Abstract
Bloodstream infections due to Candida species cause significant morbidity and mortality. Surveillance for candidemia is necessary to detect trends in species distribution and antifungal resistance. We performed prospective surveillance for candidemia at 16 hospitals in the State of Iowa from 1 July 1998 through 30 June 2001. Using U.S. Census Bureau and Iowa Hospital Association data to estimate a population denominator, we calculated the annual incidence of candidemia in Iowa to be 6.0 per 100,000 of population. Candida albicans was the most common species detected, but 43% of candidemias were due to species other than C. albicans. Overall, only 3% of Candida species were resistant to fluconazole. However, Candida glabrata was the most commonly isolated species other than C. albicans and demonstrated some resistance to azoles (fluconazole MIC at which 90% of the isolates tested are inhibited, 32 μg/ml; 10% resistant, 10% susceptible dose dependent). C. glabrata was more commonly isolated from older patients (P = 0.02) and caused over 25% of candidemias among persons 65 years of age or older. The investigational triazoles posaconazole, ravuconazole, and voriconazole had excellent in vitro activity overall against Candida species. C. albicans is the most important cause of candidemia and remains highly susceptible to available antifungal agents. However, C. glabrata has emerged as an important and potentially antifungal resistant cause of candidemia, particularly among the elderly.
Candida bloodstream infections cause significant morbidity and mortality (8, 20), and Candida species are now the fourth most common cause of hospital-acquired bloodstream infection in the United States (5). Candida albicans is the most common cause of candidemia, and in general has remained susceptible in vitro to both amphotericin B and fluconazole (11, 12). As the use of fluconazole increases (1), however, it is important to monitor for any increase in azole resistance among bloodstream isolates of C. albicans or any increase in frequency of bloodstream infection due to Candida species other than C. albicans, which have a higher incidence of in vitro azole resistance (e.g., C. glabrata and Candida krusei) (3).
Surveillance studies have provided important information about the epidemiology of candidemia, including Centers for Disease Control and Prevention (CDC) population-based surveillance (8), the National Epidemiology of Mycoses Survey (NEMIS) (2, 13, 15, 18), the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) study (5), and the SENTRY program (4, 11, 12). These studies often are done in large tertiary care hospitals and/or urban areas. We present data from the first statewide, longitudinal surveillance study of bloodstream infections caused by Candida species.
The Emerging Infections and the Epidemiology of Iowa Organisms (EIEIO) surveillance program was begun in July 1998 to monitor trends in antimicrobial resistance in the State of Iowa. As part of this study, we conducted prospective surveillance for candidemia at 16 hospitals. The hospitals participating in EIEIO were selected based upon geographic region and size, in order to evaluate isolates from all regions and hospital sizes and types (e.g., rural, rural referral, and urban) (7). Our surveillance program can, therefore, provide insight into the incidence, species distribution, and antifungal susceptibility of Candida species causing bloodstream infections in a rural state.
MATERIALS AND METHODS
Surveillance.
The EIEIO surveillance network included 15 laboratories representing 17 medical centers distributed throughout the State of Iowa. Fourteen of these participating laboratories, representing 16 medical centers, submitted consecutive unique patient blood culture isolates of Candida species. Duplicate patient isolates were not accepted. All isolates were saved on agar slants and were sent to the University of Iowa College of Medicine (Iowa City) for storage and further characterization by reference identification and susceptibility testing methods. All isolates of Candida species causing bloodstream infection at these 16 medical centers during the 3-year period from 1 July 1998 to 30 June 2001 were evaluated.
Organism identification.
All Candida species blood culture isolates were identified at the participating institution by the routine methodology in use at each laboratory. Upon receipt at the University of Iowa, the isolates were subcultured onto potato dextrose agar not containing antibiotics (Remel, Lenexa, Kans.) and CHROMagar Candida medium (Hardy Laboratories, Santa Maria, Calif.) to ensure viability and purity. All green colonies on CHROMagar were presumptively identified as C. albicans after screening by differential growth at 35 and 43°C for Candida dubliniensis (6). Other Candida species identifications were confirmed with the Vitek YBC system (bioMerieux, St. Louis, Mo.). Isolates were stored as suspensions in sterile distilled water at ambient temperature prior to susceptibility testing, which was performed within 2 weeks of receipt of all isolates.
Susceptibility testing.
Antifungal susceptibility testing was performed by the reference broth microdilution method exactly as described by the National Committee for Clinical Laboratory Standards (NCCLS) document M27-A (10). Reference powders of amphotericin B (Sigma), flucytosine (5-FC; Sigma), fluconazole (Pfizer), voriconazole (Pfizer), itraconazole (Janssen), posaconazole (Schering Plough), and ravuconazole (Bristol Myers Squibb) were obtained from their respective manufacturers. Serial twofold dilutions were prepared as outlined in the NCCLS document (10) and final dilutions were made in RPMI 1640 medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma). Aliquots (0.1 ml) of each antifungal agent at a 2× final concentration were dispensed into the wells of plastic microdilution trays with a Quick Spense II system (Dynatech Laboratories, Chantilly, Va.). The trays were sealed and frozen at −70°C until needed.
Prior to testing, each isolate was passaged at least twice on potato dextrose agar (Remel) to ensure optimal growth characteristics. A 0.1-ml yeast inoculum (concentration of 0.5 × 103 to 2.5 × 103 cells/ml) was added to each well of the microdilution trays. The trays were incubated in air at 35°C, and MIC endpoints were read after 48 h of incubation. Drug- and yeast-free controls were included on each tray. Following incubation, the broth microdilution trays were examined with a reading mirror, and the growth in each well was compared with that in the growth control well. The MIC of amphotericin B was defined as the lowest concentration resulting in 100% growth inhibition compared with the growth control. The MICs of each triazole and of 5-FC were defined as the lowest concentrations resulting in a prominent inhibition of growth (∼50%) compared with the growth control (10). The data reported represent concentrations of each antifungal agent necessary to inhibit 50% (MIC50) and 90% (MIC90) of the isolates tested. The interpretive susceptibility criteria for fluconazole, itraconazole, and 5-FC were those published by Rex et al. (16) and the NCCLS (10).
Quality control was performed by testing Candida parapsilosis ATCC 22091 and C. krusei ATCC 6258.
Statistical analyses.
The Chi-square test for trend was used to test for changes in the incidence of candidemia by year of surveillance and in the species distribution by patient age. Alpha was set at 0.05, and all reported P values are two tailed.
Crude overall incidence rates were calculated with U.S. Bureau of Census and Iowa Hospital Association data (7). The 16 EIEIO hospitals accounted for 47.9% of all acute care discharges in the State of Iowa in CY2000. Assuming that the distribution of geographic region and hospital size was representative, we estimated the population denominator to be the same fraction of the total population of the State of Iowa in CY2000 for the purpose of estimating incidence rates.
RESULTS
A total of 254 bloodstream infections caused by Candida species were identified by EIEIO centers during the study period. Most candidemias occurred in adults older than 50 years of age (67%) (Table 1). More candidemias occurred among males (59%) than females and among patients hospitalized on general wards than in intensive care units (Table 1).
TABLE 1.
Characteristics of patients with candidemia in the EIEIO program, 1 July 1998 to 30 June 2001
| Characteristic | n (%) |
|---|---|
| Age (yr) | |
| <1 | 24 (9) |
| 1-18 | 15 (6) |
| 19-49 | 43 (17) |
| 50-64 | 53 (21) |
| >64 | 117 (46) |
| Gender | |
| Male | 149 (59) |
| Female | 105 (41) |
| Location at time of candidemia: | |
| Intensive care unit | 101 (40) |
| General ward | 142 (56) |
| Outpatient setting | 10 (4) |
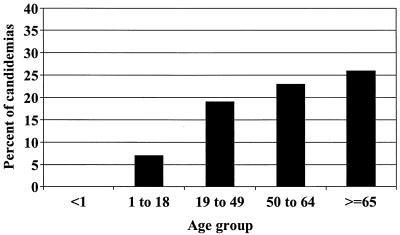
C. albicans (n = 148 [58%]) and C. glabrata (n = 51, 20%) were the most common species causing candidemia during the study period (Table 2). The proportion of Candida species other than C. albicans causing bloodstream infection did not change over time (data not shown). Two age-related trends were noted: (i) the percentage of candidemias caused by C. parapsilosis trended higher among infants less than 1 year of age (17%) than among all other age groups (6%, P = 0.06), and (ii) the percentage of candidemias caused by C. glabrata increased steadily with patient age (see Fig. 1 [P = 0.02] for trend).
TABLE 2.
Species distribution of Candida bloodstream isolates in the EIEIO program, 1998 to 2001
| Species | n (%) |
|---|---|
| C. albicans | 148 (58) |
| C. glabrata | 51 (20) |
| C. tropicalis | 28 (11) |
| C. parapsilosis | 17 (7) |
| C. krusei | 5 (2) |
| Other Candida spp.a | 5 (2) |
Includes two C. lusitaniae and two C. kefyr isolates and C. famata one isolate.
FIG. 1.
Percentage of all candidemias due to Candida glabrata in each age group. P = 0.02 for trend of increased frequency of C. glabrata with increasing age.
Based upon our estimated surveillance population, the mean annual incidence of candidemia was 6.0 per 100,000 of population. The incidence increased annually from 5.6 (1 July 1998 to 30 June 1999) to 5.9 (1 July 1999 to 30 June 2000) to 6.8 (1 July 2000 to 30 June 2001) per 100,000, but the change in incidence over time did not reach statistical significance.
Table 3 summarizes the in vitro susceptibility test results of these 254 Candida bloodstream isolates. Overall, the fluconazole resistance rate was low; three percent of all Candida species isolates were fluconazole resistant, with no fluconazole resistance detected among isolates of C. albicans, C. parapsilosis, or Candida tropicalis. Of 51 C. glabrata bloodstream isolates tested, 10% were fluconazole resistant (MIC, ≥64 μg/ml), and 10% were fluconazole susceptible dose dependent (MIC, 16 to 32 μg/ml). Itraconazole resistance was observed for 11% of all Candida isolates tested and was also highest among C. glabrata (53% resistant). For each species of Candida tested, the MIC50s and MIC90s for ravuconazole, posaconazole, and voriconazole were two- to fourfold lower than for itraconazole. Each of these investigational triazoles inhibited 98 to 99% of all Candida isolates tested at a MIC of ≤1 μg/ml, compared to 95% of isolates inhibited by itraconazole at a MIC of ≤1 μg/ml. Most Candida (87%) clustered at the amphotericin B MIC of 1 μg/ml—for only seven isolates (3%) were MICs of amphotericin B 2 μg/ml, including three of the five C. krusei isolates.
TABLE 3.
In vitro susceptibilities of bloodstream Candida spp. isolates in the EIEIO program, July 1998 to June 2001
| Species (no. of isolates tested) | Antifungal agent | MIC (μg/ml)a
|
% Resistantb | ||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| All Candida species (254) | Amphotericin B | 0.25-2.0 | 1.0 | 1.0 | |
| 5-FC | 0.06->128.0 | 0.12 | 1.0 | 3.6 | |
| Fluconazole | 0.12-128.0 | 0.25 | 8.0 | 2.8 | |
| Itraconazole | 0.007->8.0 | 0.06 | 1.0 | 11.0 | |
| Posaconazole | 0.007->8.0 | 0.03 | 0.5 | ||
| Ravuconazole | 0.007-8.0 | 0.007 | 0.25 | ||
| Voriconazole | 0.007-8.0 | 0.007 | 0.25 | ||
| C albicans (148) | Amphotericin B | 0.25-1.0 | 1.0 | 1.0 | |
| 5-FC | 0.06->128.0 | 0.25 | 1.0 | 2.7 | |
| Fluconazole | 0.12-2.0 | 0.25 | 0.5 | 0 | |
| Itraconazole | 0.007-0.25 | 0.03 | 0.06 | 0 | |
| Posaconazole | 0.007-0.12 | 0.015 | 0.06 | ||
| Ravuconazole | 0.007-0.06 | 0.007 | 0.015 | ||
| Voriconazole | 0.007-0.25 | 0.007 | 0.015 | ||
| C. glabrata (51) | Amphotericin B | 0.25-1.0 | 1.0 | 1.0 | |
| 5-FC | 0.06-16.0 | 0.12 | 0.12 | 0 | |
| Fluconazole | 1-28.0 | 8.0 | 32.0 | 9.8 | |
| Itraconazole | 0.06->8.0 | 1.0 | 2.0 | 52.9 | |
| Posaconazole | 0.06->8.0 | 0.5 | 1.0 | ||
| Ravuconazole | 0.03-8.0 | 0.25 | 1.0 | ||
| Voriconazole | 0.03-8.0 | 0.12 | 1.0 | ||
| C. tropicalis (28) | Amphotericin B | 0.5-1.0 | 1.0 | 1.0 | |
| 5-FC | 0.06->128.0 | 0.25 | 16.0 | 7.1 | |
| Fluconazole | 0.25-2.0 | 0.5 | 2.0 | 0 | |
| Itraconazole | 0.015-1.0 | 0.12 | 0.25 | 3.6 | |
| Posaconazole | 0.015-0.25 | 0.06 | 0.12 | ||
| Ravuconazole | 0.007-0.25 | 0.03 | 0.12 | ||
| Voriconazole | 0.007-0.12 | 0.03 | 0.06 | ||
| C. parapsilosis (17) | Amphotericin B | 1.0-2.0 | 1.0 | 2.0 | |
| 5-FC | 0.06-0.25 | 0.12 | 0.25 | 0 | |
| Fluconazole | 0.25-1.0 | 0.5 | 1.0 | 0 | |
| Itraconazole | 0.015-0.25 | 0.12 | 0.25 | 0 | |
| Posaconazole | 0.015-0.12 | 0.06 | 0.12 | ||
| Ravuconazole | 0.007-0.03 | 0.015 | 0.03 | ||
| Voriconazole | 0.007-0.03 | 0.015 | 0.03 | ||
50% and 90%, MIC50 and MIC90, respectively.
Percent resistant according to NCCLS breakpoints (10).
DISCUSSION
Candida albicans is the most common cause of candidemia, accounting for 58% of candidemias in Iowa hospitals surveyed. This is slightly higher than the 52 to 54% reported from surveillance studies performed in the U.S. by CDC (8), NEMIS (13), SCOPE (5) and SENTRY (11). Furthermore, our study suggests that antifungal resistance has not emerged among bloodstream isolates of C. albicans in Iowa, even with the widespread use of fluconazole. All of the C. albicans bloodstream isolates collected during this 3-year surveillance study were inhibited at fluconazole MICs of ≤2 μg/ml. So although resistance to fluconazole has been reported among mucocutaneous isolates of C. albicans (16), such resistance does not often complicate bloodstream infections due to C. albicans.
Other than C. albicans, C. glabrata is the most common species causing bloodstream infection in Iowa hospitals. In contrast to C. albicans, C. glabrata did manifest resistance to azole antifungal agents. The fluconazole MICs for 20% of C. glabrata bloodstream isolates were ≥16 μg/ml: 10% of the isolates were in the susceptible dose-dependent (S-DD) category, while 10% were fully resistant to fluconazole. This 80% rate of susceptibility to fluconazole among C. glabrata isolates in Iowa compares favorably with data from SENTRY centers in North America, where fluconazole susceptibilities among C. glabrata isolates were 63% in 1998 and 83% in 1999 (11). For no C. glabrata isolates were amphotericin B MICs >1 μg/ml. C. glabrata was also notable in EIEIO hospitals for being more common among older patients and absent among infants less than 1 year of age. These data are consistent with previous reports of the importance of C. glabrata as an emerging problem in the hospital, particularly in the elderly population (9, 11). Recent reports suggest that C. glabrata may also manifest resistance to amphotericin B (11, 17), although optimal in vitro methods for detecting such resistance are still being investigated (14).
C. tropicalis, C. parapsilosis and C. krusei together accounted for approximately 20% of Candida bloodstream infections in our study. We detected only five cases of C. krusei bloodstream infection over 3 years of surveillance. Considered inherently resistant to currently available azole antifungal agents, C. krusei isolates are also frequently resistant to amphotericin B. The amphotericin B MICs for three of the five isolates identified in this study were 2 μg/ml. C. tropicalis and C. parapsilosis were uniformly susceptible in vitro to fluconazole. Interestingly, C. tropicalis had the highest rate of in vitro resistance to 5-FC among the Candida species.
Consistent with previous reports (4, 11), each of the investigational triazoles tested (posaconazole, ravuconazole, and voriconazole) demonstrated excellent in vitro potency against Candida species. Although the MICs of these agents were higher among fluconazole- and itraconazole-resistant isolates, they still demonstrated a two- to fourfold lower MIC than itraconazole for these strains. In vitro susceptibility breakpoints for these agents have not been established, nor has their role in the management of serious infections due to Candida species. Nonetheless, they may represent future options for prophylaxis or management of selected patients at risk for or infected with Candida species (19).
Our data confirm the important role of Candida species as a cause of bloodstream infection in the health care setting. The annual incidence of 6 cases per 100,000 population in the State of Iowa is an estimate, given that our study was not designed to be population based and therefore inclusive of all cases of candidemia in the State of Iowa during the surveillance period. Our incidence estimate may be biased toward a falsely high rate, since one participating hospital is the largest tertiary care referral center in Iowa. Even so, our estimated incidence is lower than the incidence of 8 per 100,000 reported by CDC surveillance in two large urban areas (Atlanta, Ga., and San Francisco, Calif.) in 1992 to 1993. Demographic differences between the rural, elderly population of Iowa and the urban population surveyed by the CDC may explain this finding. In particular, the highest incidence of candidemia in the CDC study was found among neonates (8), and 14% of the candidemias were reported in the 0- to 12-month age group. The population of Iowa is one of the most elderly in the United States, and fewer candidemias in our study population occurred among children younger than 1 year.
In summary, we report the results of the first longitudinal statewide surveillance of bloodstream infection due to Candida species. These results demonstrate the continued prominence of C. albicans as a cause of candidemia, as well as the preserved activity of azoles against contemporary isolates of Candida species. However, C. glabrata is emerging, particularly in older adults, as an important and potentially antifungal resistance cause of candidemia. Given an aging population, widespread use of azole antifungal agents, and increasing acuity of hospitalized patients with risk factors for candidemia, we suspect that C. glabrata will continue to emerge as an important cause of serious disease in the health care setting. Ongoing surveillance of serious infections caused by Candida species will be important for tracking changes in the epidemiology and in antifungal susceptibility among these important health care-associated pathogens.
Acknowledgments
The EIEIO study was funded in part by the Prevention Epicenters Program of the Centers for Disease Control and Prevention (UR8/CCU715091-03-3), and by unrestricted educational and research grants from Abbott Laboratories, Glaxo Wellcome, Ortho McNeil, Pfizer, Smith Kline Beecham, and Wyeth-Ayerst. This study was also supported in part by research grants from Bristol Myers Squibb and Schering Plough Research Institute.
We thank the personnel at all of the EIEIO centers for their participation and support of this study: St. Luke's Regional Medical Center, Sioux City; Iowa Methodist Medical Center, Des Moines; Veteran's Affairs Medical Center, Des Moines; Laboratory Control, Ltd. (Ottumwa Regional Medical Center), Ottumwa; Mercy Medical Center, Clinton; St. Luke's Hospital, Cedar Rapids; Metropolitan Medical Laboratory (Genesis Medical Centers—East and West), Davenport; United Clinical Laboratories (The Finley Hospital and Mercy Health Center), Dubuque; Trinity Regional Hospital, Fort Dodge; University of Iowa Hospitals and Clinics, Iowa City; Spencer Municipal Hospital, Spencer; Great River Medical Center, West Burlington; Allen Memorial Hospital, Waterloo; and Mercy Medical Center, Mason City.
REFERENCES
- 1.Berrouane, Y. F., L. A. Herwaldt, and M. A. Pfaller. 1999. Trends in antifungal use and epidemiology of noscocomial yeast infections in a university hospital. J. Clin. Microbiol. 37:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, and the NEMIS Study Group. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS Prospective Multicenter Study. Clin. Infect. Dis 33:177-186. [DOI] [PubMed] [Google Scholar]
- 3.Collin, B., C. J. Clancy, and M. H. Nguyen. 1999. Antifungal resistance in non-albicans Candida species. Drug Resist. Updates 3:9-14. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, G. V. Doern, R. N. Jones, and the SENTRY Participants Group. 1999. In vitro activities of BMS-207147 against over 600 contemporary clinical bloodstream isolates of Candida species from the SENTRY Antimicrobial Surveillance Program in North America and Latin America. Antimicrob. Agents Chemother. 43:2236-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis 29:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Gales, A. C., M. A. Pfaller, A. K. Houston, S. Joly, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. Identification of Candida dubliniensis based upon temperature and utilization of xylose and α-methyl-d-glucoside as determined with the API 20C AUX and Vitek YBC systems. J. Clin. Microbiol. 37:3804-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iowa Hospital Association. 2000. Annual fact book. Iowa Hospital Association, Des Moines.
- 8.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stevens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman, C. A. 2001. Fungal infections in older adults. Clin. Infect. Dis. 33:550-555. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibility to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and the SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY Antimicrobial Surveillance Program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., S. A. Messer, A. Houston, M. S. Rangel-Frausto, T. Wiblin, H. M. Blumberg, J. E. Edwards, W. Jarvis, M. A. Martin, H. C. Neu, L. Saiman, J. E. Patterson, J. C. Dibb, C. M. Roldan, M. G. Rinaldi, and R. P. Wenzel. 1998. National Epidemiology of Mycoses Survey: a multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn. Microbiol. Infect. Dis. 31:289-296. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, and A. Bolmstrom. 1998. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn. Microbiol. Infect. Dis. 32:223-227. [DOI] [PubMed] [Google Scholar]
- 15.Rangel-Frausto, M. S., T. Wiblin, H. M. Blumberg, L. Saiman, J. Patterson, M. Rinaldi, M. Pfaller, J. E. Edwards, Jr., W. Jarvis, J. Dawson, and R. P. Wenzel. 1999. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin. Infect. Dis. 29:253-258. [DOI] [PubMed] [Google Scholar]
- 16.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 17.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 18.Saiman, L., E. Ludington, M. Pfaller, S. Rangel-Frausto, R. T. Wiblin, J. Dawson, H. M. Blumberg, J. E. Patterson, M. Rinaldi, J. E. Edwards, R. P. Wenzel, and W. Jarvis. 2000. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr. Infect. Dis. J. 19:319-324. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital acquired candidemia: attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]