Abstract
We characterized baseline and repopulating stool isolates recovered during a phase II trial of ramoplanin for the treatment of patients with stool carriage of vancomycin-resistant enterococci (VRE). Repopulation with a strain with a related genotype was found in 74, 60, and 53% of individuals in groups treated with placebo, 100 mg of ramoplanin, and 400 mg of ramoplanin, respectively. All ramoplanin-treated patients with a culture positive for VRE at day 7 had a relapse caused by a genotypically related isolate. In ramoplanin-treated patients, antibiotics with activities against anaerobic organisms were associated with positive cultures on day 7 (relative risk [RR] = 8.8; P = 0.004), and the avoidance of such antibiotics was significantly associated with culture negativity through day 21 (RR = 0.16; P = 0.02).
Vancomycin-resistant enterococci (VRE) have dramatically increased in clinical importance over the past decade (10, 16, 17), with few therapeutic options remaining (5, 16). Strains of VRE causing bacteremia in severely ill patients often originate at sites of colonization in the gastrointestinal tract (2). One strategy for the prevention of infection with VRE in at-risk, colonized patients is suppression of intestinal VRE during the periods of greatest risk. No agent has been demonstrated to have efficacy for this purpose, despite the study of several candidates (novobiocin, doxycycline, bacitracin) (14, 15, 18, 28). Ramoplanin, a glycolipodepsipeptide antimicrobial that is not systemically absorbed, has been demonstrated to have activity against VRE and has been studied as a locally active agent for the suppression of colonization (11, 13, 21).
In a multicenter, randomized, double-blind, placebo-controlled phase II trial, ramoplanin was shown to be safe and effective at suppressing VRE to undetectable levels (at day 7) in 80 to 90% of treated patients (29). Patients colonized with VRE but without evidence of active infection were randomized to receive placebo or ramoplanin at 100 or 400 mg orally twice a day for 7 days. Stool or rectal swab specimens for culture were obtained at the baseline and then at days 7 (end of treatment), 14, 21, 45, and 90. Overall antimicrobial use and use of antimicrobials with activities against anaerobic organisms during the study period were not found to be different between the three treatment groups in relation to their VRE-free status. The details of the clinical results from this phase II study have been published elsewhere (29). The purpose of the present study was to assess the molecular relatedness of paired isolates from patients whose colonization with VRE recurred after treatment with ramoplanin.
(These data were presented in part at the 1st International American Society for Microbiology Conference of Enterococci, Banff, Alberta, Canada, 2000.)
MATERIALS AND METHODS
Rectal swab specimens were obtained and directly inoculated into 1.0 ml of bile-esculin azide broth with 6 μg of vancomycin (Hardy Diagnostics, Santa Maria, Calif.) per ml, as presented elsewhere (29). The organism's genotype was determined by PCR with primers specific for vanA, vanB, vanC1, and vanC2 (6, 22). The baseline isolate from each patient and the first isolate after the baseline isolate positive for vancomycin resistance were analyzed by pulsed-field gel electrophoresis by standard techniques, as reported previously (1, 9, 26).
The following agents were considered to have significant activities against anaerobic organisms: β-lactams and β-lactamase inhibitors, cefotetan, chloamphenicol, chlorhexidine (oral) (3, 19, 24), clindamycin, imipenem, metronidazole, rifampin (7, 8), and trovafloxacin. Recent antibiotic use was defined as receipt of an antibacterial agent within the 2 weeks preceding enrollment into the study. Where appropriate, relative risks (RRs) and 95% confidence intervals (CIs) were calculated. Statistical testing was done by the two-tailed Fisher exact test or the Mantel-Haenszel chi-square test for linear trend.
RESULTS
Sixty-eight patients were enrolled in the phase II trial, and 58 were evaluable for this study (culture data were available on day 7): 19 in the placebo group, 20 in the group receiving 100 mg of ramoplanin (100-mg group), and 19 in the group receiving 400 mg of ramoplanin (400-mg group). Three patients in the ramoplanin groups did not have positive cultures upon follow-up, and two patients were not positive for an isolate of VRE until day 90. The remaining patients (n = 53) were found to be positive for VRE at day 7, 14, or 21. Thus, there were 55 pairs of patient isolates for molecular characterization. Forty-nine (88%) were Enterococcus faecium pairs, 5 (9%) were Enterococcus faecalis pairs, and 1 (2%) was an E. faecalis-E. faecium pair. Ninety-five percent of the VRE had the vanA genotype; the remainder had the vanB genotype. In the placebo group, 66 of the 77 (86%) cultures of rectal swab specimens obtained during the follow-up period demonstrated VRE. The frequency of culture positivity decreased with time, with rates of positivity of 91% (51 of 56 specimens) on days 7, 14, and 21; 83% (10 of 12 specimens) on day 45; and 56% (5 of 9 specimens) on day 90.
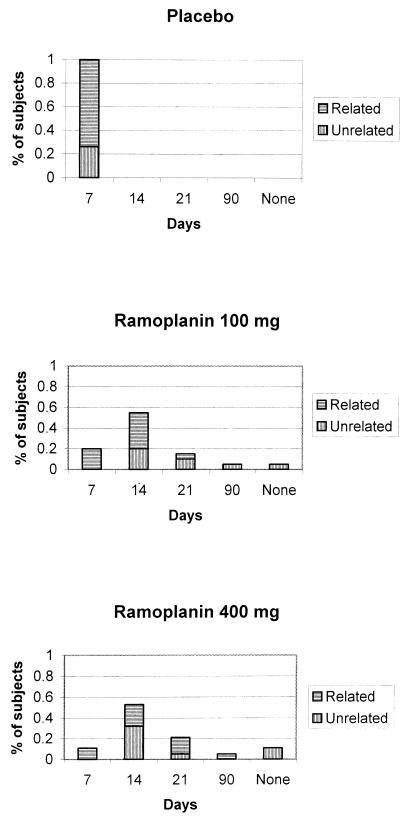
As seen in Fig. 1, the first follow-up specimen positive for VRE by culture was clonally related to a patient's baseline strain in 74% of the patients in the placebo group, 60% of the patients in the 100-mg group, and 53% of the patients in the 400-mg group (P = 0.19 for linear trend). Among the isolates of VRE in the cultures positive at day 7, 80% were clonally related to a patient's baseline isolate; for the cultures that became positive for VRE at days 14, 21, and 90, there was an approximately 50% chance that a clonally related isolate would be identified. For all six ramoplanin-treated patients who were culture positive at day 7, the isolate recovered on day 7 was clonally related to the baseline isolate. As seen in Fig. 1, patients treated with the 400-mg dose of ramoplanin tended to have a relapse caused by a clonally related strain later than the relapse in those treated with the 100-mg dose. Of those patients who were culture positive on day 7 (19 in the placebo group, 4 in the 100-mg group, and 2 in the 400-mg group), the use of antimicrobials with activities against anaerobic organisms was associated with a trend toward a relapse caused by an isolate with a related genotype (RR = 1.46; 95% CI = 0.91, 2.33; P = 0.13).
FIG. 1.
Time to first positive culture by treatment allocation and strain relatedness. The three panels demonstrate the first times to positivity for VRE of a rectal swab specimen culture and the relatedness of the relapsing strain to the patient's baseline isolate by treatment allocation.
Of the 39 ramoplanin-treated patients from whom a specimen for culture was obtained on day 7, 17 (44%) received therapy with antimicrobials with significant activities against anaerobic organisms either concurrently with the study medication or within 7 days of the beginning of treatment with the study medication. Of the 6 ramoplanin-treated patients in whose stools VRE were detected on day 7 (the end of therapy), all 6 (100%) had recently been treated with antibiotics with activities against anaerobic organisms, whereas 11 of 33 (33%) of the patients culture negative on day 7 had recently been treated with antibiotics with activities against anaerobic organisms (RR = 8.8 [the RR was estimated by adding 1 to the value for each group]; 95% CI = 1.2, 65.8; P = 0.004). Five ramoplanin-treated patients were culture negative for VRE through day 21. None of these 5 patients received therapy with antibiotics with activities against anaerobic organisms during this time period, whereas 14 of 34 (41%) of the culture-positive patients received therapy with antibiotics with activities against anaerobic organisms (RR = 0.16; 95% CI = 0.02, 1.21 [the 95% CI was estimated by adding 1 to the value for each group]; P = 0.02).
DISCUSSION
Genotypically unrelated clones of VRE were more often recovered from ramoplanin-treated patients than from placebo-treated patients (placebo group, 26%; 100-mg group, 40%; 400-mg group, 47%). Although this study did not have the power to detect a significant difference in the genotypes of the relapse strains by treatment allocation, the trend is intriguing and is supported by the suggestion of a dose-responsive pattern. These data suggest that ramoplanin may unmask baseline polyclonal colonization (23) or increase susceptibility to colonization by new enterococcal clones (25), likely through a decrement in the previous enterococcal burden, followed by reexposure to VRE from environmental reservoirs. The sensitivity for the detection of enterococcal colonization was enhanced by culture of patient samples directly into a broth medium; however, the ability to distinguish polyclonal colonization at the baseline was lost (12, 20, 27). Further research is required to determine the relative contributions of these two scenarios, as the infection control implications are different.
As all placebo-treated patients were culture positive for VRE at day 7, we analyzed the influence of antibiotic use on the time to the first positive culture in ramoplanin-treated patients. All ramoplanin-treated patients who remained culture positive at the end of treatment (day 7) had several factors in common. For each patient, the isolate recovered at the end of treatment was genotypically related to the one recovered at the baseline, and each patient had recently been treated with antimicrobial agents with activities against anaerobic organisms (whereas only 11 of the 33 subjects with negative cultures on day 7 had recently been treated with antimicrobial agents with activities against anaerobic organisms). In addition, two-thirds of these patients were receiving the lower dose of ramoplanin. The combination of these factors suggests that the predisposing factor for culture positivity at day 7 was most likely a high initial burden of VRE (1, 4). The importance of concomitant antimicrobial use and the subsequent gastrointestinal amplification of resistant pathogens is highlighted by this study, both by the significant difference in culture positivity at day 7 in those patients treated with drugs with activities against anaerobic organisms (RR = 8.8; P = 0.004) and by the culture negativity at day 21 in those patients not treated with antibiotics with activities against anaerobic organisms (RR = 0.16; P = 0.02). These findings extend the results of Wong et al. (29) and demonstrate the added challenge that concomitant use of antibiotics with activities against anaerobic organisms imparts to the suppression of excretion of VRE in patients treated with ramoplanin. It is noteworthy that we used a broader definition of timing of previous antibiotic use compared with that used by Wong et al. (29) by including antibiotic use in the 2 weeks prior to study enrollment (this was done given the data from previous studies [1, 4], which demonstrated a prolonged multilog increase in the fecal density of VRE for several weeks after the use of antimicrobials with activities against anaerobic organisms in patients colonized with VRE).
The observations made during this phase II trial afford important insights into the colonization dynamics of VRE. All placebo-treated patients were culture positive on day 7, and in the majority of these patients an isolate with a related or identical genotype was maintained, suggesting that patients are stably colonized. The 26% of placebo-treated subjects found to be colonized with a strain with a different genotype on day 7 were likely colonized with multiple clones at the baseline, although the acquisition of new clones cannot be excluded (25). During the short period of time that observations were made in this study, most placebo-treated patients persistently remained culture positive, with over 90% of specimens obtained for culture being positive at 3 weeks and over 50% being positive at 3 months.
These data highlight several important points: colonization and excretion of VRE may be prolonged; concomitant antimicrobial use increases the challenge of controlling VRE; in a patient colonized with VRE, ramoplanin use is associated with subsequent isolation of a strain of VRE with an unrelated genotype; and no evidence for ramoplanin resistance was seen in isolates that were recovered from patients treated with ramoplanin and that maintained the same genotype.
Acknowledgments
This study was funded by IntraBiotics Pharmaceuticals, Inc.
REFERENCES
- 1.Baden, L. R., W. Thiemke, A. Skolnik, R. Chambers, J. Strymish, H. S. Gold, R. C. Moellering, Jr., and G. Eliopoulos. 2001. Long term colonization with vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 33:1654-1660. [DOI] [PubMed] [Google Scholar]
- 2.Beezhold, D. W., S. Slaughter, M. K. Hayden, M. Matushek, C. Nathan, G. M. Trenholme, and R. A. Weinstein. 1997. Skin colonization with vancomycin-resistant enterococci among hospitalized patients with bacteremia. Clin. Infect. Dis. 24:704-706. [DOI] [PubMed] [Google Scholar]
- 3.Caufield, P. W., D. N. Allen, and N. K. Childers. 1987. In vitro susceptibilities of suspected periodontopathic anaerobes as determined by membrane transfer assay. Antimicrob. Agents Chemother. 31:1989-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, C. K. Hoyen, J. A. Hanrahan, A. M. Hujer, R. A. Hutton-Thomas, C. C. Whalen, R. A. Bonomo, and L. B. Rice. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliopoulos, G. M., C. B. Wennersten, H. S. Gold, T. Schulin, M. Souli, M. G. Farris, S. Cerwinka, H. L. Nadler, M. Dowzicky, G. H. Talbot, and R. C. Moellering, Jr. 1998. Characterization of vancomycin-resistant Enterococcus faecium isolates from the United States and their susceptibility in vitro to dalfopristin-quinupristin. Antimicrob. Agents Chemother. 42:1088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Free, L., and D. F. Sahm. 1996. Detection of enterococcal vancomycin resistance by multiplex PCR, p. 150-155. In D. Pershing (ed.), PCR protocols for emerging infectious diseases. ASM Press, Washington, D.C.
- 7.Fu, K. P., E. R. Lasinski, H. C. Zoganas, E. F. Kimble, and E. A. Konopka. 1984. Therapeutic efficacy and pharmacokinetic properties of rifampicin in a Bacteroides fragilis intra-abdominal abscess. J. Antimicrob. Chemother. 14:633-640. [DOI] [PubMed] [Google Scholar]
- 8.Fu, K. P., E. R. Lasinski, H. C. Zoganas, E. F. Kimble, and E. A. Konopka. 1985. Efficacy of rifampicin in experimental Bacteroides fragilis and Pseudomonas aeruginosa mixed infections. J. Antimicrob. Chemother. 15:579-585. [DOI] [PubMed] [Google Scholar]
- 9.Goering, R. V., and M. A. Winters. 1992. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion electrophoresis. J. Clin. Microbiol. 30:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold, H. S., and R. Moellering, Jr. 1996. Antimicrobial-drug resistance. N. Engl. J. Med. 335:1445-1453. [DOI] [PubMed] [Google Scholar]
- 11.Landman, D., J. M. Quale, S. Burney, B. Kreiswirth, and B. M. Willey. 1996. Treatment of experimental endocarditis caused by multidrug resistant Enterococcus faecium with ramoplanin and penicillin. J. Antimicrob. Chemother. 37:323-329. [DOI] [PubMed] [Google Scholar]
- 12.Landman, D., J. M. Quale, E. Oydna, B. Willey, V. Ditore, M. Zaman, K. Patel, G. Saurina, and W. Huang. 1996. Comparison of five selective media for identifying fecal carriage of vancomycin-resistant enterococci. J. Clin. Microbiol. 34:751-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mobarakai, N., J. M. Quale, and D. Landman. 1994. Bactericidal activities of peptide antibiotics against multidrug-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 38:385-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondy, K. E., W. Shannon, and L. M. Mundy. 2001. Evaluation of zinc bacitracin capsules versus placebo for enteric eradication of vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 33:473-476. [DOI] [PubMed] [Google Scholar]
- 15.Montecalvo, M. A., H. Horowitz, G. P. Wormser, K. Seiter, and C. A. Carbonaro. 1995. Effect of novobiocin-containing antimicrobial regimens on infection and colonization with vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 39:794.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance System, National Center for Infectious Diseases, Centers for Disease Control and Prevention. 1998. Data summary from October 1986-April 1998, p. 1-25. In National Nosocomial Infections Surveillance System report. National Nosocomial Infections Surveillance System, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga.
- 18.O'Donovan, C. A., P. Fan-Havard, F. T. Tecson-Tumang, S. M. Smith, and R. H. K. Eng. 1994. Enteric eradication of vancomycin-resistant Enterococcus faecium with oral bacitracin. Diagn. Microbiol. Infect. Dis. 18:105-109. [DOI] [PubMed] [Google Scholar]
- 19.Ohara, P., M. Torabinejad, and J. D. Kettering. 1993. Antibacterial effects of various endodontic irrigants on selected anaerobic bacteria. Endodont. Dent. Traumatol. 9:95-100. [DOI] [PubMed] [Google Scholar]
- 20.Rao, G. G., K. Ghanekar, and F. Ojo. 1996. Selective medium for screening for vancomycin-resistant enterococci in faeces. Eur. J. Clin. Microbiol. Infect. Dis. 15:175-177. [DOI] [PubMed] [Google Scholar]
- 21.Rolston, K. V., N. Dholakia, D. H. Ho, B. LeBlanc, T. Dvorak, and H. Streeter. 1996. In-vitro activity of ramoplanin (a novel lipoglycopeptide), vancomycin, and teicoplanin against gram-positive clinical isolates from cancer patients. J. Antimicrob. Chemother. 38:265-269. [DOI] [PubMed] [Google Scholar]
- 22.Sahm, D. F., L. Free, C. Smith, M. Eveland, and L. M. Mundy. 1997. Rapid characterization schemes for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 35:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoonmaker, D. J., L. H. Bopp, A. L. Baltch, R. P. Smith, M. E. Rafferty, and M. George. 1998. Genetic analysis of multiple vancomycin-resistant Enterococcus isolates obtained serially from two long-term-care patients. J. Clin. Microbiol. 36:2105-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siqueira, J. F., Jr., and M. de Uzeda. 1997. Intracanal medicaments: evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J. Endodont. 23:167-169. [DOI] [PubMed] [Google Scholar]
- 25.Tenorio, A. R., S. M. Badri, N. B. Sahgal, B. Hota, M. Matushek, M. K. Hayden, G. M. Trenholme, and R. A. Weinstein. 2001. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin. Infect. Dis. 32:826-829. [DOI] [PubMed] [Google Scholar]
- 26.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Horn, K. G., C. A. Gedris, and K. M. Rodney. 1996. Selective isolation of vancomycin-resistant enterococci. J. Clin. Microbiol. 34:924-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein, M. R., H. Dedier, J. Brunton, I. Campbell, and J. M. Conly. 1999. Lack of efficacy of oral bacitracin plus doxycycline for the eradication of stool colonization with vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 29:361-366. [DOI] [PubMed] [Google Scholar]
- 29.Wong, M. T., C. A. Kauffman, H. C. Standiford, et al. 2001. Effective suppression of vancomycin-resistant enterococcus species in asymptomatic gastrointestinal carriers by a novel glycolipodepsipeptide, ramoplanin. Clin. Infect. Dis. 33:1476-1482. [DOI] [PubMed] [Google Scholar]