Abstract
We report the development of a real-time PCR assay for the quantitative detection of Neospora caninum in infected host tissues. The assay uses the double-stranded DNA-binding dye SYBR Green I to continuously monitor product formation. Oligonucleotide primers were designed to amplify a 76-bp DNA fragment corresponding to the Nc5 sequence of N. caninum. A similar method was developed to quantify the 28S rRNA host gene in order to compare the parasite load of different samples and to correct for the presence of potential PCR-inhibiting compounds in the DNA samples. A linear quantitative detection range of 6 logs with a calculated detection limit of 10−1 tachyzoite per assay was observed with excellent linearity (R2 = 0.998). Assay specificity was confirmed by using DNA from the closely related parasite Toxoplasma gondii. The applicability of the technique was successfully tested in a variety of host brain tissues: (i) aborted bovine fetuses classified into negative or positive Neospora-infected animals according to the observation of compatible lesions by histopathological study and (ii) experimentally infected BALB/c mice, divided into three groups, inoculated animals with or without compatible lesions and negative controls. All samples were also tested by ITS1 Neospora nested PCR and a high degree of agreement was shown between both PCR techniques (κ = 0.86). This technique represents a useful quantitative diagnostic tool to be used in the study of the pathogenicity, immunoprophylaxis, and treatment of Neospora infection.
Neospora caninum is a recently discovered cyst-forming coccidian parasite that is closely related to Toxoplasma gondii. Since the first report of neosporosis (7), this parasite has been described as a major cause of abortion in the main cattle-producing countries, and the infection is also associated with neonatal mortality and encephalomyelitis in congenitally infected calves (12). Recent advances concerning the Neospora life cycle have proved dogs are both intermediate and definitive hosts. Cattle, sheep, horses, goats, foxes, coyotes, deer, buffaloes, and camels are its natural intermediate hosts and, in addition, cats, mice, rats, gerbils, and monkeys are experimental intermediate hosts (12). During the acute phase of the disease in the intermediate host, tachyzoites can be found in several organs. However, in the acute and chronic phases of the infection the brain is the most consistently affected organ and where most protozoal tissue cysts have been observed (4, 11, 25). Diagnosis is based on the detection of parasite-specific antibodies in the adult population (8) and the visualization of characteristic and consistent lesions in brain, heart, and liver in aborted fetuses combined with immunohistochemical examination of positive tissues (1, 2, 15) and fetal serology (5, 10, 26). Immunohistochemistry (IHC) is a relatively insensitive technique for detecting the parasite in host tissues due to low parasite numbers and, sometimes, due to the low quality of the fetal tissue that could be autolyzed, mummified, or macerated (6, 12). As an alternative, several PCR-based methods have been developed in the last few years targeting the parasite ITS1 region (27) and the repeated Neospora-specific Nc5 sequence (18) with different modifications, such as nested or seminested PCR test, trying to increase the sensitivity and specificity of the technique (6, 13, 14). PCR techniques have been useful as diagnostic tools for detection of the parasite in bovine aborted fetuses (6, 14, 16, 28) and to study the parasite distribution in experimentally infected mice (20, 31), as a first step to characterize an experimental model of the infection to be used in the near future in drug and vaccine testing. At present, only a quantitative-competitive PCR (QC-PCR) technique for the detection of Neospora in host tissues has been reported (19), based on the inclusion of a known competitor to the target Neospora-specific Nc5 genomic sequences. This technique is labor-intensive, low throughput, and requires post-PCR analysis. There is, therefore, a practical need for a fast and efficient method to quantify N. caninum in infected tissues.
This report describes the development of a sensitive and specific quantitative PCR based on the Nc5 sequence of N. caninum that monitors the reaction in real time, employing the double-stranded DNA-binding dye SYBR Green I. The increase in fluorescence emission detected due to the binding of SYBR Green I dye is proportional to the amount of amplified DNA, allowing a rapid and accurate quantification of initial DNA copy number. A similar method was developed to quantify the 28S rRNA host gene in order to obtain an indirect measure of the amount of tissue assayed. These real-time PCR tests, together with a well-characterized nested PCR (11), were applied to a battery of brain samples from naturally and experimentally infected hosts in order to compare the parasite loads in bovine and murine tissues with or without histological lesions.
MATERIALS AND METHODS
Parasites.
N. caninum tachyzoites (Nc-1 isolate) were maintained by continuous passage in Vero cell culture by using standard procedures described previously (22). The parasites were harvested from tissue culture and washed three times in sterile 0.3 M phosphate-buffered saline (pH 7.4) and separated from host cell debris by passaging the mixture through a 25-gauge needle following passage through a 5-μm-pore-size polycarbonate filter. T. gondii (RH strain) tachyzoites were harvested with a 25-gauge needle and syringe from peritoneal cavities of mature female Swiss White mice that had been infected 3 days earlier with 106 tachyzoites by intraperitoneal injection (cellular contamination of <2%). The average number of tachyzoites per sample was determined by counting five aliquots by using a Neubauer chamber (standard error of the mean of ≤5%). A total of 107 tachyzoites of N. caninum and of T. gondii were pelleted and cryopreserved at −80°C for later DNA extraction.
Bovine aborted fetuses and mouse samples.
Frozen and paraffin-embedded brain samples from naturally infected bovine fetuses and experimentally infected mice were used to test the applicability of the technique. Brains were aseptically recovered; one hemisphere was subsequently frozen at −80°C until analyzed, and the other was processed by routine histological methods. For the histopathological study, the tissues were fixed in 10% neutral formalin and dehydrated through graded alcohols before being embedded in paraffin wax. Bovine fetuses were classified as negative or positive based on the observation of characteristic or compatible histological lesions of neosporosis in the brain as described previously (15). A total of 12 frozen fetus brain samples (6 positive and 6 negative) and 9 frozen brain samples from experimentally infected BALB/c mice, six inoculated animals with or without compatible lesions, and three negative controls were used in this study. The mice were 6-week-old female BALB/c mice inoculated with different live N. caninum parasite doses (1 × 106 and 5 × 106) via different inoculation routes (intraperitoneally and subcutaneously) and sacrificed 2 months after infection (Table 1).
TABLE 1.
Results obtained in brain samples from bovine aborted fetuses and inoculated mice tested by real-time PCR, histopathology, and nested PCR for the N. caninum infection
| Sample | Tissue type | No. of tachyzoites/ mg of tissuea | Results of:
|
|
|---|---|---|---|---|
| HPb | Nested PCR | |||
| Fetus | ||||
| 1 | Frozen | 0 | − | − |
| Paraffin embedded | 10.2 | + | ||
| 2 | Frozen | 7.5 | − | + |
| 3 | Frozen | 3.7 | − | + |
| 4 | Frozen | 2.9 | − | + |
| 5 | Frozen | 0 | − | − |
| 6 | Frozen | 0 | − | − |
| 7 | Frozen | 13 | + | + |
| 8 | Frozen | 17.4 | + | + |
| Paraffin embedded | 0 | − | ||
| 9 | Frozen | 26.6 | + | + |
| 10 | Frozen | 13.2 | + | + |
| 11 | Frozen | 9.7 | + | + |
| Paraffin embedded | 0 | − | ||
| 12 | Frozen | 0 | + | + |
| Mousec | ||||
| 1 | Frozen | 3.1 | − | + |
| 2 | Frozen | 0 | − | − |
| Paraffin embedded | 0 | − | ||
| 3 | Frozen | 0 | − | − |
| Paraffin embedded | 0 | − | ||
| 4 | Frozen | 3 | + | + |
| Paraffin embedded | 0 | − | ||
| 5 | Frozen | 109.6d | + | + |
| 6 | Frozen | 3.5 | + | + |
| Controls | Frozen | 0 | − | − |
Values are the means of two or more assays.
HP, histopathological study; a “+” indicates that brain samples showed compatible or characteristic lesions of neosporosis.
Mice 1 and 4 were infected with 5 × 106 Nc-1 tachyzoites subcutaneously, mice 2 and 6 were infected with 106 tachyzoites intraperitoneally, and mice 3 and 5 were infected with 106 tachyzoites subcutaneously.
Parasite tissue cysts were observed in the brain during the histopathologic examination.
DNA extraction.
Genomic DNA was extracted by using the Genomic-Prep cell and tissue DNA isolation kit (Amersham Pharmacia, Uppsala, Sweden) according to the manufacturer's instructions from 107 tachyzoites of N. caninum Nc-1 isolate and T. gondii RH strain, 20 mg of mammalian brain tissue from bovine fetuses and mice, and five sections of 5-μm-thick paraffin-embedded brain sections from three aborted fetuses and three mice. The samples from animal tissue were previously subjected to treatment with proteinase K (200 μg/ml; Sigma) at 55°C for 3 h to overnight. Dilutions of Neospora DNA were done in genomic DNA from uninfected mouse brain tissue at 20 ng/μl.
Real-time PCR.
Oligonucleotide primers for N. caninum Nc5 sequence (18) (GenBank accession no. X84238) were designed to amplify a 76-bp DNA fragment. The N. caninum Nc5 forward primer spans nucleotides 248 to 257 (5′-ACTGGAGGCACGCTGAACAC-3′) and the N. caninum Nc5 reverse primer spans nucleotides 303 to 323 (5′-AACAATGCTTCGCAAGAGGAA-3′). For the quantification of host DNA and to correct for the presence of potential PCR-inhibiting compounds in the DNA samples, the 28S rRNA gene was chosen. The sequence of the forward primer 28S-PF was 5′-TGCCATGGTAATCCTGCTCA-3′, and that of the reverse primer 28S-PR was 5′-CCTCAGCCAAGCACATACACC-3′, amplifying a product of 71 bp in both bovine and murine DNA (GenBank accession no. X00525). Reactions for the Neospora Nc5 sequence and 28S rRNA gene were performed with separate tubes. Primers were designed by using Primer Express software (PE Applied Biosystems). In both cases, the PCR mixture (25-μl total volume) contained 1× SYBR Green PCR Buffer, 2 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate (dATP, dCTP, and dGTP), 400 μM dUTP, 0.625 U of AmpliTaq Gold DNA polymerase, and 0.25 U of AmpErase UNG (uracil-N-glycosilase), all of which are included in the SYBR Green PCR Core Kit (PE Applied Biosystems); 20 pmol of each primer (Amersham Pharmacia); and 5 μl of DNA template. Amplification was performed by a standard protocol recommended by the manufacturer (2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and 60°C for 1 min). All samples were always run in duplicate. Amplification, data acquisition, and data analysis were carried out in the ABI 7700 Prism Sequence Detector machine, and the calculated cycle threshold values (Ct) were exported to Microsoft Excel for analysis. The quantification was performed by experimental determination of Ct defined as the cycle at which the fluorescence exceeds 10 times the standard deviation of the mean baseline emission for the early cycles. Number of parasites and the milligrams of host tissue in the samples were calculated by interpolation on two standard curves, in which Ct values were plotted against the log of known concentration of parasites and the milligrams of host tissue, respectively. After amplifications for Neospora Nc5 sequence, the melting curves of PCR products were acquired by stepwise increase of the temperature from 55 to 95°C for 20 min. Data analyses were done by using Dissociation Curves 1.0 f. software (PE Applied Biosystems).
Nested PCR.
Nested PCR on the internal transcribed spacer 1 (ITS1) region of N. caninum (27) was carried out with four oligonucleotides as described by Buxton et al. (11). Secondary amplification product was visualized by 1.8% agarose gel electrophoresis and ethidium bromide staining.
Analysis of data.
The degree of agreement between the different techniques used was estimated by calculating the kappa value (29) in frozen brain samples from 12 fetuses and 6 inoculated BALB/c mice. Comparison of parasite numbers in brain samples from bovine fetuses with or without compatible lesions was performed by a nonparametric Mann-Whitney U-test by using STAT-VIEW version 4.0.
RESULTS
Real-time PCR assays.
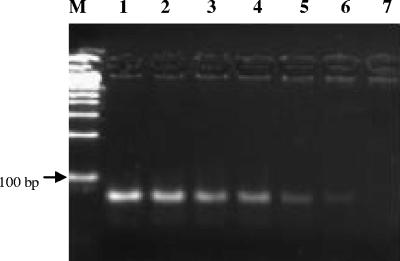
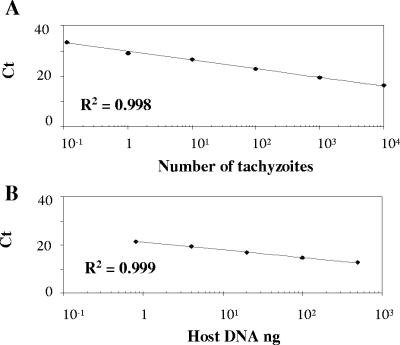
The detection limit of N. caninum by real-time PCR was determined by assaying serial 10-fold dilutions of parasite DNA equivalent to 10−2 to 104 tachyzoites in a background of 100 ng of mouse genomic DNA. PCR products were visualized on 3.5% agarose gels, and the expected 76-bp band was observed in the range of DNA dilutions equivalent to 10−1 to 104 tachyzoites (Fig. 1). Dilutions containing fewer than 10−1 tachyzoite genomes and mouse genomic DNA gave no amplification product. On the other hand, melting curve analysis detected a single product with a melting temperature of 79.0°C, only in the samples containing Neospora DNA equivalent to 10−1 to 104 tachyzoites (data not shown). A linear Neospora standard curve was routinely generated in each real-time PCR run from DNA equivalent to 10−1 to 104 tachyzoites (R2 = 0.998; slope = −3.36). Ct values increased linearly as target quantity decreased (Fig. 2), until the level of 10−1 tachyzoite equivalents was reached. The interassay coefficient of variation (CV) for six different runs was calculated, showing a value of 0.98% for the Ct of 10 tachyzoite equivalents (26.5 ± 0.3). No amplification products were detected when DNA equivalent to 104 tachyzoites of the closely related parasite T. gondii was tested with Nc5 Neospora-specific primers (data not shown). The amount of DNA per sample was normalized by the quantification of the 28S rRNA gene by real-time PCR. A standard curve was generated with fivefold serial dilutions of mouse brain DNA quantified by UV spectrophotometry (Fig. 2). The interassay CV for three independent runs was 0.6% for the Ct value of 100 ng of host DNA (14.5 ± 0.1).
FIG. 1.
Products of N. caninum real-time PCR in 3.5% agarose gel. Lanes 1 to 6, parasite DNA equivalent to 104 to 10−1 tachyzoites in 100 ng of mouse brain DNA; lane 7, DNA extracted from uninfected mouse brain; lane M, 100-bp DNA ladder (Biotools, Madrid, Spain).
FIG. 2.
Standard curves for the quantification of the N. caninum Nc5 sequence and the 28S rRNA host gene. (A) N. caninum standard curve showing Ct values plotted versus the log of the initial 10-fold dilution series of parasite DNA equivalent to 10−1 to 104 tachyzoites. (B) 28S rRNA standard curve showing Ct values plotted versus the log of the initial fivefold dilution series of host DNA (500 to 0.8 ng). All points corresponding to the Ct values represent the mean of duplicate PCR amplifications.
Parasite quantification in bovine and mouse samples.
Applicability of the technique in clinical samples was tested in brains from naturally infected bovine fetuses and experimentally infected mice. In some cases, PCR inhibitions were found in 5 μl of the templates; therefore, a 10-fold dilution was also analyzed to avoid possible inhibitions found in undiluted templates. Only the sample dilution that correctly quantified the 28S rRNA gene was subjected to Neospora Nc5 quantification. The number of N. caninum organisms and the amount of DNA present in each sample were quantified by interpolation of the corresponding Ct values in the standard curves, and a sample was considered positive when its Ct values of both duplicates were >10−1 tachyzoites. In order to estimate the amount of host DNA equivalent to a milligram of host brain tissue, five independent DNA extractions from 20 mg of uninfected frozen murine brain were done, and an average of 20 μg (20 ± 5.47) of host DNA was obtained. Based on this estimation, the final results were expressed as the number of tachyzoites per milligram of brain host tissue. The extrapolated amount of tachyzoites/milligram quantified by real-time PCR ranged from 2.9 to 26.6 in brains from aborted fetuses and from 3.0 to 109.6 in samples from mice (Table 1). Parasite numbers were significantly higher in brain samples from bovine fetuses showing compatible lesions than in samples without any detectable lesion (P < 0.05, Mann-Whitney U test). The repeatability of Neospora Nc5 and 28S rRNA real-time PCR tests in clinical samples was assessed by conducting the tests three times under identical conditions with six fetuses and three mouse brain DNA samples (Table 2).
TABLE 2.
Repeatability of Neospora Nc5 and 28S rRNA real-time PCR tests in samples of brain from bovine aborted foetuses and inoculated mice
| Sample | No. of tachyzoitesa
|
Amt of brain tissue (mg)a
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | % CV | Mean | SD | % CV | |
| Fetus | ||||||
| 1 | 0 | 0 | 0 | 21.9 | 0.8 | 3.5 |
| 2 | 13.3 | 0.6 | 4.3 | 1.7 | 0.1 | 5.4 |
| 3 | 16.7 | 3.1 | 18.3 | 4.6 | 0.8 | 17.6 |
| 4 | 43.3 | 12.7 | 29.2 | 14.9 | 0.8 | 5.1 |
| 7 | 36 | 1.0 | 2.8 | 2.8 | 0.2 | 8.3 |
| 10 | 46 | 8.5 | 18.6 | 3.5 | 0.4 | 13.0 |
| Mouse | ||||||
| 1 | 22.7 | 4.2 | 18.4 | 7.4 | 1.2 | 16.7 |
| 4 | 19.7 | 7.2 | 36.8 | 6.6 | 1.5 | 22.9 |
| 5 | 265.3 | 18.1 | 6.8 | 2.4 | 0.2 | 10.5 |
The given values are the means of the duplicates of the three different PCR runs.
Comparison of real-time PCR, nested PCR, and histology analyses.
Agreement (measured as κ [kappa]) (29) between real-time PCR and nested PCR analyses was almost perfect (κ = 0.86). However, when histology was compared with either the real-time PCR or the nested PCR, only a moderate degree of agreement was obtained (κ = 0.44 and 0.55, respectively) and DNA from Neospora was detected in several brain samples in the absence of microscopic lesions.
DISCUSSION
In the present study, we describe the development and standardization of a real-time PCR assay for the quantitative detection of N. caninum in infected host tissues. Quantification by PCR of parasite levels in different host tissues would assist in the near future in the investigation of infection and disease and in the evaluation of vaccines or therapeutic drugs to control Neospora infection in cattle and dogs. The use of real-time PCR to detect and measure the parasite load has several advantages over conventional PCR techniques since amplification of the product, detection, and quantification take place within a single tube, obviating the need for post-PCR manipulation and reducing the risk of contamination. In addition, SYBR Green I methodology does not require the use of gene-specific hybridization probes, providing a flexible, easy, and fast method.
The sequence Nc5 was chosen as the target for our assay for several reasons: this sequence has apparently not been found in other taxa; it is a repetitive sequence (18, 31); and, finally, it has shown excellent sensitivity when used in a quantitative-competitive detection system for Neospora in mice (19) and for the diagnoses of bovine aborted fetuses (6, 16). In the real-time PCR for the Nc5 sequence, as little as 0.1 tachyzoites per reaction, which is approximately equal to 10 fg of genomic Neospora DNA, could be detected. Furthermore, differences in Ct values in samples containing 0.1 tachyzoites and no template control (33.2 versus 40) allows an accurate discrimination between infected and noninfected tissues. This sensitivity is comparable to that reported for other real-time PCR protocols for the parasite T. gondii (0.05 parasites/reaction) (21) and that described for the Nc5 Neospora PCR, both as a QC-PCR (one parasite/0.45 mg of mouse tissue) (19) and as a conventional PCR, where the detection limit was a single tachyzoite in the presence of 2 mg of brain tissue (31). In the present study, the sensitivity in clinical samples was compared with an ITS1 Neospora nested PCR, and a high degree of agreement was shown between both PCR techniques. Discrepant results (i.e., fetus 12) may be due to a false-positive result or to very low parasite numbers in the sample detectable only by nested PCR. Using SYBR Green I as detection reagent in real-time PCR, the specificity issue may be an open question. In our case, the designed primers were species specific, and no signals were obtained with DNA of the closely related parasite T. gondii or with mammalian host DNA. In addition, as shown in Fig. 1, nonspecific DNA bands and primer dimers were not perceptible on agarose gels, and only the expected 76-bp DNA fragment was observed when Neospora DNA was present in the template.
Variation in quantity and quality of DNA extracted from samples of diverse origin (frozen versus paraffin embedded, cattle versus mice) hampers direct quantification in terms of parasites per milligram of tissue. In order to obtain an indirect measure of the amount of tissue assayed that allowed a proper comparison of the number of N. caninum organisms per sample in different host tissues, a parallel real-time PCR was developed to quantify the amount of host DNA in the sample, targeting the 28S rRNA host gene. The combination of several factors, such as a high conservation among species, the presence of the target in bovine and murine tissues, and a high copy number, made the 28S rRNA host gene a worthwhile candidate for our assay. In addition, such analysis should compensate for the presence of potential PCR-inhibiting compounds and exclude false-negative results due to poor-quality DNA. Another criterion for the design of both 28S rRNA and Neospora Nc5 primers was that the resultant amplicon should be as short as practical since DNA in some clinical samples may be degraded due to autolysis, aging, or chemical fixation (14).
To prove the applicability of the technique, tissues from naturally and experimentally infected host brain tissues were selected. Brain is the organ most consistently parasitized by the two Neospora phases in the intermediate host and the target organ to detect specific and/or consistent lesions both in aborted fetuses (2, 6, 17) and infected mice (23, 24). PCR quantification by real-time was performed on frozen and paraffin-embedded brain tissue sections. The data obtained suggest a positive relationship between parasite burden and the presence of lesions and parasite tissue cysts observed in brain during the histopathologic examination. Besides, lower parasite numbers were detected in brain tissues in the absence of lesions. In our study, the presence of parasite DNA was also confirmed in a majority of cases by nested PCR. In contrast, a weak agreement was observed between these techniques and histopathological studies. Previous histological results concerning parasite presence in areas with lesions are controversial. Some studies in Neospora bovine aborted fetuses revealed the presence of Neospora in the necrotic and inflammatory areas (1, 3, 9). Other studies, however, showed that the location of the lesions did not correlate with the presence of tachyzoites and/or tissue cysts (17, 30). Regarding the parasite level in murine samples, similar parasite numbers were observed in brains from experimentally infected mice and from naturally infected bovine fetuses, which suggests that the mouse is an appropriate model for studying the distribution and level of parasites.
In summary, we demonstrate here a rapid and flexible method for quantifying N. caninum by using continuous fluorescence monitoring of a double-stranded DNA-specific dye, a method that is capable of testing a large number of samples, and the results can be obtained in a short time and statistically analyzed. Thus, this technique can be used as an alternative to the tedious visual counting of parasite forms or to labor-intensive QC-PCR. The novel procedure reported here allows accurate quantification of N. caninum in tissue samples by a reproducible and sensitive technique. The ability to quantify N. caninum in tissue samples can be a useful quantitative diagnostic tool for use in the study of the pathogenicity, immunoprophylaxis, and treatment of Neospora infection in cattle and murine models.
Acknowledgments
We thank the Centro de Secuenciación de DNA (U.C.M.) for allowing us to use an ABI 7700 Prism Sequence Detector machine and Vanesa Navarro for technical assistance.
This work was supported by a grant from the Spanish Government (CICYT-AGF98-0804-CO2) and E.C.-F. was financed by Ministerio de Ciencia y Tecnología (Spain).
REFERENCES
- 1.Anderson, M. L., P. C. Blanchard, B. C. Barr, P. A. Conrad, J. P. Dubey, and R. L. Hoffman. 1991. Neospora-like protozoal infection as a major cause of abortion in California dairy cattle. J. Am. Vet. Med. Assoc. 198:241-244. [PubMed] [Google Scholar]
- 2.Barr, B. C., M. L. Anderson, P. C. Blanchard, B. M. Daft, H. Kinde, and P. A. Conrad. 1990. Bovine fetal encephalitis and myocarditis associated with protozoal infections. Vet. Pathol. 27:354-361. [DOI] [PubMed] [Google Scholar]
- 3.Barr, B. C., M. L. Anderson, J. P. Dubey, and P. A. Conrad. 1991. Neospora-like protozoal infections associated with bovine abortions. Vet. Pathol. 28:110-116. [DOI] [PubMed] [Google Scholar]
- 4.Barr, B. C., J. D. Rowe, M. L. Anderson, K. W. Sverlow, R. H. BonDurant, A. A. Ardans, M. N. Oliver, and P. A. Conrad. 1994. Experimental reproduction of bovine fetal Neospora infection and death with a bovine Neospora isolate. J. Vet. Diagn. Investig. 6:207-215. [DOI] [PubMed] [Google Scholar]
- 5.Barr, B. C., J. D. Rowe, M. L. Anderson, K. Sverlow, and P. A. Conrad. 1995. Diagnosis of bovine fetal Neospora infection with an indirect fluorescent antibody test. Vet. Rec. 137:611-613. [PubMed] [Google Scholar]
- 6.Baszler, T. V., J. C. Lawrence, T. L. Maureent, and B. Mathison. 1999. Detection by PCR of Neospora caninum in fetal tissues from spontaneous bovine abortions. J. Clin. Microbiol. 37:4059-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjerkas, I., S. F. Monh, and J. Presthus. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 70:271-274. [DOI] [PubMed] [Google Scholar]
- 8.Björkman, C., and A. Uggla. 1999. Serological diagnosis of Neospora caninum infection. Int. J. Parasitol. 29:1497-1507. [DOI] [PubMed] [Google Scholar]
- 9.Bryan, L. A., A. A. Gajadhar, J. P. Dubey, and D. M. Haines. 1994. Bovine neonatal encephalomyelitis associated with a Neospora sp. protozoan. Can. Vet. J. 35:11-13. [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton, D., G. L. Caldow, S. W. Maley, J. Marks, and E. A. Innes. 1997. Neosporosis and bovine abortions in Scotland. Vet. Rec. 141:649-651. [PubMed] [Google Scholar]
- 11.Buxton, D., S. W. Maley, S. Wright, K. M. Thomson, A. G. Rae, and E. A. Innes. 1998. The pathogenesis of experimental neosporosis in pregnant sheep. J. Comp. Pathol. 118:267-279. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, J. P. 1999. Recent advances in Neospora and neosporosis. Vet. Parasitol. 84:349-367. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, J. T. 1998. Polymerase chain reaction approaches for the detection of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 28:1053-1060. [DOI] [PubMed] [Google Scholar]
- 14.Ellis, J. T., D. McMillan, C. Ryce, S. Payne, R. Atkinson, and P. A. W. Harper. 1999. Development of a single tube nested polymerase chain reaction assay for the detection of Neospora caninum DNA. Int. J. Parasitol. 29:1589-1596. [DOI] [PubMed] [Google Scholar]
- 15.González, L., D. Buxton, R. Atxaerandio, S. Aduriz, S. W. Maley, J. C. Marco, and L. A. Cuervo. 1999. Bovine abortion associated with Neospora caninum in northern Spain. Vet. Rec. 144:145-150. [DOI] [PubMed] [Google Scholar]
- 16.Gottstein, B., B. Hentrich, R. Wyss, B. Thür, A. Busato, K. D. C. Stärk, and N. Müller. 1998. Molecular and immunodiagnostic investigations on bovine neosporosis in Switzerland. Int. J. Parasitol. 28:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattel, A. L., M. D. Castro, J. D. Gummo, D. Weinstock, J. A. Reed, and J. P. Dubey. 1998. Neosporosis-associated bovine abortion in Pennsylvania. Vet. Parasitol. 74:307-313. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann, H., M. Yamage, I. Roditi, D. Dobbelaere, J. P. Dubey, O. J. M. Holmdahl, A. Trees, and B. Gottstein. 1996. Discrimination of Neospora caninum from Toxoplasma gondii and other apicomplexan parasites by hybridization and PCR. Mol. Cell. Prob. 10:289-297. [DOI] [PubMed] [Google Scholar]
- 19.Liddell, S., M. C. Jenkins, and J. P. Dubey. 1999. A competitive PCR assay for quantitative detection of Neospora caninum. Int. J. Parasitol. 29:1583-1587. [DOI] [PubMed] [Google Scholar]
- 20.Liddell, S., M. C. Jenkins, and J. P. Dubey. 1999. Vertical transmission of Neospora caninum in BALB/c mice determined by polymerase chain reaction detection. J. Parasitol. 85:550-555. [PubMed] [Google Scholar]
- 21.Lin, M. H., T. C. Chen, T. T. Kuo, C. C. Tseng, and C. P. Tseng. 2000. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 38:4121-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsay, D. S., and J. P. Dubey. 1989. In vitro development of Neospora caninum (Protozoa: Apicomplexa) from dogs. J. Parasitol. 75:163-165. [PubMed] [Google Scholar]
- 23.Lindsay, D. S., S. D. Lenz, R. A. Cole, J. P. Dubey, and B. L. Blagburn. 1995. Mouse model for central system Neospora caninum infections. J. Parasitol. 81:313-315. [PubMed] [Google Scholar]
- 24.Long, M. T., T. V. Baszler, B. A. Mathison. 1998. Comparison of intracerebral parasite load, lesion development and systemic cytokines in mouse strains infected with Neospora caninum. J. Parasitol. 84:316-320. [PubMed] [Google Scholar]
- 25.McAllister, M. M., E. M. Huffman, S. K. Hietala, P. A. Conrad, M. L. Anderson, and M. D. Salman. 1996. Evidence suggesting a point source exposure in an outbreak of bovine abortion due to neosporosis. J. Vet. Diagn. Investig. 8:355-357. [DOI] [PubMed] [Google Scholar]
- 26.Paré, J., S. K. Hietala, and M. C. Thurmond. 1995. An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J. Vet. Diagn. Investig. 7:352-359. [DOI] [PubMed] [Google Scholar]
- 27.Payne, S., and J. Ellis. 1996. Detection of Neospora caninum DNA by the polymerase chain reaction. Int. J. Parasitol. 26:347-351. [DOI] [PubMed] [Google Scholar]
- 28.Schock, A., D. Buxton, J. A. Spence, J. C. Low, and A. Baird. 2000. Histopathological survey of aborted bovine fetuses in Scotland with special reference to Neospora caninum. Vet. Rec. 147:687-688. [PubMed] [Google Scholar]
- 29.Thrusfield, M. 1995. Diagnostic testing, p. 266-285. In M. Thrusfield (ed.), Veterinary epidemiology, 2nd ed. Blackwell Science, Ltd., Oxford, England.
- 30.Venturini, M. C., L. Venturini, D. Bacigalupe, M. Machuca, I. Echaide, W. Basso, J. M. Unzaga, C. Di Lorenzo, A. Guglielmone, M. C. Jenkins, and J. P. Dubey. 1999. Neospora caninum infections in bovine fetuses and dairy cows with abortions in Argentina. Int. J. Parasitol. 29:1705-1708. [DOI] [PubMed] [Google Scholar]
- 31.Yamage, M., O. Flechtner, and B. Gottstein. 1996. Neospora caninum: specific oligonucleotide primers for detection of brain “cyst” DNA of experimentally infected nude mice by the polymerase chain reaction. J. Parasitol. 82:272-279. [PubMed] [Google Scholar]