Abstract
OBJECTIVES: To study the epidemiology of gait disorders in community-residing older adults and their association with death and institutionalization.
DESIGN: Community-based cohort study.
SETTING: Bronx County and the research center at Albert Einstein College of Medicine.
PARTICIPANTS: The Einstein Aging study recruited 488 adults aged 70 to 99 between 1999 and 2001. At entry and during annual visits over 5 years, subjects received clinical evaluations to determine presence of neurological or nonneurological gait abnormalities.
MEASUREMENTS: Prevalence and incidence of gait disorders based on clinical evaluations and time to institutionalization and death.
RESULTS: Of 468 subjects (95.9%) with baseline gait evaluations, 168 had abnormal gaits: 70 neurological, 81 nonneurological, and 17 both. Prevalence of abnormal gait was 35.0% (95% confidence interval (CI)=28.6-42.1). Incidence of abnormal gait was 168.6 per 1,000 person-years (95% CI=117.4-242.0) and increased with age. Men had a higher incidence of neurological gait abnormalities, whereas women had a higher incidence of nonneurological gaits. Abnormal gaits were associated with greater risk of institutionalization and death (hazard ratio (HR)=2.2, 95% CI=1.5-3.2). The risk was strongly related to severity of impairment; subjects with moderate to severe gait abnormalities (HR=3.2, 95% CI=1.9-5.2) were at higher risk than those with mild gait abnormalities (HR=1.8, 95% CI=1.0-2.8).
CONCLUSION: The incidence and prevalence of gait disorders are high in community-residing older adults and are associated with greater risk of institutionalization and death.
Keywords: gait, incidence, prevalence, death, institutionalization
Gait is routinely examined in clinical practice to assess the health of older adults.1 Abnormal gait has been associated with greater risk for adverse outcomes in older adults such as immobility, falls, and dementia, which in turn lead to loss of functional independence and death.2-7 Abnormal gait may result from neurological (e.g., strokes) or nonneurological (e.g., arthritis) conditions.1,4 Neurological gait abnormalities result from focal or diffuse lesions occurring in the neural pathways linking the cortical motor centers to the peripheral neuromuscular systems.1,4,8,9 Nonneurological gait abnormalities usually present with slow gait due to limitations resulting from musculoskeletal, cardiac, or respiratory diseases. Some gait patterns, such as slowing or short steps, that were previously considered to be part of normal aging5 are now being recognized as disease markers.
Although abnormal gait is said to be common with advancing age,1-9 the epidemiology of gait disorders in older adults is not well established. Incidence data of gait disorders, which are crucial for assessing the association between sociodemographic and medical risk factors and abnormal gait, are lacking. Previous prevalence estimates are unlikely to capture the full spectrum of gait disorders in the community, because they are derived from clinic samples,8,9 focus on specific gait subtypes,6 or use functional assessments and self-report.2,7
The Einstein Aging Study,10,11 a longitudinal study of community-residing older adults, provided the opportunity to study the prevalence and incidence of gait disorders and report their association with risk of death and institutionalization over a 5-year study period. Defining the epidemiology of abnormal gait offers the possibility of introducing therapeutic interventions early to reduce adverse outcomes in older adults.
METHODS
Study Population
Subjects were participants in the Einstein Aging Study.10,11 Eligibility criteria for the study were aged 70 and older, community residing, and English speaking. Exclusion criteria included severe audiovisual disturbances and bedbound because of illness. Individuals were identified from Medicare lists of older adults in Bronx County. From this list, 31,335 potential subjects were selected randomly and contacted with a letter explaining the purpose and nature of the study. They were then contacted by telephone (46% successfully contacted). The telephone interview included oral consent, medical history questionnaire, and cognitive screening tests.10 At the end of the interview, a computerized randomization procedure chose an age-stratified sample of 613 subjects from those who gave oral consent, oversampling from older age groups. Potential subjects were invited for further evaluation at the clinical research center based at the Albert Einstein Medical College. Written informed consent was obtained according to protocols approved by the local institutional review board. Between 1999 and 2001, 488 subjects were enrolled. Subjects returned at 12- to 18-month intervals. Follow-up is reported through May 2004. Mean age ± standard deviation at enrollment was 77.4 ± 5.2 (range 70-99). The subjects in the inception cohort were mostly women (59%); 67% were white (black 27%).
Clinical Gait Assessment
At each visit, study clinicians performed structured neurological evaluations, which included assessment of cranial nerves, muscle strength, sensation, and deep tendon reflexes.4,10,11 Gait patterns and turns were observed while subjects walked up and down a well-lit hallway at their normal pace. Study clinicians determined whether gaits were normal or abnormal after visual inspection of gait. Abnormal gaits were further subtyped as nonneurological (from causes such as arthritis, cardiac disease, chronic lung disease, and peripheral vascular disease) or neurological (unsteady, ataxic, frontal, parkinsonian, neuropathic, hemiparetic, and spastic).4 Neurological gait abnormalities were subtyped as unsteady if subjects experienced marked swaying or losing balance under two or more of the following conditions: while walking in a straight line or in tandem or while making turns. Ataxic (cerebellar) gait is wide based, with other cerebellar signs such as intention tremor. Ataxic and unsteady gaits were combined, because they share clinical features such as wide base and poor balance. Patients with neuropathic gaits have foot drop, sensory loss, and depressed deep tendon reflexes. Short steps, wide base, and difficulty lifting the feet off the floor characterize frontal gait. Older people with parkinsonian gaits have small shuffling steps, flexed posture, absent arm swing, en bloc turns, and festination. Patients with hemiparetic gait swing a leg outward and in a semicircle from the hip (circumduction). In spastic gait, both legs circumduct and when severe cross in front of one another (scissoring). See4 for Web links to videos of abnormal neurological gait subtypes.
Study clinicians ranked multiple subtypes if present within neurological or nonneurological categories. For this analysis, the highest-ranked subtype was used, although if abnormal gaits resulted from both neurological and nonneurological causes, they were classified and reported as “combined.” Abnormalities were clinically graded as mild (walks without assistance), moderate (uses walking aids), or severe (uses a wheelchair or stands with assistance).
Clinicians who evaluated gaits at study visits were blinded to results of previous assessments. Eighty-nine percent agreement (kappa = 0.6) was reported on gait classification as neurological or not neurological during study evaluations conducted a year apart in 189 subjects in the Bronx Aging Study.4 Interrater reliability, studied prospectively, between two study clinicians who independently assessed gait (normal vs any abnormal gait) in 30 subjects from the present cohort was good (kappa = 0.8).11
Covariates
Subjects, family members, and caregivers were interviewed at study visits about sociodemographic variables, medical illnesses, medications, and activities of daily living. Cognition was assessed using a neuropsychological test battery.10,11Herein, Blessed Information Memory Concentration test,12a general mental status test, is reported. Depressive symptoms were assessed using the Geriatric Depression Scale.13Information on outcomes such as hospitalizations and institutionalization was collected. Medical records and primary care providers were also consulted for additional information.
Data Analysis
Baseline characteristics were compared using descriptive statistics, applying nonparametric tests as appropriate.14 To adjust for the age-stratified sampling design, sampling weights based on age at enrollment were used. Age- and sex-specific prevalence rates were estimated using a weighted logistic regression model. Incidence rates were estimated using a Poisson regression model accounting for person-years of follow-up of each subject. Standard errors for prevalence and incidence rates were calculated using replication-based variance estimation.15 Incident cases were defined as when abnormal gait was first diagnosed on follow-up. Subjects with prevalent gait abnormalities (pure and combined) were excluded for the incidence analysis. Tests for trends in incidence and prevalence were calculated from age-specific estimates.16
Cox proportional hazards models were used to compute hazard ratios (HRs, with 95% confidence intervals (CIs)) to compare risk of death, institutionalization (nursing home or assisted living facility placement), or both in subjects with baseline abnormal gaits or subtypes with risk in unaffected people, adjusted for age and sex.17 Time to event was from entry to death, institutionalization, or final contact.
RESULTS
Baseline Characteristics
Of the 468 subjects (95.9%) with gait evaluations at entry, 168 were diagnosed with abnormal gaits: 70 neurological, 81 nonneurological, and 17 combined. Unsteady gait (46.6%) was the most common neurological gait abnormality subtype, followed by hemiparetic (26.4%), frontal (12.6%), parkinsonian (9.3%), neuropathic (4.1%), and spastic (1.0%). Causes of pure nonneurological gait abnormalities included arthritis or joint deformities (84.8%), chronic lung disease (6.2%), angina pectoris (4.0%), cardiac failure (3.1%), and peripheral vascular disease (1.9%). Prevalence of moderate to severe abnormal gait was higher in subjects with nonneurological gait abnormalities than in those with neurological gait abnormalities (33.3% vs 17.1%, P=.02). Table 1 shows that subjects with abnormal gait were older (P<.001), had higher Blessed test scores (P=.01), and had more depressive symptoms (P<.001) at entry. There were no significant sex or race differences by gait status. Subjects with abnormal gaits had a higher prevalence of hypertension (P=.002), diabetes mellitus (P=.01), myocardial infarctions (P=.02), and strokes (P<.001).
Table 1.
Baseline Characteristics of Subjects with and without Gait Abnormalities
| Characteristic | Normal (n=300) | Abnormal (n=168) | Neurological* (n=70) | Nonneurological* (n=81) |
|---|---|---|---|---|
| Age, mean ± SD | 76.8 ± 4.9 | 78.6 ± 5.7† | 78.7 ± 5.6 | 78.5 ± 5.7 |
| Female, % | 58.0 | 58.8 | 57.6 | 64.9 |
| Race, % | ||||
| White | 72.0 | 72.8 | 77.4 | 69.1 |
| Black | 23.8 | 22.8 | 20.2 | 24.7 |
| Blessed test score, mean ± SD (range 0-32,>7 abnormal) | 2.5 ± 2.8 | 3.2 ± 2.9‡ | 3.5 ± 3.1 | 3.1 ± 2.9 |
| Geriatric Depression Scale score, mean ± SD (range 0-15) | 2.1 ± 2.2 | 3.5 ± 2.7† | 3.4 ± 2.6 | 3.7 ± 2.9 |
| Medical illnesses, % | ||||
| Hypertension | 52.3 | 64.8† | 60.0 | 65.4 |
| Previous myocardial infarction | 9.3 | 16.7‡ | 20.0 | 18.8 |
| Cardiac failure | 3.7 | 5.9 | 7.1 | 3.7 |
| Chronic lung disease | 11.7 | 15.5 | 12.9 | 17.3 |
| Diabetes mellitus | 12.0 | 20.8‡ | 17.1 | 20.9 |
| Strokes | 6.0 | 16.1† | 18.6 | 12.3 |
| Parkinson's disease | 0.0 | 5.4 | 9.3 | 1.2 |
| Depression | 8.0 | 8.9 | 5.7 | 8.6 |
| Arthritis | 56.0 | 72.6† | 58.6 | 85.1 |
Note: Statistical comparisons are between subjects with normal and abnormal gaits at baseline.
Includes pure cases only, with combined gait abnormalities excluded.
P< .05
.01
SD = standard deviation.
Prevalence
The weighted overall prevalence estimate of any abnormal gait was 35.0% (95% CI=28.6-42.1). The prevalence of any neurological gait abnormalities, including combined, was 15.7% (95% CI=11.1-21.7) and of any nonneurological gait abnormalities was 20.8% (95% CI=15.9-26.7). Age-specific prevalence rates are shown in Table 2. Prevalence of abnormal gaits was not different between men (prevalence 35.5%, 95% CI=26.1-46.2) and women (prevalence 34.7%, 95% CI=27.0-43.3). There were also no significant sex differences in prevalence of gait subtypes. Most abnormal gaits were mild, with an overall prevalence of 25.8% (95% CI=20.2-32.4). Prevalence of moderate abnormalities was 6.0% (95% CI=3.7-9.6). Severe abnormalities were less common in this community-based sample, with an overall prevalence of 3.2% (95% CI=1.4-7.3).
Table 2.
Age-Specific Prevalence of Gait Disorders, Adjusted for Study Design
| Age |
|||||
|---|---|---|---|---|---|
| 70-74 (n = 171) |
75-79 (n = 141) |
80-84 (n = 98) |
≥85 (n = 58) |
Total (N = 468) |
|
| Gait Status | % (95% Confidence Interval) | ||||
| Abnormal (n = 168) | 24.3 (16.4-34.50) | 40.1 (29.0-52.4) | 59.4 (46.5-71.2) | 45.9 (26.5-66.7) | 35.0 (28.6-42.1) |
| Neurological (n = 87) | 12.3 (6.5-22.1) | 9.8 (4.6-19.7) | 24.8 (14.5-39.1) | 28.6 (12.7-52.5) | 15.7 (11.1-21.7) |
| Nonneurological (n = 98) | 12.3 (7.1-20.3) | 30.4 (19.9-43.4) | 34.6 (23.3-47.9) | 17.3 (6.1-40.3) | 20.8 (16.0-26.7) |
Incidence
Of 300 subjects with normal gaits at baseline, 73 developed abnormal gaits over 345 person-years: 29 neurological, 34 nonneurological, and 10 combined. The weighted incidence of any abnormal gait was 168.6 per 1,000 person-years (Table 3), and increased with age (P for trend = .005). The incidence of abnormal gaits in men (165.6 per 1,000 person-years) and women (170.5 per 1,000 person-years) was similar, although men had a higher incidence of neurological gait abnormalities (153.5 per 1,000 person-years, 95% CI=92.7-254.2) than women (73.8 per 1,000 person-years, 95% CI=41.4-131.6), whereas women (132.7 per 1,000 person-years, 95% CI=82.9-212.6) had a higher incidence of nonneurological gait abnormalities than men (57.8 per 1,000 person-years, 95% CI=25.4-131.2).
Table 3.
Age-Specific Incidence per 1,000 Person-Years by Gait Status, Adjusted for Study Design
| Age |
|||||
|---|---|---|---|---|---|
| 70-74 (n = 171) |
75-79 (n = 141) |
80-84 (n = 98) |
≥85 |
Total |
|
| Gait Status | Cases/N (Incidence) 95% Confidence Interval | ||||
| Abnormal | 25/136 (116.4) 62.3-217.5 | 21/89 (247.6) 149.0-411.4 | 17/50 (321.4) 188.6-547.7 | 9/25 (502.8) 284.5-888.6 | 73/300 (168.6) 117.4-242.0 |
| Neurological | 20/159 (74.7) 36.9-150.9 | 19/115 (126.4) 65.3-244.6 | 17/69 (155.3) 78.7-306.5 | 7/35 (397.2) 203.1-776.9 | 63/378 (103.1) 72.2-147.1 |
| Nonneurological | 22/157 (79.7) 40.6-156.4 | 14/106 (156.5) 80.7-303.5 | 9/70 (114.3) 46.4-218.7 | 6/37 (233.3) 70.5-771.8 | 51/370 (103.1) 68.4-155.3 |
Of the 378 subjects with normal gait or pure nonneurological gait abnormality (excluding combined) at baseline, 63 developed neurological gait abnormalities over 452 person-years (incidence 103.1 per 1,000 person-years). Of the 370 subjects with normal gait or pure neurological gait abnormalities (excluding combined) at baseline, 51 developed nonneurological gait abnormalities over 421 person-years (incidence 103.1 per 1,000 person-years). The incidence of neurological gait abnormalities was higher than nonneurological gait abnormalities in subjects aged 80 and older (Table 3).
The association between chronic medical illnesses (Table 1) and incident neurological or nonneurological gait abnormalities was also examined, adjusted for age, sex, and Blessed score. Stroke (HR = 2.6, 95% CI = 1.3-5.1) and hypertension (HR = 1.9, 95% CI = 1.1-3.2) at baseline independently predicted risk of incident neurological abnormality. Only arthritis (HR = 3.0, 95% CI = 1.4-6.2) predicted risk of nonneurological gait abnormalities. Excluding subjects who died or using age as the time scale in the model to better account for differing risk based on age at entry did not change predictors or their significance.
Gait, Institutionalization, and Death
Subjects were censored at last contact or at study outcome (mean follow-up 1.7 ± 0.8 years). Of the 468 subjects, 422 returned for follow-up gait evaluations 1 year later. Of the 44 subjects who did not return after their first visit, six died and four were institutionalized. Over the 5-year study period, 30 subjects died (15 with abnormal gait at baseline), 75 were institutionalized (43 abnormal gaits), 15 relocated, 33 were lost to contact, 16 stopped participation because they had become caregivers for family members, and 59 refused to continue. Mean follow-up in subjects who dropped out without reaching a study outcome was 1.3 ± 0.9 years. Table 4 and Figure 1 show that risk of death and institutionalization for those with abnormal gaits was 2.16 times that of those without it (95% CI = 1.45-3.24), which was mainly due to institutionalization over this short study period (HR = 2.12, 95% CI = 1.33-3.37). Neurological but not nonneurological gait abnormalities were associated with greater risk of institutionalization.
Table 4.
Risk of Death, Institutionalization, or Both over the 5-Year Study Period by Baseline Gait Status, Adjusted for Age and Sex
| Institutionalization (n = 75) |
Death (n = 30) |
Institutionalization or Death (n = 99) |
||
|---|---|---|---|---|
| Gait | N = 468 | Hazard Ratio (95% Confidence Interval) | ||
| Any abnormality | ||||
| Overall | 168 | 2.12 (1.33-3.37) | 1.63 (0.79-3.36) | 2.16 (1.45-3.24) |
| Normal | 300 | 1 (reference) | 1 (reference) | 1 (reference) |
| Mild | 118 | 1.99 (1.18-3.36) | 0.89 (0.32-2.51) | 1.76 (1.01-2.84) |
| Moderate-severe | 50 | 2.67 (1.47-4.84) | 3.66 (1.62-8.29) | 3.18 (1.94-5.21) |
| Neurological | ||||
| Overall | 87 | 2.35 (1.46-3.81) | 2.02 (0.92-4.48) | 2.39 (1.55-3.61) |
| Normal/nonneurological | 381 | 1 (reference) | 1 (reference) | 1 (reference) |
| Mild | 63 | 2.38 (1.35-4.20) | 1.17 (0.34-3.95) | 2.07 (1.23-3.51) |
| Moderate-severe | 24 | 2.31 (1.15-4.66) | 3.32 (1.27-8.69) | 2.85 (1.62-5.03) |
| Nonneurological | ||||
| Overall | 98 | 1.29 (0.79-2.13) | 1.99 (0.94-4.22) | 1.55 (1.02-2.36) |
| Normal/neurological | 370 | 1 (reference) | 1 (reference) | 1 (reference) |
| Mild | 61 | 0.98 (0.51-1.88) | 0.79 (0.23-2.71) | 1.00 (0.66-1.79) |
| Moderate-severe | 37 | 1.87 (0.99-3.53) | 4.13 (1.80-9.48) | 2.54 (1.52-4.23) |
Figure 1.
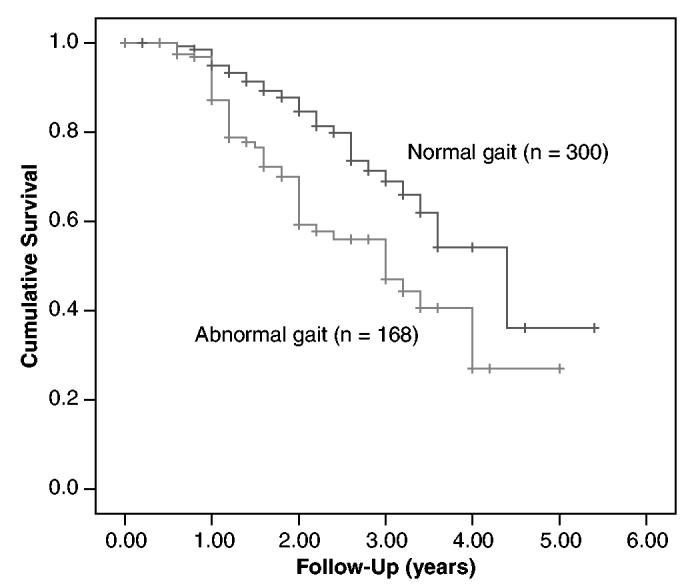
Kaplan-Meier survival curves comparing risk of death and institutionalization over the 5-year study period of subjects with abnormal gaits at enrollment and subjects with normal gaits.
Potential confounders for the association between gait and the study outcomes included dementia and vascular risk factors such as hypertension and stroke,4,18-21 although the results were unchanged when the models were adjusted for medical illnesses and Blessed scores.12 There was no significant interaction between age and gait (P = .60). When subjects who developed incident gait abnormalities by the second year were excluded, the associations between neurological (HR = 2.32, 95% CI = 1.02- 5.27) and nonneurological gait abnormalities (HR = 2.22, 95% CI = 1.01-4.86) and risk of death were strengthened. Excluding subjects who died before institutionalization strengthened the association between nonneurological gait abnormality and institutionalization, but the results were not significant (data not shown).
The effect of the severity of gait impairment on outcomes was also examined (Table 4). Risk of institutionalization and death was greater in subjects with moderate to severe abnormalities (HR = 3.18, 95% CI = 1.94-5.21) than in those with mild abnormalities (HR = 1.76, 95% CI = 1.01-2.84). Subjects with all grades of severity of neurological gaits were at greater risk of institutionalization. Although the overall association with death was not significant for both gait subtypes, moderate to severe abnormalities of both gait subtypes at baseline showed strong associations with the risk of death.
DISCUSSION
This is the first study, to the authors' knowledge, to report the prevalence and incidence of gait disorders in community-residing older adults using systematic assessments of neurological and nonneurological gait abnormalities. A high incidence of abnormal gait, which increased with advancing age, was found. Although there were no sex differences in the incidence of abnormal gaits overall, men had a higher incidence of neurological gait abnormalities, and women had a higher incidence of nonneurological gait abnormalities. This sex difference may relate to differing medical risk factors. For instance, arthritis is more common in older women and is a major contributor to nonneurological gait abnormalities.22,23 Incidence data based on comprehensive gait evaluations are lacking. The cumulative incidence of severe walking disability (gait velocityo < 40 cm/ s or inability to walk one quarter of a mile) over 3 years in the Women's Health and Aging Study was 22.8%,7 although women with mild or moderate disability at baseline were not excluded from the estimates.
The prevalence estimates in the current study are compatible with previous studies.6,7,18,19,24 The weighted prevalence of abnormal gait was lower in subjects aged 85 and older, which the lower prevalence of nonneurological gait abnormalities in this age group explains. The lower prevalence may reflect participation bias or poor survival in the community, as suggested by the higher risk of adverse outcomes with increasing gait severity. Moreover, nonneurological gaits were more severe than neurological gait abnormalities in this sample. Incidence of nonneurological gait abnormalities showed the expected increasing trend with age.
As previously reported,18-20 subjects with neurological gait abnormalities had a higher prevalence of cardiovascular diseases. Moreover, hypertension and strokes predicted risk of incident neurological gait abnormality. Only arthritis predicted incident nonneurological gait abnormalities. Although these findings suggest causality, a caveat is that evidence of medical illness during evaluation could influence subtyping of abnormal gaits, although knowledge of medical illnesses did not appear to unduly bias study clinicians, as suggested by the high frequency of etiological causes of nonneurological gait abnormalities such as arthritis (59%) and chronic lung disease (13%) in subjects diagnosed with pure neurological gait abnormalities.
Of the subjects with neurological gait abnormalities at enrollment, 55% remained stable, 19% worsened, 8% had milder impairment, and 18% were normal by their second follow-up visit. It is likely that the gait fluctuations may influence point estimates of incidence, although the low back-conversion rate of abnormal gaits to normal suggests that these fluctuations may not have a significant effect on estimates. More-closely spaced examinations may better identify fluctuations in gait status but may not be feasible in cohort studies with large number of subjects because of resource constraints. The gait classification procedures in the current study evolved out of assessment techniques employed in clinical practice. Hence, neurological gait abnormalities were subtyped syndromically, whereas nonneurological gait abnormalities were classified etiologically. Nonneurological gait abnormalities are generally, but not invariably (as in the case of long-standing joint deformities), associated with slow gait that may result from symptoms such as pain, stiffness, or reduced exercise tolerance.18,24,25 Nonneurological gait abnormalities might, hence, be considered as limitations in mobility rather than true gait disorders resulting from lesions in gait pathways as seen with neurological gait abnormalities. Many studies have used slow gait without reporting underlying neurological or nonneurological causes to determine mobility limitations and predict adverse outcomes.2,7,26 The classification in the current study, which includes causes for nonneurological gait impairments besides arthritis, will facilitate comparisons with previous studies. Although other classifications for neurological gait abnormalities have been described,1,27 they have not been validated in clinical settings or do not consider nonneurological causes.
A hierarchical progression of risk for death and institutionalization was found with increasing severity of gait abnormality. The results remained significant even after adjusting for cognitive impairment, which independently predicts death.28 Institutionalization mostly explained the association between abnormal gait and the study outcomes over the short follow-up, but moderate to severe neurological and nonneurological gait abnormalities were associated with greater risk of death. Like in other cohort studies, attrition may result from informative (death) and noninformative (relocation) censoring. The latter does not require correction, whereas the former may affect results. For instance, the association between gait and institutionalization may be underestimated because of deaths before institutionalization. The high incidence of abnormal gaits can also lead to underestimation of effects. The association between abnormal gait and death was strengthened when subjects who developed incident gait abnormalities in the first year were excluded. Although risk of death has been related to specific neurological gait abnormalities,6,29 a greater risk of death with neurological gaits overall is reported.
The strengths of this study included the populationbased recruitment from a defined geographic area, systematic gait evaluation using procedures validated in this population, and weighting of the estimates to account for the study design and recruitment. A limitation was the modest sample size, which resulted in wide confidence intervals for the estimates. The study design may have led to underestimation of severe gait abnormalities, although subjects with severe gait abnormalities are more likely to be seen in institutional settings.30 It is likely that the associations between institutionalization and death in this study might have been strengthened if there had been more subjects with severe gait abnormalities.
This study has important implications for public health and clinical practice. This and previous studies have reported that abnormal gait predicts greater risk of falls, dementia, institutionalization, and death.4,31 The high prevalence and incidence of abnormal gait and its association with multiple adverse outcomes in older adults requires urgent attention. Although subjects with abnormal gaits were only one-third of the sample, 58% of the overall number of deaths and institutionalizations over the 5-year study period occurred in these subjects. Abnormal gaits, hence, are a proxy measure for underlying diseases, which in themselves are the underlying cause of adverse outcomes. Risk factors such as hypertension, stroke, and arthritis were more common in subjects with abnormal gaits, suggesting possible targets for intervention. Risk of adverse outcomes was directly related to the severity of gait impairment. Hence, identifying gait abnormalities early may help identify high-risk older adults and provide a window of opportunity to institute interventions to slow the progression of gait impairment or reduce risk of secondary outcomes.
ACKNOWLEDGMENTS
Financial Disclosure: The Einstein Aging Study is supported by National Institute on Aging (NIA) program project grant AGO3949. Dr. Verghese is supported by a Paul B. Beeson Career Development Award (NIA-K23 AG024848) and NIA Grant RO1 AGO25119. The other authors have no financial conflict of interest.
Author Contributions: Dr. Verghese was responsible for development of the study design, acquisition of data, analysis and interpretation of data, and initial draft of the manuscript. Ms. Katz and Dr. Lipton were responsible for development of the study design, acquisition of data, interpretation of data, and critical revisions of the manuscript. Dr. Ambrose was responsible for analysis and interpretation of data and preparation of the manuscript. Mr. LeValley and Dr. Hall were responsible for acquisition of data, analysis and interpretation of data, and critical revisions of the manuscript.
Sponsor's Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis, or preparation of the manuscript.
REFERENCES
- 1.Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689–703. [PubMed] [Google Scholar]
- 2.Guralnik J, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 4.Verghese J, Lipton RB, Hall CB, et al. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 5.Critchley M. The neurology of old age: Clinical manifestations in old age. Lancet. 1931;1:1221–1230. [Google Scholar]
- 6.Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 7.Chaves PH, Garrett ES, Fried LP. Predicting the risk of mobility difficulty in older women with screening nomograms. The Women's Health and Aging Study II. Arch Intern Med. 2000;160:2525–2533. doi: 10.1001/archinte.160.16.2525. [DOI] [PubMed] [Google Scholar]
- 8.Bloem BR, Haan J, Lagaay AM, et al. Investigation of gait in elderly subjects over 88 years of age. J Geriatr Psychiatry Neurol. 1992;5:78–84. doi: 10.1177/002383099200500204. [DOI] [PubMed] [Google Scholar]
- 9.Fuh JL, Lin KN, Wang SJ, et al. Neurologic diseases presenting with gait impairment in the elderly. J Geriatr Psychiatry Neurol. 1994;7:91–94. doi: 10.1177/089198879400700204. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Katz MJ, Derby CA, et al. Reliability and validity of a telephonebased mobility assessment questionnaire. Age Ageing. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- 12.Blessed G, Tomlinson E, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 14.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd Ed. John Wiley; New York: 1981. [Google Scholar]
- 15.Beckett LA, Scherr PA, Evans DA. Population prevalence estimates from complex samples. J Clin Epidemiol. 1992;45:393–402. doi: 10.1016/0895-4356(92)90040-t. [DOI] [PubMed] [Google Scholar]
- 16.Rosner B. Fundamentals of Biostatistics. Duxbury Press; Belmont, CA: 2000. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;74:187–220. [Google Scholar]
- 18.Ferrucci L, Bandinelli S, Cavazzini C, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116:807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Geroldi C, Ferrucci L, Bandinelli S, et al. Mild cognitive deterioration with subcortical features: Prevalence, clinical characteristics, and association with cardiovascular risk factors in community-dwelling older persons (The InCHIANTI Study) J Am Geriatr Soc. 2003;51:1064–1071. doi: 10.1046/j.1532-5415.2003.51353.x. [DOI] [PubMed] [Google Scholar]
- 20.Pugh KG, Lipsitz LA. The microvascular frontal—subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 21.Waite LM, Broe GA, Grayson DA, et al. Preclinical syndromes predict dementia: The Sydney Older Persons Study. J Neurol Neurosurg Psychiatry. 2001;71:296–302. doi: 10.1136/jnnp.71.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maillefert JF, Gueguen A, Monreal M, et al. Sex differences in hip osteoarthritis: Results of a longitudinal study in 508 patients. Ann Rheum Dis. 2003;62:931–934. doi: 10.1136/ard.62.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs J, Hughes S, Dunlop D, et al. Joint impairment and ambulation in the elderly. J Am Geriatr Soc. 1993;41:1205–1211. doi: 10.1111/j.1532-5415.1993.tb07304.x. [DOI] [PubMed] [Google Scholar]
- 24.Odenheimer G, Funkenstein HH, Beckett L, et al. Comparison of neurologic changes in ‘successfully aging’ persons vs the total aging population. Arch Neurol. 1994;51:573–580. doi: 10.1001/archneur.1994.00540180051013. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 26.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—Fresults from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 27.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43:268–279. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- 28.Aronson MK, Ooi WL, Geva DL, et al. Dementia: Age-dependent incidence, prevalence, and mortality in the old old. Arch Intern Med. 1991;151:989–992. doi: 10.1001/archinte.151.5.989. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RS, Schneider JA, Beckett LA, et al. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- 30.Jones A. The National Nursing Home Survey: 1999 summary. Vital Health Stat 13. 2002;152:1–116. [PubMed] [Google Scholar]
- 31.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]


