Abstract
A cosmid library of Shiga toxigenic Escherichia coli (STEC) O157:H7 strain EDL933 DNA was screened for clones capable of reacting with convalescent-phase serum from a patient with hemolytic-uremic syndrome (HUS), in an attempt to identify candidate virulence genes. One of the immunoreactive clones contained a portion of the large plasmid pO157, and the immunoreactive gene product was identified as TagA. The function of this 898-amino-acid protein is unknown, but it exhibits 42% amino acid sequence identity and 63% similarity to a 312-amino-acid region of a ToxR-regulated lipoprotein of Vibrio cholerae. Antibodies to E. coli O157 TagA were detected in sera from other HUS patients with O157 STEC infection but not in those from patients whose illnesses were caused by other STEC types or in healthy controls. These data demonstrate that TagA is expressed in vivo and provide circumstantial evidence for a role in the pathogenesis of the disease. The tagA gene is present only in STEC strains belonging to serogroup O157, and so antibodies to TagA are a potentially useful serological marker for infections due to such strains.
Shiga toxigenic Escherichia coli (STEC) organisms are an important cause of gastrointestinal disease in humans, particularly since these infections may result in life-threatening sequelae such as the hemolytic-uremic syndrome (HUS) (13, 17, 23). The STEC family is very diverse, and strains belonging to a broad range of O:H serotypes have been associated with human disease. However, certain STEC subsets account for a disproportionately high number of serious infections. Members of one such subset have the capacity to produce attaching and effacing (A/E) lesions on intestinal mucosa, a property encoded on a pathogenicity island termed the locus for enterocyte effacement (LEE). LEE encodes a type III secretion system and E. coli-secreted proteins (Esp) which deliver effector molecules to the host cell and disrupt the host cytoskeleton (4, 6, 25). LEE also carries eae, which encodes an outer membrane protein (intimin) required for intimate attachment to epithelial cells via a translocated intimin receptor (Tir), which is also LEE encoded. Most STEC strains isolated from humans (both LEE positive and LEE negative) also carry large (>90-kb) plasmids encoding proteins such as the enterohemorrhagic E. coli enterohemolysin (EhxA) (26) and an extracellular serine protease (EspP) (2), both of which may be accessory virulence factors.
STEC strains belonging to serogroup O157 appear to be of particular virulence for humans. Although epidemiological data may have been skewed by the fact that they are much easier to detect than other STEC strains (because they are sorbitol negative), this serogroup (particularly serotype O157:H7) has been historically responsible for most major outbreaks of serious human STEC disease (13, 17, 23). For this reason, O157:H7 STEC strains have been the subject of intensive study in recent years. Indeed, the complete genome sequences of two O157:H7 STEC strains have recently been published (9, 24); the sequences of the large plasmids (designated pO157) from the same two strains had been reported separately (3, 16). The sequenced strains were EDL933, which was responsible for an outbreak of hemorrhagic colitis in 1983, and RIMD0509952, which was associated with a massive outbreak of hemorrhagic colitis and HUS in Sakai, Japan, in 1996. These studies have provided a valuable resource for STEC research. In particular, they have demonstrated that the O157:H7 STEC genome contains approximately 1,400 genes not present in the genome of E. coli K-12. However, determining which of these, including many with no homology to known virulence genes of other bacteria, actually function in the pathogenesis of human disease is a difficult undertaking, particularly given the paucity of suitable animal models.
One potentially useful approach to the identification of virulence-related gene products is to determine which STEC-specific proteins elicit a host immune response during infection. Indeed, convalescent-phase sera from HUS patients have been shown to contain antibodies to several proteins already strongly implicated in pathogenesis, including the LEE-encoded proteins intimin, Tir, EspA, and EspB (11, 15, 20, 28), as well as the plasmid-encoded hemolysin EhxA and the serine protease EspP (2, 26). In an attempt to identify additional virulence-related gene products of O157:H7 STEC, we have screened a cosmid library of EDL933 DNA for clones reacting with convalescent HUS patient sera. Mapping of sequence data generated from these clones on the genome facilitates characterization of the full repertoire of targets of the human immune response to STEC infection.
MATERIALS AND METHODS
Bacterial strains and cloning vectors.
The O157:H7 STEC strain EDL933 and a sorbitol-fermenting, nontoxigenic O157:H20 isolate were provided by R. Robins-Browne, Royal Children's Hospital, Melbourne, Australia. All other strains used in this study were clinical isolates from the Women's and Children's Hospital, North Adelaide, Australia. E. coli K-12 strains DH1 and JM109 have been described previously (7, 29). The cosmid vector pHC79 has also been described previously (10). The phagemids pBluescript KS (encoding ampicillin resistance) and pBC SK (encoding chloramphenicol resistance) were obtained from Stratagene, La Jolla, Calif. All E. coli strains were routinely grown in Luria-Bertani (LB) medium with or without 1.5% Bacto-Agar (Difco Laboratories, Detroit, Mich.). Where appropriate, ampicillin or chloramphenicol were added to growth media at a concentration of 50 or 40 μg/ml, respectively.
Construction of EDL933 cosmid bank.
To construct a cosmid gene bank of E. coli EDL933 DNA, high-molecular-weight genomic DNA was digested partially with Sau3A1 in order to optimize the yield of fragments in the size range of 35 to 40 kb. This was ligated with a fivefold molar excess of BamHI-digested pHC79 DNA and was packaged into lambda heads using a Packagene kit (Promega Biotec, Madison, Wis.). This mix was then transfected into E. coli DH1, which had been grown in LB broth supplemented with 2% maltose. Bacteria were then plated onto LB agar supplemented with 50 μg of ampicillin per ml, and after incubation, cosmid clones were routinely stored in LB supplemented with ampicillin with 15% glycerol in microtiter trays at −70°C.
Screening of cosmid clones with convalescent-phase HUS patient serum.
Cosmid clones were grown overnight at 37°C in 150 μl of LB with ampicillin in microtiter trays and then spotted onto nitrocellulose filters. Filters were then fixed, blocked, and reacted with convalescent-phase serum (diluted 1:6,000) from an HUS patient who was culture positive for O157 STEC. This was followed by incubation with goat anti-human immunoglobulin G conjugated to alkaline phosphatase (Bio-Rad Laboratories, Hercules, Calif.). Immunoreactive clones were visualized by using a chromogenic substrate (4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate).
Western blot analysis was used to confirm the seroreactivity of dot immunoblot-positive cosmid clones. Lysates from fresh overnight cultures were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14), and proteins were electrophoretically transferred onto nitrocellulose filters (27). Immunoreactive bands were detected by using convalescent-phase serum as described above.
DNA sequencing.
For sequencing, cosmid DNA was purified from seroreactive clones by using a QIAPrep Spin miniprep kit (Qiagen, Hilden, Germany). The sequences of 5′ and 3′ termini of the EDL933 DNA inserts were then determined by using dye-terminator chemistry and custom-made oligonucleotide primers 5′-CGTATCACGAGGCCCTTTC-3′ and 5′-CCACCGGAAGGAGCTGAC-3′, which flank the insertion site in pHC79, on an Applied Biosystems model 3700 automated DNA sequencer. A series of deletion derivatives of each cosmid, as well as random subclones of insert DNA in pBC (in E. coli JM109), were also constructed and subjected to sequence analysis using the above primers, as well as universal M13 sequencing primers, respectively. The program BLASTN (1) was used to identify the region of the EDL933 genome sequence from which the cosmid (or subclone) insert was derived.
RESULTS
Analysis of immunoreactive cosmid clones.
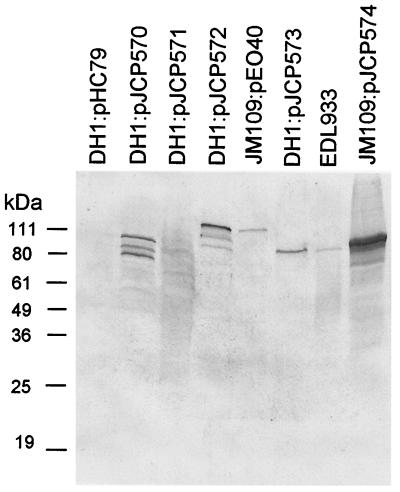
Screening of the EDL933 cosmid gene bank by dot immunoblot using convalescent-phase serum from an HUS patient with culture-confirmed O157 STEC infection identified four seropositive cosmid clones (designated pJCP570 through pJCP573), which were confirmed by Western blot analysis. These expressed immunoreactive protein species ranging in size from approximately 54 to 110 kDa, which were absent in the lysate of DH1:pHC79 (Fig. 1). Cosmid DNA was then purified from each of the seroreactive clones, and the sequences of 5′ and 3′ termini of the EDL933 DNA inserts were then determined, as described in Materials and Methods. The program BLASTN was used to identify the region of the EDL933 genome sequence from which the cosmid insert was derived, and these regions were then examined for open reading frames (ORFs) capable of encoding a protein of a size similar to that of the respective immunoreactive species.
FIG. 1.
Western blot analysis. Lysates of E. coli DH1 carrying the indicated cosmids, E. coli JM109:pEO40, E. coli O157:H7 strain EDL933, or E. coli JM109:pJCP574 were subjected to SDS-PAGE, electroblotted, and probed with convalescent-phase serum from a patient with HUS caused by an O157:H− STEC strain, as described in the text. The mobilities of protein size markers are also indicated.
The insert of cosmid pJCP570 was derived from the region of the EDL933 genome contained on contig 3 of section 214 (GenBank accession number AE00595). This contig includes the portion of the LEE locus encoding Tir and intimin, with which the sizes of the major immunoreactive species (approximately 75 and 100 kDa, respectively) (Fig. 1) are consistent. Both of these proteins are known to react with convalescent-phase sera from patients with STEC disease (11, 15, 20, 28). The cosmid pJCP571 directed expression of several weakly reactive species (Fig. 1). However, DH1:pJCP571 grew poorly in vitro, and the clone yielded poor-quality sequence data, preventing localization of the insert in the genome sequence. Interestingly, the inserts of both pJCP572 and pJCP573 originated from the large virulence plasmid of EDL933 (referred to as pO157) (GenBank accession number AF074613). Cosmid pJCP572 contained a 37-kb insert incorporating the region from nucleotides (nt) 23172 to 60261. E. coli DH1:pJCP572 expressed a major immunoreactive protein species of approximately 110 kDa (Fig. 1). Examination of the respective portion of the pO157 sequence indicated that the only predicted ORF of this size was the enterohemolysin EhxA, which is also known to react with HUS patient sera (26). Furthermore, E. coli JM109 carrying the EhxA-encoding plasmid pEO40 (26) also expressed an immunoreactive protein of the same size (Fig. 1). Cosmid pJCP573 contained an insert of similar size (>35 kb), which partially overlapped that of pJCP572. The 5′ terminus corresponds to nt 8671 of pO157, and a random subclone corresponded to nt 19622 to 21839. However, the 3′ terminus of the pJCP573 insert contained a separate portion of the plasmid, presumably due to coinsertion of a discontiguous Sau3A fragment during the original cloning step. Thus, the extent of the overlap between the inserts of the two clones was uncertain. E. coli DH1:pJCP573 expressed a single immunoreactive protein species of approximately 95 kDa, which appeared to comigrate with the major immunoreactive species in the lysate of EDL933 (Fig. 1). Examination of the respective portion of the pO157 sequence indicated that it contains the gene encoding EspP, which has been shown previously to react with HUS patient sera (2). EspP is 1,300 amino acids (aa) in length, but it is cleaved during secretion, resulting in a mature protein with an apparent size of 104 kDa, as judged by SDS-PAGE (2). Although this is not dissimilar in size to the immunoreactive species produced by E. coli DH1:pJCP573, a random pBC subclone containing pO157 nt 10718 to 15165 (incorporating the complete espP ORF and promoter region) did not direct expression of an immunoreactive species (result not shown). The only other candidate ORF of the appropriate size in the region of pO157 contained in pJCP573 was TagA, a 99.5-kDa protein with partial similarity to a ToxR-regulated lipoprotein of Vibrio cholerae (8).
Subcloning of EDL933 tagA and seroreactivity of TagA.
To examine whether the immunoreactive protein expressed by E. coli DH1:pJCP573 was TagA, the region of pO157 from nt 22903 to 25789 (which contains the complete tagA ORF and promoter region) was amplified by PCR using EDL933 genomic DNA as a template and primers 5′-GGTCAGGATATATTTACGAAACAG-3′ and 5′-CTGGACGCTACTTTATGTTTTG-3′. The resultant 2,887-bp PCR product was blunt cloned into pBluescript KS and transformed into E. coli JM109. Western blot analysis using the O157 HUS patient serum indicated that the lysate of JM109 carrying the recombinant plasmid (designated pJCP574) contained a strongly immunoreactive species of approximately 95 kDa, which comigrated with the immunoreactive protein in lysates of DH1:pJCP573 and EDL933 (Fig. 1). This apparent size is compatible with that predicted for the 99.5-kDa TagA protein after cleavage of its signal peptide, which is predicted to occur between aa 35 and 36 by the program SignalP V1.1 (18).
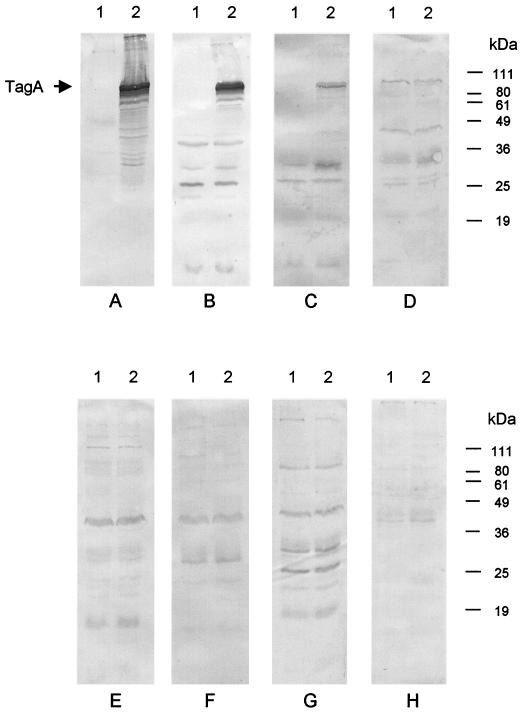
Lysates of JM109:pJCP574 or JM109:pBluescript were then subjected to Western blot analysis using convalescent-phase sera from various HUS patients or healthy control sera (Fig. 2). Sera were available from two HUS patients with culture-confirmed O157 STEC infection, and both of these labeled the 95-kDa species in the JM109:pJCP574 lysate. Both sera labeled a number of smaller species in the JM109:pJCP574 lysate and these were presumed to be TagA degradation products, because there was negligible reactivity in the respective JM109:pBluescript lysate lanes (Fig. 2A and B). A slightly weaker TagA-specific response was also detected when using the convalescent-phase serum of an HUS patient from whom an O111 STEC strain had originally been isolated (Fig. 2C). Interestingly, however, no TagA-specific immune response was detected in convalescent-phase sera from two other patients with HUS caused by O111 STEC strains (Fig. 2D and E) or in serum from a patient with HUS caused by an O113 STEC (Fig. 2F). Similarly, no differences in the labeling pattern for JM109:pJCP574 and JM109:pBluescript lysates were observed when using sera from four healthy controls (results for two of these are shown in Fig. 2G and H).
FIG. 2.
Reactivity of HUS patient sera with TagA-producing E. coli JM109. Lysates of E. coli JM109 carrying either pBluescript (lane 1) or pJCP574 (lane 2) were separated by SDS-PAGE, electroblotted, and probed with convalescent-phase sera from patients with HUS caused by STEC strains belonging to serotype O157:H− (A and B), O111:H− (C, D, and E), or O113:H21 (F), or with sera from healthy controls (G and H). The expected mobilities of TagA and various protein size markers are indicated.
Distribution of tagA among STEC strains.
The distribution of tagA among a wide range of STEC strains was then examined by Southern hybridization analysis using digoxigenin-labeled 2,887-bp tagA PCR product as a probe, under both high- and low-stringency conditions. A strong hybridization signal was obtained at high stringency with DNA from the O157:H7 STEC EDL933 and two O157:H− STEC strains. However, even at low stringency, no hybridization was detected with DNA from any of 37 non-O157 STEC strains in our collection or with an enteropathogenic E. coli strain (result not shown). The strains tested included 15 LEE-positive STEC belonging to serogroups O111 and O26, and 22 LEE-negative STEC, including representatives of serogroups O6, O23, O48, O91, O113, O123, O128, O141, and O159, as well as rough or O nontypeable strains. All of the LEE-positive and 16 of the LEE-negative STEC strains were presumed to carry a large plasmid, on the basis of a positive PCR with primers specific for the ehxA gene (21). The presence of tagA was also examined by PCR analysis (using the primers described above) in an additional 2 O157:H7 and 19 O157:H− STEC strains, as well as in a sorbitol-fermenting non-Shiga toxigenic E. coli O157:H20 strain. All of these strains were tagA PCR positive, except for the non-STEC O157:H20 strain and one of the O157: H− STEC strains which had lost its large plasmid (as judged by negative PCR for ehxA) (results not shown). Thus, presence of tagA appears to be strictly associated with O157 STEC strains.
Given the apparent absence of tagA-related genes in non-O157 STEC strains, the fact that the convalescent-phase serum tested in Fig. 2C appeared to react with TagA was unexpected, because it came from an HUS patient from whom an O111:H− STEC strain was isolated. However, this patient was one of 21 HUS cases associated with an outbreak of HUS caused by contaminated fermented sausage (22), and although all of these were confirmed to have been infected with an O111:H H− STEC strain, an O157:H− STEC strain was also isolated from two of the other patients. Thus, it is possible that this patient had also been coinfected with an O157:H− STEC strain. Although over 100 stx-positive E. coli colonies had been isolated from this patient at the time of the outbreak, only 6 were sent for serotyping and these were all O111:H− (22).
DISCUSSION
Recent genome sequence analyses of O157:H7 STEC strains have identified a large number of genes (approximately 1,400), which are not present in E. coli K-12. These are found in roughly 190 discrete clusters, several of which are known to be associated with virulence (e.g., the Shiga toxin-converting prophages and the LEE pathogenicity island) (9, 24). However, many of the O157:H7-specific genes have no known function and their contribution to pathogenesis is unclear. Patients with severe STEC disease are known to mount an immune response to proven or putative virulence factors, such as LEE-encoded proteins (11, 15, 20, 28) or plasmid-encoded factors such EhxA or EspP (2, 26). In the present study, we used immunoblot analysis of a cosmid library with convalescent-phase serum from an HUS patient to identify additional putative O157:H7 STEC virulence factors. Detection of immunoreactive clones expressing intimin, Tir, and EhxA was consistent with previous studies. However, we also isolated an immunoreactive clone expressing TagA, a hitherto cryptic 898-aa protein encoded by pO157, so named because it exhibits 42% identity and 63% similarity with a 312-aa portion of the TagA lipoprotein of V. cholerae (8). In V. cholerae, expression of tagA is regulated by ToxR (19) and the gene is part of a 39.5-kb pathogenicity island associated with epidemic and pandemic strains (12). However, little is known regarding the structure of Vibrio TagA or its role (if any) in virulence. The C-terminal portion of E. coli O157 TagA also exhibits similarity (53% identity and 66% similarity over 106 aa) with a hemolysin-related protein of V. cholerae (GenBank accession number AAF94092). E. coli O157 TagA has a predicted signal peptidase cleavage site between aa 35 and 36 and so is likely to be exported from the cell, but it lacks the lipoprotein signal sequence LXXC found in the V. cholerae protein. The lack of an appropriate animal model for STEC disease complicates direct molecular genetic assessment of the contribution of TagA to virulence. Nevertheless, detection of antibodies to TagA in convalescent-phase sera from patients with HUS caused by O157 STEC indicates that the protein is expressed in vivo and provides circumstantial evidence that it may function in the pathogenesis of disease. This possibility is also supported by the recent finding that expression of tagA is upregulated by the LEE-encoded transcriptional activator ler (5).
A second finding of the present study is that tagA appears to be present only in STEC strains belonging to serogroup O157. The absence of the gene from all other STEC serogroups tested was unexpected. In view of the strong immune response mounted by patients with O157 STEC disease, antibodies to TagA may be a useful serological marker for O157 STEC infection, particularly in young children who may respond poorly to O157 O antigen. PCR assays specific for tagA may also be a useful adjunct to existing multiplex PCR assays (for an example, see reference 21) used for detection and characterization of STEC in clinical and environmental samples. The STEC family is very diverse and includes representatives of more than 200 E. coli O:H serotypes (17), yet in spite of this, strains belonging to serogroup O157 are the most common causes of serious disease in most parts of the world. Furthermore, O157 STEC strains have been responsible for the vast majority of the major outbreaks of such infections (13, 17, 23). There is no evidence that O157 STEC strains produce larger amounts or more-potent subtypes of Shiga toxin, and proven or putative accessory virulence factors such as the LEE locus, EhxA, or EspP are known to be produced by a number of STEC serotypes. To date, TagA is the only O157-specific protein for which there is even circumstantial evidence of involvement in the pathogenesis of disease. Thus, we hypothesize that TagA may directly contribute to the enhanced human virulence of O157 STEC strains.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 3.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L.-C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 8.Harkey, C. W., K. D. Everiss, and K. M. Peterson. 1995. Isolation and characterization of a Vibrio cholerae gene (tagA) that encodes a ToxR-regulated lipoprotein. Gene 153:81-84. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 10.Hohn, B., and J. Collins. 1980. A small cosmid for efficient cloning of large DNA fragments. Gene 11:291-298. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins, C., H. Chart, H. R. Smith, E. L. Hartland, M. Batchelor, R. M. Delahay, G. Dougan, and G. Frankel. 2000. Antibody response of patients infected with verocytotoxin-producing Escherichia coli to protein antigens encoded on the LEE locus. J. Med. Microbiol. 49:97-101. [DOI] [PubMed] [Google Scholar]
- 12.Karaolis, D. K., J. A. Johnson, C. C. Bailey, E. C. Boedeker, J. B. Kaper, and P. R. Reeves. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc. Natl. Acad. Sci. USA 95:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali, M. A. 1989. Infection by verotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Li, Y., E. Frey, A. M. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makino, K., K. Ishii, T. Yasunaga, M. Hattori, K. Yokoyama, H. C. Yutsudo, Y. Kubota, Y. Yamaichi, T. Iida, K. Yamamoto, T. Honda, C. G. Han, E. Ohtsubo, M. Kasamatsu, T. Hayashi, S. Kuhara, and H. Shinagawa. 1998. Complete nucleotide sequences of 93-kb and 3.3-kb plasmids of an enterohemorrhagic Escherichia coli O157:H7 derived from Sakai outbreak. DNA Res. 5:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 19.Parsot, C., E. Taxman, and J. J. Mekalanos. 1991. ToxR regulates the production of lipoproteins and the expression of serum resistance in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:1641-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton, A. W., P. A. Manning, M. C. Woodrow, and J. C. Paton. 1998. Translocated intimin receptors (Tir) of Shiga-toxigenic Escherichia coli isolates belonging to serogroups O26, O111, and O157 react with sera from patients with hemolytic-uremic syndrome and exhibit marked sequence heterogeneity. Infect. Immun. 66:5580-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton, A. W., R. Ratcliff, R. M. Doyle, J. Seymour-Murray, D. Davos, J. A. Lanser, and J. C. Paton. 1996. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol. 34:1622-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 25.Perna, N. T., G. F. Mayhew, G. Posfai, S. J. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss, E., A. W. Paton, P. A. Manning, and J. C. Paton. 1998. Molecular analysis of Shiga toxigenic Escherichia coli O111:H− proteins which react with sera from patients with hemolytic-uremic syndrome. Infect. Immun. 66:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13 mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]