Abstract
The InPouch TV is a method which combines a wet preparation and a culture method to detect Trichomonas vaginalis. The top portion of the InPouch TV essentially functions as a slide to be examined under the microscope. If the initial examination is negative, the specimen is pushed down into the bottom pouch, which serves as a broth for cultivation. The issue of timing has not been specifically addressed for optimal processing. To assess the effect of timing on the inoculation of the bottom pouch, we conducted a study designed to determine which procedure had better sensitivity, that of delaying inoculation of the bottom pouch until the initial examination on the top pouch is performed (method A) or that of immediately inoculating the bottom pouch (method B). In addition, we compared the sensitivity of the InPouch TV to that of the traditional wet mount. Fifty of 498 specimens were positive. Methods A and B had identical results: 31 specimens were initially positive regardless of transit time, and incubation yielded another 19 positives. The wet preparation detected 36 positive specimens. The sensitivities of the methods were 100% for the InPouch TV (including examination on receipt and after incubation) and 72% for the traditional wet mount. In conclusion, the InPouch TV method is more sensitive than the traditional method and no detectable differences were observed with timing of the inoculation of the top or bottom pouch.
Trichomonas vaginalis is the second most common sexually transmitted disease (10). Clinical syndromes in females vary from asymptomatic presentations to (more commonly) vaginitis with copious discharge. Infections can be associated with serious sequelae such as preterm labor, premature rupture of membranes, and low birth weight (3, 9) as well as increased risk of transmission of other sexually transmitted diseases, including human immunodeficiency virus (6).
The InPouch TV (BioMed Diagnostics, San Jose, Calif.) is a commercially available method which combines a wet preparation and a culture method to detect T. vaginalis. The InPouch TV is a double-pouched container made of soft plastic (Fig. 1). The top pouch is inoculated with genital secretions suspected of having trichomonads. When the specimen is initially received in the laboratory, the top portion of the InPouch TV essentially functions as a slide to be examined under the microscope. If the initial examination is negative, the specimen is pushed down into the bottom pouch, which serves as a container for the culture broth during subsequent incubation.
FIG. 1.
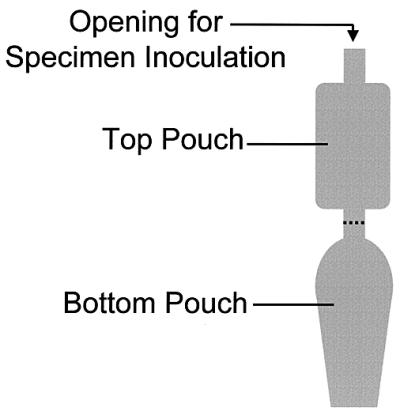
Diagram of InPouch TV.
The issue of timing has not been specifically addressed in the optimal processing of the InPouch TV test. The instructions provided by the manufacturer say to inoculate the bottom pouch immediately if the specimen will not be observed microscopically within 15 min of collection. However, these instructions do not directly address what to do if one does not know how long a delay is expected. The question remains: is it better to inoculate the bottom pouch if a substantial delay is expected before performing the initial examination? One reference (4) indicated that the bottom pouch was inoculated within 6 h after specimen collection, but other references do not address this potentially important issue (2, 3, 7).
In order to assess the effect of timing on the inoculation of the bottom pouch, we conducted the present study to determine which procedure had better sensitivity, (i) delaying inoculation of the bottom pouch until the initial examination of the top pouch is performed or (ii) inoculating the bottom pouch of the InPouch TV if a delay is anticipated before initial examination. In addition, we compared the sensitivity of the InPouch TV to that of the traditional wet mount.
(The present study was presented at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla., 20 to 24 May 2001.)
MATERIALS AND METHODS
Specimens from women with the signs and/or symptoms of vaginitis, vaginal discharge, dysuria, dyspareunia, and/or “strawberry” cervix were obtained from the emergency department of the Memorial Medical Center, Springfield, Ill., and from various clinics in the region, including an urgent care center, a family practice clinic, and a women's health center. Vaginal secretions suspended in a few milliliters of nonbacteriostatic saline were handled three different ways. For method A, the secretions were introduced into the bottom pouch only after the microscopic examination of the top pouch was completed, no matter what the transit time to the laboratory was. For method B, the specimen was introduced into the bottom pouch immediately after collection; microscopic examination of the bottom pouch was conducted when the specimen arrived in the laboratory. For method B, no examination was made of the top pouch because its contents had been immediately pushed into the bottom pouch. Finally, the vaginal secretions were examined by the traditional wet preparation method. For this method, 2 to 3 drops of genital secretions were placed on a glass slide, covered, and then examined microscopically.
When possible, the microscopic examinations were done in a blinded manner. Namely, different technologists performed an examination without knowledge of the result of the corresponding specimens on a given patient. Most of the time, this completely blinded technique was not possible because only one technologist was available. In that case, if several specimens arrived at the same time, the specimens were mixed so that method B specimens were examined without knowledge of the results of the method A specimens on a given patient; the wet preparation was examined similarly.
On day 1, the specimens were collected from the patient and examined upon arrival in the laboratory. Results of the wet preparations were final on day 1. For method A, if the result was initially negative, the specimen was pushed to the bottom pouch and incubated. For method B, since the specimen was already in the bottom pouch, it was simply incubated after the initial examination. For the wet mount, the test was terminated after the initial examination regardless of result. The InPouch TV-incubated specimens were examined on days 2, 3, and 5. (No examination was performed on day 4.) For all methods, if the results were positive, the test was terminated. In determining sensitivity, a positive result from either the wet preparation or the InPouch TV was considered a true positive.
Transit time was defined as the time elapsed between the time of collection of the specimen from the patient and the time the specimen was examined under the microscope in the laboratory.
RESULTS
Four hundred ninety-eight specimens were examined. Table 1 shows the distribution of the transit time for all specimens. Two-thirds of the specimens arrived less than 1 h after collection. The time of collection was not recorded for 45 of 498 (9%) of the specimens, so transit time remained undetermined. The recorded transit times ranged from 4 to 570 min, with the exception of two specimens with transit times of 960 and 1,200 min. The mean transit time of all recorded specimens was 76 min.
TABLE 1.
Distribution of specimens by transit time
| Type of specimens | No. (%) of specimens with indicated transit time (h)
|
Total no. (%) of specimens | ||||
|---|---|---|---|---|---|---|
| <1 | 1-2 | 2-3 | >3 | Undetermined | ||
| All | 337 (67.7) | 31 (6.2) | 18 (3.6) | 67 (13.5) | 45 (9.0) | 498 (100.0) |
| Negativea | 305 (61.2) | 30 (6.0) | 16 (3.2) | 59 (11.8) | 38 (7.6) | 448 (90.0) |
| Positivea | 32 (6.4) | 1 (0.2) | 2 (0.4) | 8 (1.6) | 7 (1.4) | 50 (10.0) |
Result by InPouch TV method.
Fifty of the specimens were examined in a blinded manner; 448 were not examined in a blinded manner (i.e., the same technologist examined the traditional wet preparation, method A, and method B). A total of 50 out of 498 specimens were positive. Methods A and B had identical results (Table 2). Upon receipt of the specimen in the laboratory, 31 specimens were positive for T. vaginalis by both methods A and B regardless of transit time. If the initial examination was negative, incubation yielded another 19 positive specimens with both methods A and B. The traditional wet preparation detected 36 positive specimens. Of the five specimens that were positive by the wet preparation but initially negative by the InPouch TV method, each specimen became positive upon follow-up culture. Four became positive by day 2 and one became positive by day 5. The mean transit time of the positives by the InPouch TV method was 81.2 min, with a range of 5 to 510 min. The mean transit time of the positives by the traditional method was 91.7 min, with a range of 8 to 405 min.
TABLE 2.
Distribution of positive specimens by method and incubation status
| Incubation status | No. (%) of positive specimens
|
||
|---|---|---|---|
| Top pouch inoculated (method A) | Bottom pouch inoculated (method B) | Traditional method | |
| Not incubated | 31 (62.0) | 31 (62.0) | 36 (72.0) |
| Incubated | 19 (38.0) | 19 (38.0) | Not applicable |
The sensitivities of the three methods were as follows: 62% for the InPouch TV upon receipt, 100% for the InPouch TV (including examination on receipt and after incubation), and 72% for the traditional wet mount. Of the positive specimens by the InPouch TV method, 31 of 50 (62%) were detected on day 1, 6 of 50 (12%) were detected on day 2, 8 of 50 (16%) were detected on day 3, and 5 of 50 (10%) were detected on day 5.
The relationship of specimen result to transit time for the InPouch TV can be seen in Table 1. While over half of all the specimens and all of the positive specimens arrived at the laboratory within 1 h after collection, the transit times for the rest of the positive samples were spread out over all the time periods. Although the mean transit time for all specimens with known times was 76 min, the mean transit time for the false negatives by the traditional method was 112 min.
Although the InPouch TV is not intended to function as a wet mount to detect clue cells and yeasts, these cells could be seen. The traditional wet preparation detected clue cells in 27.8% of the specimens and the InPouch TV detected 20.4%. The traditional wet preparation detected yeasts in 7.7% of the specimens and the InPouch TV detected yeasts in 3.0%.
DISCUSSION
The InPouch TV detected 28% more positives than the traditional wet mount and the timing of inoculation of the upper or lower pouch did not affect its performance. In spite of its lower sensitivity overall (compared to the InPouch TV method upon receipt and following incubation), the wet mount detected five positive specimens which the InPouch TV method did not initially detect. The most likely explanation is that the organisms were not evenly distributed between containers for all three methods due to their low numbers. Each of these five specimens had very few organisms seen on the wet mount, and each subsequently became positive by the culture method.
It was not necessary to resolve the discrepancies for specificity since the presence of trichomonads with characteristic size, shape, and motility was the determining factor in both tests. The specificity was therefore considered the same for both the wet mount and the InPouch TV method.
Approximately one-third of the specimens arrived more than 1 h after being collected, which is in excess of the maximum time recommended for transit time by the traditional method (8). The detrimental effect of prolonged transit time is borne out by the present study; while the mean transit time for all specimens was 76 min, the mean transit time for the false negatives by the traditional method was 112 min. This indicates that the prolonged transit time of the false-negative specimens contributed to their being falsely negative. The extra cost of the InPouch TV method over the traditional method can be justified by its superiority in accommodating unavoidable transit delays and its increased sensitivity. Other nutrient broths intended to grow T. vaginalis are commercially available, but we have had insufficient experience with them on which to comment (1, 5).
In conclusion, because of decreased sensitivity and unacceptable transit times, we switched the detection method for T. vaginalis from the traditional wet preparation to the InPouch TV. The InPouch TV method is more sensitive than the traditional method (100 and 72%, respectively). No detectable differences were observed with timing of the inoculation of the top or bottom pouch.
Acknowledgments
This work was supported in part by BioMed Diagnostics, San Jose, Calif.
REFERENCES
- 1.Beal, C., R. Goldsmith, M. Kotby, M. Sherif, A. El-Tagi, A. Farid, S. Zakaria, and J. Eapen. 1992. Plastic envelope method, a simplified technique for culture diagnosis of trichomoniasis. J. Clin. Microbiol. 30:2265-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchardt, K. A., S. Al-Haraci, and N. Maida. 1991. Prevalence of Trichomonas vaginalis in a male sexually transmitted disease clinic population by interview, wet mount microscopy, and the InPouch TV test. Genitourin. Med. 76:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotch, M. F., J. G. Pastorek, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Exchenbach, R. Edelman, C. Carey, J. A. Regan, M. A. Drohn, M. A. Kebanoff, A. Vijaya, G. G. Rhoads, and the Vaginal Infections and Prematurity Study Group. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Draper, D., R. Parker, E. L. Patterson, W. Jones, M. Beutz, J. French, K. Borchardt, and J. McGregor. 1993. Detection of Trichomonas vaginalis in pregnant women with InPouch TV culture system. J. Clin. Microbiol. 31:1016-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelbart, S. M., J. L. Thomason, P. J. Osypowski, A. V. Kellett, J. A. James, and F. F. Broekhuizen. 1990. Growth of Trichomonas vaginalis in commercial culture media. J. Clin. Microbiol. 28:962-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laga, M., A. Manoka, and M. Kivuvu. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 7.Levi, M. H., J. Torres, C. Pina, and R. Klein. 1997. Comparison of the InPouch TV culture system and Diamond's modified medium for detection of Trichomonas vaginalis. J. Clin. Microbiol. 35:3308-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, J. M. 1999. A guide to specimen management in clinical microbiology, 2nd ed., p. 168-173. ASM Press, Washington, D.C.
- 9.Minkoff, H., A. N. Grunebaum, R. H. Schwarz, J. Feldman, M. Cummings, W. Crumbleholme, L. Clark, G. Pringle, and W. M. McCormack. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150:965-972. [DOI] [PubMed] [Google Scholar]
- 10.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]


