Abstract
Since vancomycin-intermediate Staphylococcus aureus (VISA) was first reported in Japan in 1997, there has been great concern that heterogeneous vancomycin-intermediate S. aureus (hetero-VISA) is the putative precursor of VISA. To investigate the prevalence, clinical significance, and molecular epidemiology of S. aureus with reduced susceptibility to vancomycin, all consecutive isolates of S. aureus isolated from clinical specimens from December 1998 to August 1999 at Asan Medical Center were screened for VISA and hetero-VISA by using brain heart infusion agar containing 4 μg of vancomycin/ml. Screen-positive isolates were confirmed by susceptibility testing and population analysis of subpopulations with reduced susceptibility to vancomycin. The isolates confirmed as hetero-VISA were typed by pulsed-field gel electrophoresis (PFGE). Medical records were reviewed to evaluate the clinical significance and risk factors for the acquisition of hetero-VISA. Of the 4,483 isolates that were tested, 53 were screen positive; no VISA was detected, but 24 isolates (0.54%) from 22 patients were hetero-VISA. All but two strains appeared to be clones of the Korean VISA strain, AMC11094, in the PFGE analysis. A total of 18 patients were in intensive care units, and 16 underwent major surgeries during the same admission. Only 10 of the 22 patients had previous methicillin-resistant S. aureus infections and 11 had previous vancomycin or teicoplanin therapy. Only 7 of the 22 patients from whom hetero-VISA strains were isolated were infected, and the remaining 15 patients were colonized. All seven infected patients were successfully treated with vancomycin. These results suggest that hetero-VISA can be treated with vancomycin, but the spread of hetero-VISA clonal to VISA is of concern, since many believe that VISA can arise from hetero-VISA, although this phenomenon was not observed in this study.
Following reports describing the emergence of vancomycin-intermediate Staphylococcus aureus (VISA) in Japan (10), another type of vancomycin resistance in S. aureus called heterogeneous resistance (hetero-VISA) has been reported (12). First described by Hiramatsu et al. (11), hetero-VISA strains are susceptible to vancomycin (MIC ≤ 4 μg/ml) but contain subpopulations at a frequency of 10−6 or higher that grow at a vancomycin concentration of 4 μg/ml and have an MIC of >4 μg/ml (11). There has been a growing concern over recent reports from many countries suggesting that hetero-VISA is prevalent (3, 4, 6, 8, 11, 15, 20, 26, 28, 29), responsible for the failure of vancomycin therapy (3, 28, 29), and a potential source of VISA subclones in patients given vancomycin for a prolonged period (12). With the extremely high prevalence of methicillin-resistant S. aureus (MRSA) strains in Korea (7), it is not surprising that Korea is among the few countries where VISA has emerged (10, 12, 19, 21, 22). Furthermore, there have been reports from tertiary-care hospitals in Korea suggesting that hetero-VISA is prevalent (H. Kim et al., unpublished data). However, these reports were based on the screening of only a few hundred selected MRSA strains, and the reliability of the laboratory methods used to confirm hetero-VISA has been questioned. Whereas several lines of evidence indicate that VISA is responsible for the clinical failure of vancomycin treatment in S. aureus infection (10, 24, 25), the clinical significance of hetero-VISA has yet to be defined (3, 12, 13, 28).
In this study, we screened all consecutive isolates of S. aureus from clinical specimens at a large university hospital in Korea for VISA and hetero-VISA to investigate their prevalence, molecular epidemiology, and clinical significance.
MATERIALS AND METHODS
Screening for S. aureus with reduced susceptibility to vancomycin.
During the 9-month period from December 1998 to August 1999, all S. aureus isolates from clinical specimens at Asan Medical Center, a 2,200-bed university hospital in Seoul, were screened for VISA and hetero-VISA. Asan Medical Center is a large tertiary-care hospital with 139 beds in intensive care units (ICUs), and a VISA strain had been isolated previously from a patient admitted to surgical and oncology wards (14). All isolates of S. aureus except for blood culture isolates were identified by conventional biochemical tests and a PS Latex (Eiken Co.) test for staphylococcal coagulase and protein A; susceptibility tests were performed by the NCCLS disk diffusion test. Blood culture isolates were identified and tested for susceptibility by using MicroScan PosCombo 12 (Dade-Behring).
Consecutive isolates of S. aureus were screened by a method described previously (11). Briefly, overnight cultures were adjusted to 0.5 McFarland turbidity and 10 μl of the cell suspension was inoculated onto brain heart infusion (BHI) agar (BBL; Becton Dickinson) plates containing 4 μg of vancomycin (BHI-V4)/ml. The plates were incubated at 37°C for 48 h. If cell growth was not apparent within 48 h, the isolate was considered susceptible to vancomycin. If confluent growth was seen within 24 h, the isolate was considered potentially VISA. If a countable number (1 to 30) of colonies were apparent within 48 h, the isolate was designated as possibly hetero-VISA (11).
Potential VISA strains were tested immediately for vancomycin MIC by using an E Test Strip (AB Biodisk) to notify the hospital infection control office in case any isolate turned out to be a VISA strain. VISA was defined as an S. aureus strain for which the vancomycin MIC was >4 to ≤16 μg/ml (17). All screen-positive isolates were stored at −70°C and were tested later for vancomycin MIC by the NCCLS agar dilution method (18). They were also tested by using the aztreonam disk method, which is based on the antagonism of vancomycin and β-lactam for hetero-VISA (2, 6, 28); test strains in 0.5 McFarland turbidity were swabbed over the entire surface of brain heart infusion (BHI) agar plates containing 4 μg of vancomycin/ml, and aztreonam disks (30 μg) were applied. Hetero-VISA strains produced dense growth around the disks.
Population analysis.
To confirm hetero-VISA strains, screen-positive strains were tested by population analysis (11). A 100-μl aliquot of the starting cell suspension at 0.5 McFarland turbidity and serial 10-fold dilutions were spread over BHI agar plates containing vancomycin at concentrations ranging from 1 to 10 μg/ml. After incubation at 37°C for 48 h, the numbers of viable cells contained in 100 μl of the starting cell suspension growing on the different concentrations of vancomycin were calculated and plotted on a semilogarithmic scale. S. aureus strains AMC11094 (VISA [Korea]), Mu50 (VISA [Japan]), Mu3 (hetero-VISA [Japan]), and H1 (vancomycin-susceptible MRSA [VSMRSA; Japan]) were used as controls (11, 14). To compare with hetero-VISA, 13 screen-negative MRSA strains isolated from blood cultures during the same study period were also tested.
Hetero-VISA was defined as any screen-positive strain that contained subpopulations with intermediate susceptibility to vancomycin at a frequency of 1 in 10−6 or higher. The MICs of subpopulations were tested with mature colonies grown on a BHI agar plate containing 4 μg of vancomycin/ml by the NCCLS agar dilution method (11, 18)
Epidemiological typing.
Hetero-VISA strains were typed by using pulsed-field gel electrophoresis (PFGE). Chromosomal DNA was prepared by using a 1-day protocol described previously (M. N. Kim, J. S. Park, J. S.Choi, and C. H. Pai, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 538, 1998) and digested with SmaI endonuclease (Takara-Korea Biomedicals). The DNA fragments were separated by PFGE by using program 11 with the GenePath apparatus (Bio-Rad Laboratories). For comparison with hetero-VISA, 13 screen-negative MRSA strains isolated from blood cultures during the same study period were also typed by PFGE.
Medical record review.
To evaluate the clinical relevance of hetero-VISA and the risk factors for its acquisition, patients' medical records were reviewed for demographic findings, clinical diagnoses, underlying diseases, hospital stay, invasive procedures, antibiotic treatment, and clinical outcome. Nosocomial infections were determined by the Centers for Disease Control definition (9).
RESULTS
Detection of S. aureus with reduced susceptibility to vancomycin.
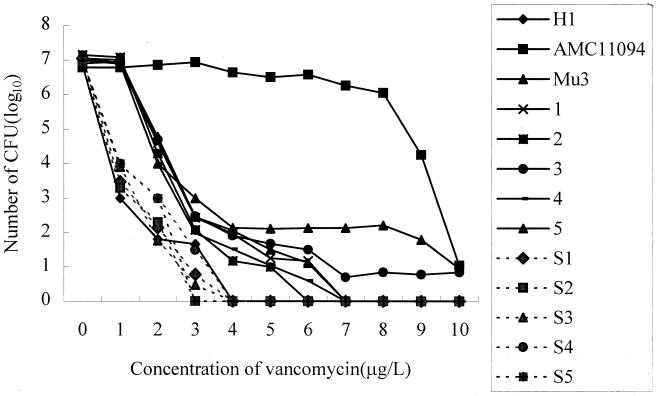
Of 4,483 consecutive isolates (from 1,709 patients), 3,363 (75%) of which were MRSA, 58 (1.29%) showed various amounts of growth on the screening plates; these included 10 potential VISA and 48 possible hetero-VISA isolates. They were all MRSA, but none were VISA since they were susceptible to vancomycin with MICs of 1 to 2 μg/ml. Population analysis confirmed 24 isolates (from 22 patients) as hetero-VISA since they produced subclones capable of growing on BHI-V4 at a frequency of 10−6 or higher (Fig. 1). Thirteen VSMRSA strains showed curves similar to that of the H1 strain (Fig. 1).
FIG. 1.
Population analysis profile of VISA, hetero-VISA, and VSMRSA. Hetero-VISA strains 1 to 5 and Mu3 contained the subpopulation that grew at the vancomycin concentration of 4 μg/ml at a frequency of 10−6 and higher. VSMRSA strains S1 to S5 and H1 showed curves that were similar to each other.
The vancomycin MICs of the subclones ranged from 4 to 8 μg/ml. The amount of growth produced by S. aureus on the screening plate had no predictive value as to whether the organism was a hetero-VISA, since 5 of 10 potential VISA isolates were hetero-VISA compared to 19 of 48 possible hetero-VISA isolates (P > 0.05). Of the 58 screening-positive isolates 45, including all 24 hetero-VISA strains, showed dense growth around aztreonam disks.
Epidemiological typing.
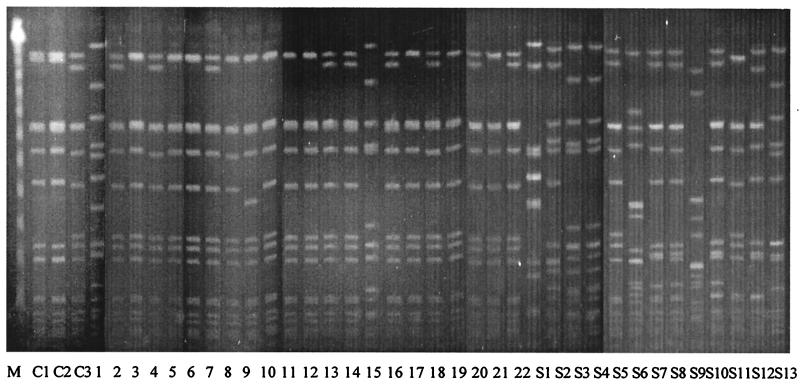
The PFGE patterns of the 22 hetero-VISA strains from 22 patients and 13 VSMRSA strains isolated from blood cultures during the study period are shown in Fig. 2. Nine (type A0) of the 22 hetero-VISA strains showed a banding pattern identical to that of AMC11094, the VISA isolate reported from the study hospital (14). Ten isolates (type A1) showed a one-band difference from AMC 11094, and one isolate (type A2) showed a three-band difference. Only two isolates (types B and C) differed from the VISA isolate by more than six bands. In contrast, all but one VSMRSA strain showed various banding patterns and differed from AMC11094 by more than six bands.
FIG. 2.
PFGE patterns of hetero-VISA and VSMRSA. Lanes; M, Lambda Ladder PFG Marker (New England Biolabs); lanes C1 to C3, control strains Mus3 (hetero-VISA [Japan]), Mu50 (VISA [Japan]), and AMC11094 (VISA [Korea]), respectively; lanes 1 to 22, hetero-VISA isolates 1 to 22 from the study; lanes S1 to S13, VSMRSA isolates S1 to S13 from this study. Hetero-VISA isolates 2, 4, 7, 13, 14, 16, 18, 20, and 22, are designated as type A0, show a PFGE pattern identical to that of the Korean VISA. Isolates 3, 5, 6, 8, 10, 11, 12, 17, 19, and 21, designated as type A1, show a 1-band difference; hetero-VISA isolate 9, type A2, shows a 3-band difference (a two-band difference from type A1); hetero-VISA isolates 1 and 15, type B and C, respectively, show a >6-band difference. All VSMRSA isolates, except S5 (type A), show various patterns and differ from type A by more than six bands.
Epidemiological and clinical significance.
The 22 patients from whom hetero-VISA strains were isolated ranged from 31 to 77 years of age (median, 59.5 years); 8 patients were female. They were hospitalized for periods ranging from 3 to 360 days (median, 45.5 days), and 18 were in the ICU. Underlying conditions were present in all 22 patients (malignancy, 6 patients; intracranial hemorrhage, 6 patients; bone fracture, 4 patients; acute pancreatitis, 2 patients; liver cirrhosis, 1 patient; diabetes, 1 patient; gall bladder stone, 1 patient; asthma, 1 patient), and 16 underwent major surgery (neurosurgery, 9 patients; gastrointestinal surgery, 3 patients; above-the-knee amputation, 2 patients; prostatectomy, 1 patient; and fasciotomy, 1 patient) during the same admission as that in which the hetero-VISA strain was identified. Only 10 of the 22 patients had previous MRSA infections, and only 11 had received vancomycin or teicoplanin therapy previously, including 3 patients who had had vancomycin therapy for 3 days or less. Since these hetero-VISA patients might have been cross-contaminated from other patients or medical staff, we checked their admission units, periods of hospitalization, surgeons, and operating room numbers. Five patients in the medical ICU, six in the surgical ICU, and six in the neurosurgical ICU were hospitalized during the same or overlapping periods. In addition, two patients were in the surgical ICU briefly for postoperative care before returning to the medical ICU. Of the 16 patients who had major surgery, 2 patients in each of three groups were operated on by the same neurosurgeons and in the same operating rooms.
A total of 7 patients were infected, and 15 were colonized. Infections in all seven patients were nosocomial in origin, including three surgical-site infections, two cases of pneumonia, and one case each of urinary tract infection and central venous line site infection (Table 1). The patients were treated with vancomycin for periods ranging from 9 to 63 days. The dose of vancomycin was dosed at 1 g every 12 h (one patient) or 500 mg every 6 h (five patients). Vancomycin levels were monitored in two patients; peak and trough blood levels were 30.2 and 8.9 μg/ml and 39.6 and 6.2 μg/ml in one patient and 26.7 and 18.2 μg/ml in the other. Clinical improvement was seen in all seven patients, including four patients with bacteriological cure, although one patient died from a complication of the underlying disease. Of the 15 colonized patients, 8 were identified from the cultures of nasal swabs taken as MRSA surveillance cultures in the ICU. Other colonized patients were identified from cultures of sputum (three patients), urine (two patients), bed sore swab (one patient), and percutaneous transhepatic biliary drainage (one patient).
TABLE 1.
Clinical features of patients infected with hetero-VISAa
| Age/sexb | Ward | Underlying disease | ICU stay/ hospital stay (duration in days) | Infection | Specimen type | Surgical operation | Previous MRSA infection | Previous GP (duration in days) | VAN treatment (duration in days) | Clinical outcome | PFGE type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 63/M | OS/94w | Trauma with tibial fracture | 0/38 | Wound infection | Pus | AK amputation | No | No | 7+7c | Improved | C |
| 58/M | SICU | Biliary adenocarcinoma | 50/56 | Pneumonia | Bronchial aspirates | No | Pneumonia | VAN (3) | 9 | Improved | A |
| 73/M | NSICU | Intracranial hemorrhage | 18/18 | Urinary tract infection | Urine | Craniotomy | Pneumonia | VAN (3) | 25 | Death | A |
| 31/M | SICU | Trauma with femur fracture | 16/360 | Wound infection | Pus | AK amputation | Wound infection | VAN (7), TEC (7) | 63 | Improved | A |
| 75/M | SICU | Rectal cancer | 8/55 | Pneumonia | Sputum | Colon resection | No | No | 28 | Improved | A |
| 34/M | NSICU | Thoracic vertebrae fracture | 16/16 | Wound infection | Pus | Dorsal spine fusion | No | No | 14 | Improved | A |
| 58/F | NSICU | Schwannoma | 13/13 | C-line site infection | Pus | Excision of schwannoma | No | No | 16 | Improved | A |
Abbreviations: OS, orthopedic surgery; SICU, surgical intensive care unit; NSICU, neurosurgical intensive care unit; AK, above the knee; GP, glycopeptide treatment; VAN, vancomycin; TEC, teicoplanin.
M, male; F, female.
Includes also teicoplanin treatment.
DISCUSSION
This study examined the prevalence of S. aureus with reduced susceptibility to vancomycin by screening consecutive isolates from clinical specimens over a 9-month period at a large university hospital in Korea. Since a VISA strain had been reported previously and MRSA was reported to be highly prevalent in the hospital, the study was initiated with the expectation of finding more VISA strains and a significant number of hetero-VISA strains. However, we found no VISA strains and that the prevalence of hetero-VISA was only 0.5% of the S. aureus or 0.7% of the MRSA isolates screened. Even if only one isolate per patient was counted, the prevalence was 1.3% of S. aureus. This is much lower than the prevalences reported elsewhere: 9.3% of MRSA isolates at university hospitals in Japan (11) and 0.6 to 65% in the reports from Spain, Germany, France, Italy, Brazil, Thailand, and Hong Kong (3, 4, 6, 8, 15, 20, 26, 28). These data are suggestive of regional differences in the prevalence of hetero-VISA. However, it is interesting that a prevalence of 0.6%, similar to our results, was reported in a study involving two French hospitals, where consecutive isolates of S. aureus were screened in the same manner as in our study (20). A few studies involving a selected population of S. aureus or MRSA strains reported higher prevalence rates. The rate was 65% in a Spanish study, in which only MRSA isolates from surgical-site infections of orthopedic operations were screened (3), and 20% in a French study in which S. aureus isolates from nosocomial infections were examined (6). The difference in the study populations of the S. aureus strains may also account for the differences in the reported prevalence.
Of 22 hetero-VISA strains isolated in our study, 20 showed no difference or less than a three-band difference from AMC11094 in the PFGE profile, suggesting a clonal relationship between the VISA and most of the hetero-VISA strains isolated from in our hospital (23). In contrast, the PFGE patterns of VSMRSA strains isolated during the same study period were very different from that of VISA. Clonal relationships between VISA and hetero-VISA strains isolated from the same hospital (11), country (6), or region (4) have also been reported. Also reported was a clonal relationship between the VISA and the preceding MRSA isolated consecutively from a single patient (21, 22). These findings support the hypothesis that hetero-VISA gives rise to VISA with prolonged exposure to glycopeptides. This hypothesis is also consistent with the in vitro finding that the vancomycin resistance of hetero-VISA can be enhanced with stepwise exposure to higher concentrations of vancomycin (1, 5, 13).
All seven infections with hetero-VISA were treated successfully with vancomycin (for as long as 63 days in one patient) with no emergence of VISA. This finding is not consistent with previous reports that hetero-VISA is associated with a high rate of failure of vancomycin therapy (3, 28). These cases, however, were severe infections such as surgical infections, infections involving orthopedic implants, or bacteremia (3, 28), whereas the hetero-VISA infections in our study were not deep-tissue infections, although three of seven were surgical-site infections. The clinical outcome of hetero-VISA infection may depend on the severity, the site of the infections, and the presence of a foreign body. This finding suggests that, in addition to the prolonged exposure to vancomycin, the in vivo transition from hetero-VISA to VISA requires an infection site with suboptimal concentrations of vancomycin.
As for the risk factors for the acquisition of hetero-VISA, ICU stay and surgery seemed to be important. Of the 22 patients, 18 stayed in the ICU and 16 underwent major surgery. Admission to the ICU was reported as a risk factor in another study (28). In addition, about half of the patients, including four of the seven infected patients, had had neither previous MRSA infections nor any previous vancomycin therapy, suggesting that cross-contamination plays a major role in the spread of hetero-VISA rather than the acquisition of heterogeneous vancomycin resistance by MRSA colonization or infection during glycopeptide therapy. Although we found no direct evidence in our study suggesting the spread of hetero-VISA from one patient to another, it should be noted that many of the patients were hospitalized in the medical, surgical, or neurosurgical ICU during the same or overlapping periods and that some patients were operated on by the same surgeons in the same operating rooms.
To select appropriate antimicrobials and start infection control, a reliable screening method for S. aureus with reduced susceptibility to vancomycin is warranted as a part of the routine susceptibility testing of clinical isolates at hospitals where hetero-VISA strains are prevalent. Since we found that all of the hetero-VISA isolates were MRSA, as reported elsewhere (4, 6, 8, 11, 15, 26, 28), it appears that only MRSA needs to be screened. However, one should consider screening all S. aureus isolates rather than only MRSA for the following reasons. First, it is more practical to screen all S. aureus isolates because screening of MRSA only would require a pretesting for oxacillin susceptibility, causing a delay in the final report. Second, there are recent reports that hetero-VISA or VISA can develop from methicillin-susceptible S. aureus (5, 16). The BHI-V4 screen has been widely used because it is an original method that is consistent with the definition of hetero-VISA (11), and it is relatively sensitive (27) and easy to perform as a screening test (4, 6, 8, 15, 26). As to the aztreonam disk method, we find it as sensitive as the BHI-V4 method, but we are not able to comment on its specificity because we did not compare the two methods in a parallel study.
In conclusion, the prevalence of hetero-VISA in our study was low, in spite of the fact that the study was performed in a hospital where a VISA strain had been isolated previously. Most hetero-VISA strains were clonal to the VISA strain, but all infections with hetero-VISA were treated successfully with vancomycin with no emergence of a VISA strain. Mere exposure to vancomycin may not be sufficient for the transition of hetero-VISA to VISA. Further studies are warranted to clarify the clinical significance of hetero-VISA.
Acknowledgments
This study was supported by the Korean Research Foundation.
We are grateful to K. Hiramatsu for providing the control S. aureus strains H1, Mu3, and Mu50.
REFERENCES
- 1.Aeschlimann, J. R., E. Hershberger, and M. J. Rybak. 1999. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aritaka, N., H. Hanaki, L. Cui, and K. Hiramatsu. 2001. Combination effect of vancomycin and β-lactams against a Staphylococcus aureus strain, Mu3, with heterogeneous resistance to vancomycin. Antimicrob. Agents Chemother. 45:1292-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariza, J., M. Pujol, J. Cabo, C. Peña, N. Fernández, J. Liñares, J. Ayats, and F. Gudiol. 1999. Vancomycin in surgical infections due to methicillin-resistant Staphylococcus aureus with heterogeneous resistance to vancomycin. Lancet 353:1587-1588. [DOI] [PubMed] [Google Scholar]
- 4.Bierbaum, G., K. Fuchs, W. Lenz, C. Szekat, and H.-G. Sahl. 1999. Presence of Staphylococcus aureus with reduced susceptibility to vancomycin in Germany. Eur. J. Microbiol. Infect. Dis. 18:691-696. [DOI] [PubMed] [Google Scholar]
- 5.Bobin-Dubreux, S., M. E. Reverdy, C. Nervi, M. Rougier, A. Bolmstrom, F. Vandenesch, and J. Etienne. 2001. Clinical isolate of vancomycin-heterointermediate Staphylococcus aureus susceptible to methicillin and in vitro selection of a vancomycin-resistant derivative. Antimicrob. Agents Chemother. 45:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesneau, O., A. Morvan, and N. El Solh. 2000. Retrospective screening for heterogeneous vancomycin resistance in diverse Staphylococcus aureus clones disseminated in French hospitals. J. Antimicrob. Chemother. 45:887-890. [DOI] [PubMed] [Google Scholar]
- 7.Chong, Y., K. Lee, J. W. Shin, H. B. Shin, and J. B. Lim. 1997. Activities of arbekacin against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. J. Korean Soc. Chemother. 15:319-327. [Google Scholar]
- 8.Dos Santos Soares, M. J., M. C. dal Silva-Carvalho, and B. T. Ferreira-Carvalho. 2000. Spread of methicillin-resistant Staphylococcus aureus belonging to the Brazilian epidemic clone in a general hospital and emergence of heterogeneous resistance to glycopeptide antibiotics among these isolates. J. Hosp. Infect. 44:301-308. [PubMed] [Google Scholar]
- 9.Gaynes, R. P., and T. C. Horan. 1999. Surveillance of nosocomial infections, p. 1285-1307. In C. G. Mayhall (ed.), Hospital epidemiology and infection control, 2nd ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 10.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K. 1998. The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Am. J. Med. 104:7S-10S. [DOI] [PubMed] [Google Scholar]
- 13.Howe, R. A., M. Wooton, T. R. Walsh, P. M. Bennet, and A. P. MacGowan. 1999. Expression and detection of hetero-vancomycin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 44:675-678. [DOI] [PubMed] [Google Scholar]
- 14.Kim, M. N., C. H. Pai, J. H. Woo, J. S. Ryu, and K. Hiramatsu. 2000. Vancomycin-intermediate Staphylococcus aureus in Korea. J. Clin. Microbiol. 38:3879-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchese, A., G. Balistreri, E. Tonoli, E. A. Debbia, and G. C. Schito. 2001. Heterogeneous vancomycin resistance in methicillin-resistant Staphylococcus aureus strains in a large Italian hospital. J. Clin. Microbiol. 39:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlowe, E. M., M. D. Cohen, J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2001. Practical strategies for detecting and confirming vancomycin-intermediate Staphylococcus aureus: a tertiary-care hospital laboratory's experience. J. Clin. Microbiol. 39:2637-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing: 11th informational supplement. Approved standard M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212.. [DOI] [PubMed] [Google Scholar]
- 20.Reverdy, M. E., S. Jarraud, S. Bobin-Dubreux, E. Burel, P. Girardo, G. Lina, E. Vandenesch, and J. Etienne. 2001. Incidence of Staphylococcus aureus with reduced susceptibility to glycopeptides in two French hospitals. Clin. Microbiol. Infect. 7:267-272. [DOI] [PubMed] [Google Scholar]
- 21.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:519-523. [DOI] [PubMed] [Google Scholar]
- 22.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz. M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphyococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover, F. C., M. V. Lancaster, B. C. Hill, C. D. Steward, S. A. Stocker, G. A. Hancock, C. M. O'Hara, S. K. McAllister, N. C. Clark, and K. Hiramatsu. 1998. Characterization of staphylococci with reduced susceptibility to vancomycin and other glycopeptides. J. Clin. Microbiol. 36:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenover, F. C. 1999. Implication of vancomycin-resistant Staphylococcus aureus. J. Hosp. Infect. 43:S3-S7. [DOI] [PubMed] [Google Scholar]
- 26.Trakulsomboon, S., S. Danchaivijitr, Y. Rongrungruang, C. Dhiraputra, W. Susaemgrat, T. Ito, and K. Hiramatsu. 2001. First report of methicillin-resistant Staphylococcus aureus with reduced susceptibility to vancomycin in Thailand. J. Clin. Microbiol. 39:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh, T. T., A. Bolmstrom, A. Qwarnstrom, P. Ho, M. Wootton, R. A. Howe, A. P. MacGowan, and D. Diekema. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, S. S. Y., P. L. Ho, P. C. Y. Woo, and Y. K. Yuen. 1999. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29:760-767. [DOI] [PubMed] [Google Scholar]
- 29.Wong, S. S. Y., P. L. Ho, P. C. Y. Woo, and Y. K. Yuen. 2000. Bacteremia caused by staphylococci with reduced susceptibility to vancomycin. Diagn. Microbiol. Infect. Dis. 36:261-268. [DOI] [PubMed] [Google Scholar]