies/ml. It might be difficult for M. genitalium, which requires strict conditions for growth, to colonize and proliferate in the urethra infected with N. gonorrhoeae. The development of urethritis after treatment of GU with penicillin and cephalosporin antibiotics, post-GU (PGU), was previously noted in a large proportion of men with chlamydia-negative GU (16). Like all mycoplasmas, M. genitalium lacks a cell wall and thus is insensitive to penicillins or cephalosporins. Such characteristics of M. genitalium along with the documented presence of this mycoplasma in the urethra of men with GU have suggested that M. genitalium may be one of the causes of PGU (23). Use of the TaqMan assay to monitor changes in the load of the mycoplasma after eradication of N. gonorrhoeae in the urethra will be helpful in clarifying whether M. genitalium plays a pathogenic role in men who develop this condition.
The various results reported to date tend to support the proposition that M. genitalium is an important pathogen of NGU independent of C. trachomatis (19). The prevalence of M. genitalium in patients with acute NCNGU has ranged from 18.4 to 45.5% (2, 12). Although M. genitalium also has been detected in men without urethritis, its prevalence has remained as low as 0.8% to 9.1% (1, 11). In most of the reports, M. genitalium is found significantly more often in patients with NCNGU than in control subjects without urethritis (1, 2, 7, 9, 11, 13, 20). In addition, our present results showed that M. genitalium loads in men with NGU were significantly higher than those in asymptomatic men. In particular, all the men with NCNGU had detectable levels of M. genitalium DNA in their first-pass urine, which were significantly higher than those in asymptomatic men. Higher loads of M. genitalium in the first-pass urine specimens would be associated with inflammation responses in the urethra, resulting in manifestation of clinical symptoms and signs of acute urethritis. Such a possible association of higher M. genitalium loads with symptomatic urethritis strengthens the proposition that M. genitalium is a pathogen of NGU.
Savio et al. (18) and Martinelli et al. (15) have reported an increased frequency of M. genitalium (37.5 and 56.0%, respectively) in the urethra of AIDS patients without urethral symptoms. Wang et al. (24) also reported a higher prevalence of antibodies specific for M. genitalium in asymptomatic human immunodeficiency virus (HIV)-positive patients (32.3%) and AIDS patients (44.0%) than in healthy blood donors (5.5%). Our present study lacked information regarding HIV serological status of the subjects; therefore, we were unable to determine what role M. genitalium might play in HIV-positive men or AIDS patients. However, further studies involving the quantitative assessment of M. genitalium loads by the TaqMan assay will provide useful information for understanding roles of the mycoplasma in such subjects.
Previous studies have suggested that M. genitalium may play a significant pathogenic role in chronic NGU (5, 6). We also reported that, in men with NGU, the persistence of M. genitalium in the urethra after antimicrobial chemotherapy was associated with the recurrence of NGU (14). The TaqMan assay will make it possible to monitor longitudinal changes in M. genitalium loads in such cases and to examine patients with chronic NGU for association of M. genitalium loads with clinical findings and inflammatory responses.
The TaqMan assay presents several important advantages over conventional DNA probe- or PCR-based assays. The simultaneous amplification and quantification eliminate the need for further manipulation of PCR products, resulting in a reduced risk of contamination and the ability to process a large number of specimens rapidly. The assay is sensitive, and its working range for quantification of M. genitalium DNA is broad. Further studies with the TaqMan assay should be performed for quantitative detection of M. genitalium in men with various conditions related to PGU, HIV serological status, or chronic NGU. Nevertheless, this study provides sufficient promising, albeit preliminary, data to suggest that the TaqMan assay will be a relevant tool for establishing the pathogenic role of M. genitalium in the urogenital tract.
REFERENCES
- 1.Björnelius, E., P. Lidbrink, and J. S. Jensen. 2000. Mycoplasma genitalium in non-gonococcal urethritis—a study in Swedish male STD patients. Int. J. STD AIDS 11:292-296. [DOI] [PubMed] [Google Scholar]
- 2.Deguchi, T., H. Komeda, M. Yasuda, K. Tada, H. Iwata, M. Asano, T. Ezaki, and Y. Kawada. 1995. Mycoplasma genitalium in non-gonococcal urethritis. Int. J. STD AIDS 6:144-145. [DOI] [PubMed] [Google Scholar]
- 3.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 4.Holland, P. M., R. D. Abramson, R. Watson, and D. H. Gelfand. 1991. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 88:7276-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooton, T. M., M. C. Roberts, P. L. Roberts, K. K. Holmes, W. E. Stamm, and G. E. Kenny. 1988. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet i:266-268. [DOI] [PubMed] [Google Scholar]
- 6.Horner, P., B. Thomas, C. B. Gilroy, M. Egger, and D. Taylor-Robinson. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin. Infect. Dis. 32:995-1003. [DOI] [PubMed] [Google Scholar]
- 7.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 8.Jensen, J. S., H. T. Hansen, and K. Lind. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen, J. S., R. Orsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind. 1993. Mycoplasma genitalium: a cause of male urethritis? Genitourin. Med. 69:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen, J. S., S. A. Uldum, J. Sondergard-Andersen, J. Vuust, and K. Lind. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J. Clin. Microbiol. 29:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannisson, G., R. Enstrom, G. B. Lowhagen, V. Nagy, K. Ryberg, S. Seeberg, and C. Welinder-Olsson. 2000. Occurrence and treatment of Mycoplasma genitalium in patients visiting STD clinics in Sweden. Int. J. STD AIDS 11:324-326. [DOI] [PubMed] [Google Scholar]
- 12.Keane, F. E., B. J. Thomas, C. B. Gilroy, A. Renton, and D. Taylor-Robinson. 2000. The association of Chlamydia trachomatis and Mycoplasma genitalium with non-gonococcal urethritis: observations on heterosexual men and their female partners. Int. J. STD AIDS 11:435-439. [DOI] [PubMed] [Google Scholar]
- 13.Maeda, S., M. Tamaki, M. Nakano, M. Uno, T. Deguchi, and Y. Kawada. 1998. Detection of Mycoplasma genitalium in patients with urethritis. J. Urol. 159:405-407. [DOI] [PubMed] [Google Scholar]
- 14.Maeda, S. I., M. Tamaki, K. Kojima, T. Yoshida, H. Ishiko, M. Yasuda, and T. Deguchi. 2001. Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex. Transm. Dis. 28:472-476. [DOI] [PubMed] [Google Scholar]
- 15.Martinelli, F., E. Garrafa, A. Turano, and A. Caruso. 1999. Increased frequency of detection of Ureaplasma urealyticum and Mycoplasma genitalium in AIDS patients without urethral symptoms. J. Clin. Microbiol. 37:2042-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oriel, J. D., P. Reeve, B. J. Thomas, and C. S. Nicol. 1975. Infection with Chlamydia group A in men with urethritis due to Neisseria gonorrhoeae. J. Infect. Dis. 131:376-382. [DOI] [PubMed] [Google Scholar]
- 17.Palmer, H. M., C. B. Gilroy, P. M. Furr, and D. Taylor-Robinson. 1991. Development and evaluation of the polymerase chain reaction to detect Mycoplasma genitalium. FEMS Microbiol. Lett. 61:199-203. [DOI] [PubMed] [Google Scholar]
- 18.Savio, M. L., A. Caruso, R. Allegri, F. Fallacara, C. P. Pollara, I. Foresti, E. Comberti, F. Gargiulo, F. Dima, G. P. Cadeo, and A. Turano. 1996. Detection of Mycoplasma genitalium from urethral swabs of human immunodeficiency virus-infected patients. New Microbiol. 19:203-209. [PubMed] [Google Scholar]
- 19.Taylor-Robinson, D., and P. M. Furr. 1998. Update on sexually transmitted mycoplasmas. Lancet 351(Suppl. 3):12-15. [DOI] [PubMed] [Google Scholar]
- 20.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 21.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 22.Tully, J. G., D. Taylor-Robinson, D. L. Rose, P. M. Furr, C. E. Graham, and M. F. Barile. 1986. Urogenital challenge of primate species with Mycoplasma genitalium and characteristics of infection induced in chimpanzees. J. Infect. Dis. 153:1046-1054. [DOI] [PubMed] [Google Scholar]
- 23.Uno, M., T. Deguchi, H. Komeda, M. Yasuda, M. Tamaki, S. Maeda, I. Saito, and Y. Kawada. 1996. Prevalence of Mycoplasma genitalium in men with gonococcal urethritis. Int. J. STD AIDS 7:443-444. [DOI] [PubMed] [Google Scholar]
- 24.Wang, R. Y., T. Grandinetti, J. W. Shih, S. H. Weiss, C. L. Haley, M. M. Hayes, and S. C. Lo. 1997. Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol. Med. Microbiol. 19:237-245. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida, T., S.-I. Maeda, T. Deguchi, and H. Ishiko. 2002. Phylogeny-based rapid identification of mycoplasmas and ureaplasmas from urethritis patients. J. Clin. Microbiol. 40:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]