Abstract
Induction of heme oxygenase-1 (HO-1) expression can be achieved by stimulation with cobalt protoporphyrin (CoPPIX) or cobalt chloride (CoCl2). HO-1 has been recently implicated in regulation of angiogenesis and CoCl2 is known to potently activate hypoxia inducible factor-1 (HIF-1) transcription factor, a key regulator of angiogenic response in hypoxia. Here we determined the effect of CoPPIX and CoCl2 on the expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8), the two major angiogenic mediators, in human microvascular endothelial cells (HMEC-1). CoPPIX induced HO-1 expression and strongly enhanced VEGF and IL-8 synthesis, through the activation of VEGF and IL-8 promoters. Inhibition of HO activity by SnPPIX decreased VEGF production, while, interestingly, it did not affect IL-8. CoCl2 activated hypoxia-responsive element (HRE) and consequently VEGF generation via the enhancement of production of reactive oxygen species (ROS). On the other hand, CoCl2 did not influence IL-8 expression, while CoPPIX did not induce ROS elevation neither it affected HRE activity in VEGF promoter. Our data show that although both CoCl2 and CoPPIX induce HO-1, the influence of CoCl2 on VEGF does not involve HO-1 and is HIF-1-dependent, while the effect of CoPPIX does not involve HIF-1 but relies on HO-1.
Keywords: VEGF, IL-8, heme oxygenase, HIF-1, hypoxia, angiogenesis, reactive oxygen species
INTRODUCTION
Vascular endothelial growth factor (VEGF) is the major angiogenic mediator generated by different cell types, including microvascular endothelial cells, smooth muscle cells and fibroblasts (10). It exerts a wide range of actions being a specific mitogen for endothelial cells, increasing their migration and inhibiting apoptosis (35). IL-8, a member of chemokine family, is not only a pro-inflammatory agent but also plays an important role in angiogenesis (23). Both these factors are crucial in various angiogenic-dependent disorders, including tumors and cardiovascular diseases (3).
Heme oxygenases (HO) are the rate limiting enzymes that catalyze the conversion of heme into biliverdin, carbon monoxide (CO) and free iron (24). Two isoforms are known. HO-2 is constitutively expressed in many cells, including endothelial cells and neurons, while HO-1 is the inducible protein. Up-regulation of HO-1 may be an adaptive mechanism protecting cells from stress, in particular during hypoxia or inflammation (25).
Several reports have demonstrated that increased HO-1 expression and activity can upregulate synthesis of VEGF both in vitro (8, 15, 16,26) and in vivo (5,21 ,29). It has been shown that enhancement of VEGF production in response to hemin in rat vascular smooth muscle cells (VSMC) and endothelial cells was dependent on HO-1 activity, as it was completely reversed by HO inhibitor, SnPPIX (8, 15). Also 15-deoxy12, 14 Δ-prostaglandin-J2 (15d-PGJ2) augmented the expression of VEGF in an HO-1 dependent manner in human microvascular endothelial cell line (HMEC-1) (16). Interestingly, in the same cells the production of IL-8 was not affected by hemin or SnPPIX (16), indicating that its synthesis does not involve HO-1 pathway.
However, the stimulation of cells with exogenous heme involves not only the HO-1 induction, but may represent the complex activity of heme as a pro-oxidant and iron-releasing compound. Therefore, in the present study we aimed to validate the role of HO-1 activation in regulation of VEGF and IL-8 synthesis, comparing the effects of two other potent HO-1 inducers, CoPPIX and CoCl2.
MATERIALS AND METHODS
Reagents
Luciferase Assay Reagents, oligo(dT) primers, dNTPs and MMLV reverse transcriptase were purchased from Promega (Madison, WI, USA). The ELISA kit for human VEGF was procured from R&D Systems Europe (Abingdon, UK). BCA-1 protein determination kit, epidermal growth factor (EGF), hydrocortisone and the cell culture medium (MCDB 131) were obtained from Sigma-Aldrich (Poznan, Poland). Tin protoporphyrin-IX (SnPPIX) and cobalt protoporhyrin (CoPPIX) were purchased from Porphyrin Products. The SuperFect transfection reagent was bought from Qiagen. Fetal calf serum was procured from ICN (Irvine, CA, USA). The ELISA-based Trans-Am HIF-1 kit was procured from Active Motif (Belgium).
Cell culture and incubation experiments
Human microvascular endothelial cells (HMEC-1) were obtained from Centers for Disease Control and Prevention (Atlanta, GA, USA) and cultured in a MCDB 131 medium containing 10% FCS, L-glutamine (2 mM), EGF (10 ng/ml) and hydrocortisone (1 μg/ml).
Cells were seeded in 24-well or 6-well plates and grown to full confluence. Next, medium was changed and stimulants were added for 6 or 24 hr. After this time media were collected for determining VEGF and IL-8 protein concentration, while cells were washed twice with cold PBS and then subjected to RNA or protein isolations for RT-PCR analysis or Western blotting, respectively. CoPPIX, SnPPIX and CoCl2 were prepared freshly and added to cells for indicated time. SnPPIX and N-acetylcysteine were applied 1 hr before stimulation. Solvent (0.1 M NaOH for CoPPIX and SnPPIX) at appropriate final dilution was included in control.
Reverse transcription-polymerase chain reaction
RT-PCR was performed on 2 μg of total RNA which was isolated from the cells by acid guanidinium thiocyanate-phenol-chloroform extraction. Reverse transcription was carried out with oligo-dT primers for 1 hr at 42°C using MMLV reverse transcriptase, according to vendor's instruction. PCR amplification with Taq polymerase was performed for 28 cycles (EF2), 30 cycles (HO-1, IL-8) and 35 cycles (VEGF) using the following protocol: 95°C - 40 sec, 58°C - 40 sec and 72°C - 50 sec. The primers specific for VEGF (5'-CAC CGC CTC GGC TTG TCA CAT-3' and 5'-CTG CTG TCT TGG GTG CAT TGG-3'), for HO-1 (5'-GTG GAG ACG CTT TAC GTA GTG C-3' and 5'-CTT TCA GAA GGG TCA GGT GTC C-3'), for IL-8 (5'-CTC TCT TGG CAG CCT TCC TGA-3' and 5'-CCC TCT GCA CCC AGT TTT CCT T-3'), and for housekeeping gene EF2 (5'-GCG GTC AGC ACA ATG GCA TA and 5'-GAC ATC ACC AAG GGT GTG CAG) were used. PCR products were analyzed by electrophoresis in 2% agarose gel. The product length for the VEGF120 was 431 bp, for VEGF164 - 563 bp, for HO-1 - 250 bp, for IL-8 - 240 bp and for EF2 - 218 bp.
Transfection with reporter plasmids
Cells were transfected with constructs containing a full-length human VEGF promoter (−2279 to +54) or HRE fragment of this promoter (−1014 to −903) cloned into the luciferase reporter plasmid pGL2 and inserted upstream of the thymidine kinase promoter of pT81luc0 plasmid. Both constructs were kindly provided by Dr. Hideo Kimura (Chiba, Japan). The full-length promoter of the IL-8 gene was cloned upstream of the firefly luciferase coding region in the pGL2-basic vector and was kindly supplied by Dr. Rainer de Martin (Vienna, Austria) (34). HMEC-1 cells growing to 70% confluence in 24-well plates were transfected with 0.25 μg of DNA and 1,25 μl of SuperFect Reagent per well, according to vendor's protocol. After 24 hr cells were treated with indicated stimulants for next 24 hr. The activity of reporter genes was determined in cell lysates at 48 hr after transfection and normalized to the total protein content.
Western blotting
The total cellular protein from HMEC-1 cells was isolated using ice-cold lysis buffer (1 × PBS, 10 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM leupeptine, 10 mM aprotinin and 1% Triton X-100). Samples were centrifuged (20 min, 8000 g, 4°C) and clear supematants were collected. After measurement of protein content with bicinchoninic acid protein assay kit (BCA, Sigma) 10 μg of protein was loaded on 12% SDS-PAGE gel followed by transfer to nitrocellulose membrane Hybond™ECL™ (Amersham Pharmacia Biotech, Buckinghamshire, UK). Membranes were probed with polyclonal antibodies against heme oxygenase-1 (Stressgen Biotech, Canada) followed by biotin-conjugated secondary antibodies at the dilution 1:2500 in TBS with 3% albumin. Alkaline Phosphatase-Conjugated Streptavidin (Dako) at the dilution 1:5000 in TBS was used and the visualisation was performed using the BCIP/NBT Blue liquid substrate for membranes.
Determination of intracellular reactive oxygen species generation using DCFH-DA oxidation
HMEC-1 cells were stimulated for 24 hr and DCFH—DA (10 μM) was added for the last hour of incubation. The fluorescence (excitation 485 nm, emission 535 nm) was measured directly from the plate. Data were normalized to the control values.
Measurement of HIF-1 binding
Binding of HIF-1 (hypoxia inducible factor-1) to the hypoxia responsive element (HRE) was measured with the ELISA-based Trans-Am HIF-1 kit using nuclear extracts lysates prepared from HMEC-1 cells. The Trans-Am kit employs 96-well microtier plates coated with an oligonucleotide containing the HRE consensus sequence. Transcription factor binding to HRE sequence is detected by anti-HIF specific antibody followed by colorimetric measurement. Preparation of nuclear extracts was done exactly as recommended by the manufacturer. Specificity was checked by measuring the ability of soluble wild-type or mutated oligonucleotides to inhibit binding.
Luciferase assay
Luciferase assay was done according to the manufacturer's protocol as described previously (14).
ELISA assays
Cell culture media were collected and concentration of VEGF and IL-8 protein was quantified following the manufacturer's protocol.
Statistical analysis
All experiments were performed in duplicates or triplicates and if not reported otherwise they were repeated at least three times. Data are presented as mean ± SD. Statistical evaluation was done with Student's t test. Differences were accepted as statistically significant at p<0.05.
RESULTS
Effect of CoPPIX and CoCl2 on HO-1 expression
CoPPIX and CoCl2 can induce HO-1 in many cell types (2,3,30). Our experiments confirm that these compounds are strong activators of HO-1 expression also in microvascular endothelium. We observed a potent upregulation of HO-1 in CoPPIX- and CoCl2-treated HMEC-1 both at the mRNA and protein levels, as demonstrated by RT-PCR (Fig. 1A) and Western-blotting (Fig. 1B), respectively.
Fig. 1.

Effect of CoPPIX and CoCl2 on HO-1 expression and ROS generation in HMEC-1 cells. Induction of HO-1 expression in HMEC-1 after 24 hr treatment with CoPPIX (10 μM) and CoCl2 (250 μM) measured by RT-PCR (A) and Western blot analysis (B). EF2 was used as a housekeeping gene (A). ROS generation was increased after CoCl2 (250 μM) but not by CoPPIX (10 μM). Moreover, pretreatment with NaC (1 mM) reversed stimulatory effect of CoCl2 on ROS generation (C). Mean of three independent experiments, *p<0.05 vs control, #p<0.05 in comparison to cells treated with CoCl2.
Additionally, using DCFH-DA oxidation assay, we found that exposure of HMEC-1 to CoCl2 leads to a strong elevation of reactive oxygen species (ROS). Fluorescence of DCFH-DA oxidation product was inhibited by preincubation of cells with N-acetylcysteine (NAC), confirming the specificity of assay. Of note, CoPPIX did not affect ROS generation in HMEC-1 (Fig. 1C). At the doses used, CoPPIX and CoCl2 did not influence HMEC-1 viability (data not shown). Thus, apart from induction of HO-1 these two cobalt compounds exert distinct effects on the cells.
Effect of CoPPIX and CoCl2 on VEGF synthesis
Basal production of VEGF in HMEC-1 is usually low and ranges around 10-30 pg/105 cells. Along with induction of HO-1, CoPPIX and CoCl2 upregulated by approximately three-fold the release of VEGF into the culture media, as measured by ELISA (Fig. 2A). Augmented synthesis of VEGF protein was paralleled by similar extent of activation of VEGF transcription, as determined by luciferase assay in cells transfected with a reporter plasmid containing a human full-length VEGF promoter (Fig. 2B). It may suggest that regulation of VEGF synthesis by both compounds is exerted at the transcription level.
Fig. 2.

Effect of CoPPIX and CoCl2 on VEGF synthesis (A), activity of a full length VEGF promoter (B) and HRE sequence of the human VEGF promoter (C) in HMEC-1 cells. Cells were treated with HO-1 inducers and VEGF synthesis was measured by ELISA (A). Moreover, cells were transfected with reporter plasmids, then treated with CoPPIX and CoCl2 and after 24 hr subjected to luciferase activity assay. Both CoPPIX and CoCl2 potently upregulated VEGF synthesis (A), enhanced VEGF promoter (B) but they affected HRE sequence in a completely different way (C). Mean of three independent experiments. *p<0.05 in comparison to control.
Despite the similar stimulatory effects of CoPPIX and CoCl2 on VEGF transcription, the underlying pathways appear to be different. Using the cells transfected with a reporter construct regulated by the hypoxia responsive element (HRE) from the VEGF promoter, we demonstrated that CoCl2 strongly activated HRE. In contrast, CoPPIX did not exert any influence (Fig. 2C). Thus, we can suppose that increased transcription of VEGF in response to CoCl2 results mostly from activation of hypoxia inducible factor-1 (HIF-1) and direct stimulation of VEGF promoter through binding of HIF-1 to HRE.
Lack of effect of the second HO-1 activator, CoPPIX, on HRE suggested that HO-1 is not involved in regulation of HIF-1 binding activity. To check this supposition we measured activation of HIF-1 in cells treated with hemin, the most common HO-1 inducer. Analysis done using a TransAm assay confirmed that modulation of HO-1 activity does not affect the HIF-1 binding to the target DNA sequences (data not shown, 101.15 ± 4.03% of control, n=2 independent experiments).
Finally, we evaluated the involvement of HO-1 pathway in CoPPIX- and CoCl2-induced upregulation of VEGF expression. To this aim we pretreated the cells with SnPPIX, a competitive inhibitor of HO-1, and then stimulated them with CoPPIX or CoCl2. As shown at Fig. 3, inhibition of HO-1 significantly reversed the increase in VEGF synthesis induced by CoPPIX. It was demonstrated both at the mRNA (Fig. 3A) and protein (Fig. 3B) level. In contrast, the upregulation of VEGF in response to CoCl2 was not significantly affected by SnPPIX.
Fig. 3.
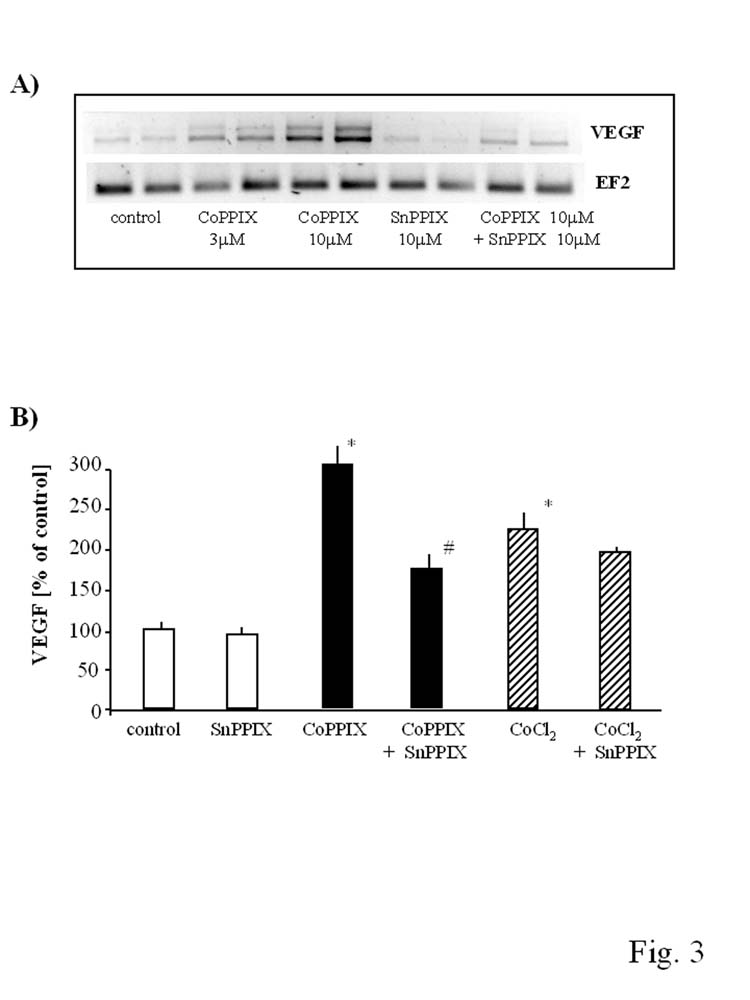
Comparison of the involvement of HO-1 pathway in CoPPIX- and CoCl2-induced upregulation of VEGF expression. HMEC-1 were pretreated with SnPPIX (10 μM) 1 hr before stimulation with CoPPIX (10 μM) and CoCl2 (250 μM). Inhibition of HO-1 by SnPPIX significantly reversed the increase in VEGF synthesis induced by CoPPIX both at the mRNA (A) and protein (B) level but did not influence CoCl2-induced increase in VEGF synthesis (B). Representative RT-PCR result after 6 hr incubation (A), result of ELISA for VEGF after 24 hr incubation (B). *p<0.05 in comparison to control, #p<0.05 in comparison to cells treated with CoPPIX.
Because treatment of HMEC-1 with CoCl2 elevated the generation of ROS, the effect reversed by NAC (Fig. 1C), we checked whether NAC can modulate the CoCl2-induced production of VEGF. Actually, we found that such a treatment significantly reduced the synthesis of VEGF protein (Fig. 4A) and decreased the activity of HRE part of VEGF promoter (Fig. 4B). Thus, we postulate, that although both CoPPIX and CoCl2 strongly induce HO-1 expression, only in the case of the former compound this induction is an important mediator of VEGF augmentation. In upregulation of VEGF by CoCl2, the concomitant induction of HO-1 does not play a significant role, as much more important appears the activation of HIF-1, a direct inducer of VEGF transcription. Stimulation of HIF-1 in response to CoCl2 may result from the generation of ROS (Fig. 1C).
Fig. 4.
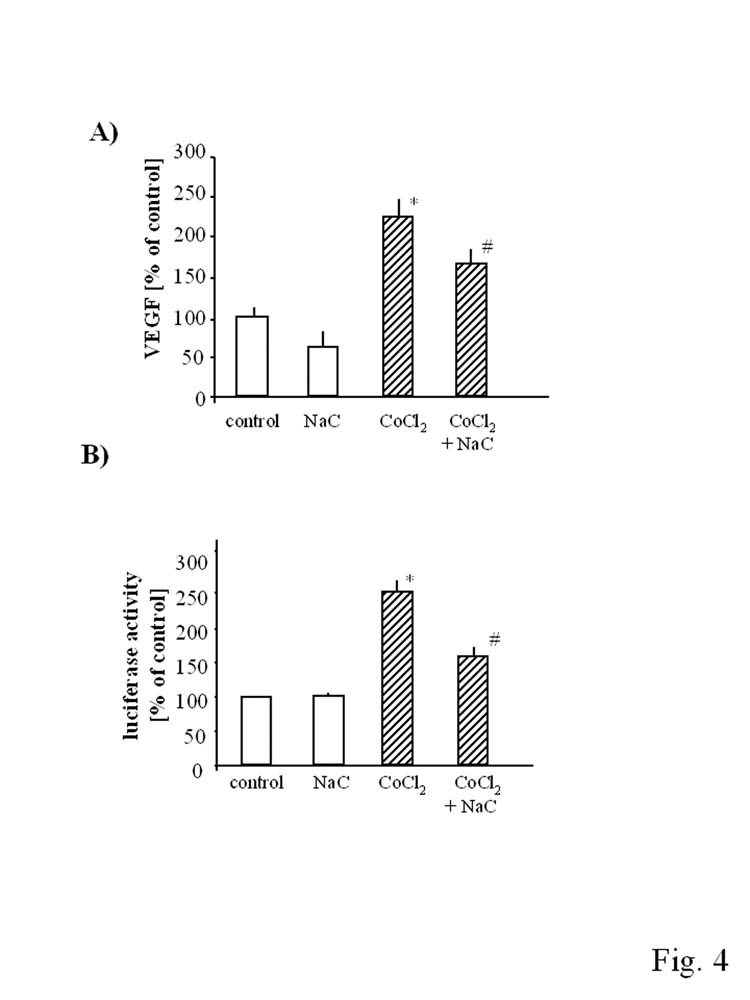
Effect of N-acetylcysteine on CoCl2-induced VEGF synthesis (A) and HRE activation (B) in HMEC-1. Cells were pretreated with NaC (1 mM) 1 hr before stimulation with CoPPIX (10 μM) and CoCl2 (250 μM). NaC reversed stimulatory effect of CoCl2 on VEGF synthesis (A) and HRE activation (B) in HMEC-1. Mean of three independent experiments. *p<0.05 in comparison to control, #p<0.05 in comparison to cells treated with CoCl2.
Effect CoPPIX and CoCl2 on IL-8 synthesis
IL-8 is a pro-angiogenic cytokine able to enhance the proliferation of endothelial cell and promote their survival (23). Basal production of IL-8 by HMEC-1 is relatively high, within the range of ∼1000 pg/105 cells. We found that in response to CoPPIX, expression of IL-8 mRNA (Fig. 5A) and synthesis of IL-8 protein (Fig. 5B) were strongly augmented. Similarly as in the case of VEGF, the increased generation of IL-8 was associated with a higher transcription rate from the IL-8 promoter (Fig. 5C).
Fig. 5.
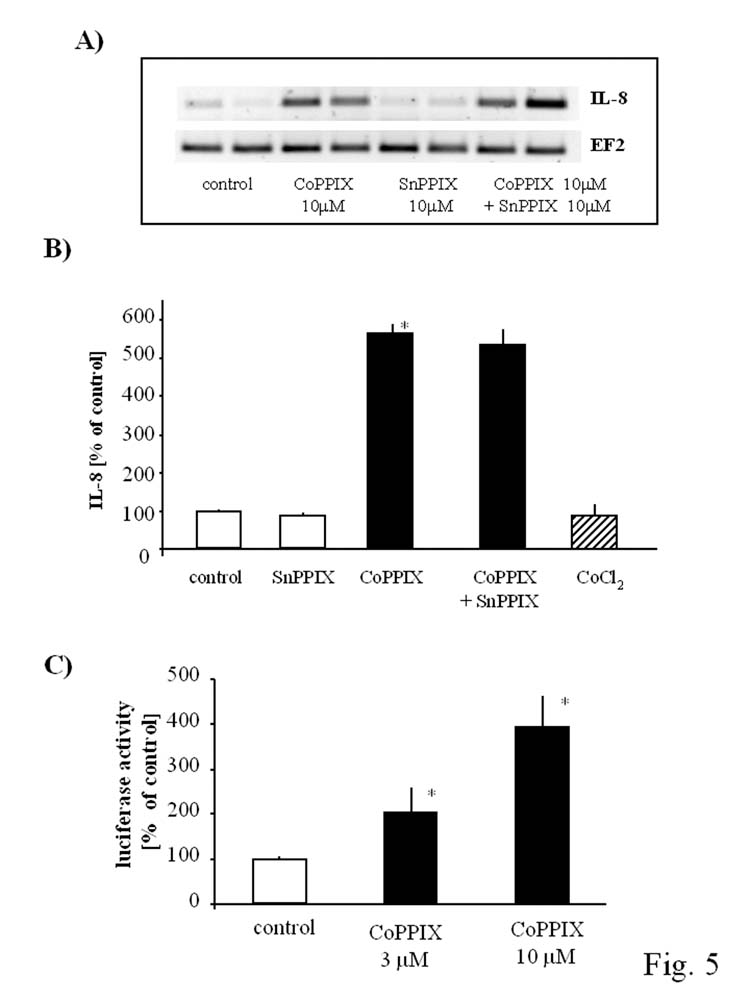
Opposite effect of CoPPIX and CoCl2 on IL-8 expression in HMEC-1 cells. IL-8 production is strongly upregulated by CoPPIX (10 μM) at mRNA (A) and protein level (B) but CoCl2 (250 μM) does not influence its production in HMEC-1 after 24 hr incubation (B). CoPPIX-induced IL-8 synthesis was not diminished after SnPPIX treatment (A, B). IL-8 promoter activity is increased in a concentration-dependent manner after 24 hr stimulation with CoPPIX (C). Mean of three (B) and two (C) independent experiments. *p<0.05 in comparison to control.
However, pretreatment of cells with SnPPIX did not modulate the response to CoPPIX (Fig. 5A, 5B). Therefore, differently from VEGF expression, the upregulation of IL-8 in HMEC-1 exposed to CoPPIX does not involve HO-1 pathway. This observation was further confirmed by treatment of the cells with CoCl2. In spite of the strong HO-1 induction, CoCl2 did not influence the generation of IL-8 protein (Fig. 5B).
DISCUSSION
The salient finding of the present study is the demonstration that synthesis of VEGF in HMEC-1 can be enhanced by induction of HO-1, while the synthesis of IL-8 is not reliant on this pathway. However, induction of HO-1 by various chemical stimuli does not necessarily lead to increased VEGF synthesis. Thus, although both CoCl2 and CoPPIX induce HO-1, the effect of CoCl2 on VEGF does not involve HO-1 and is dependent on HIF-1, while the effect of CoPPIX does not involve HIF-1 but relies on HO-1.
HO-1 is an inducible enzyme converting heme to CO, free iron and biliverdin which is reduced to bilirubin by biliverdin reductase (24). The products of this enzymatic reaction have important biological effects, including antioxidant, anti-inflammatory and cytoprotective functions (25). Induction of HO-1 is an adaptive response to several injurious stimuli including heme, heavy metals, nitric oxide (NO) or cytokines. Such induction has been implicated in numerous diseases including transplant rejection, hypertension, atherosclerosis, lung injury, endotoxic shock and others (25).
CoPPIX is used as an inducer of HO-1 activity and its action is confirmed by many studies. It was used to up-regulate HO-1 in the stateotic rat liver model of ex vivo cold ischemia/reperfusion injury, where it was able to improve portal venous blood flow, increase bile production and decrease hepatocyte injury (2). Moreover, it can prevent graft-versus-host disease after experimental allogenic bone marrow transplantation (13) and improve survival after rat renal allografts (32).
CoCl2 has been also reported to up-regulate HO-1 (6,17,30). Additionally, the important mechanism of its action is the stimulation of HIF-1 transcription factor (33). HIF-1 is a heterodimeric protein composed of the HIF-1α and HIF-1β subunits. HIF-1β subunit is constitutively expressed whereas the α subunit is unstable at normal oxygen concentration, while low oxygen tension stabilizes it (for a review see 36). CoCl2 which is often used as a hypoxia mimic can potently induce HIF-1 by stabilization and accumulation of HIF-1α protein (18). After this stabilization active HIF-1 heterodimer is formed and binds to HRE present in the regulatory regions of many genes including VEGF (19).
In our studies pharmacological activators of HO-1, CoPPIX and CoCl2, induced VEGF synthesis. VEGF is a potent angiogenic mediator that plays an important role in both physiological as well as pathological vasculogenesis and angiogenesis (10). It stimulates endothelial cell proliferation and migration in vitro and exerts anti-apoptotic activities by inducing Akt phosphorylation (1). In addition, VEGF acts as a proinflammatory cytokine due to its chemoattractant properties, by increasing endothelial cell pemeability or augmenting the expression of endothelial cell adhesion molecules (20). Taken together, VEGF is likely a key intermediary between cell-mediated immune inflammation and the associated angiogenesis reaction.
In our previous papers we have demonstrated that HO-1 expression and activity can enhance synthesis of VEGF in vitro (8,15,16). We have demonstrated earlier that SnPPIX, an inhibitor of HO activity, completely blocked cytokine-induced VEGF expression, whereas stimulation of HO-1 with hemin increased its expression. Similar effect was obtained by overexpressing ho-1 gene in VSMC (8) and HMEC-1 (16). Also in vivo studies clearly showed that VEGF synthesis can be dependent on HO-1 activity (21,29). Here we have obtained analogous results using CoPPIX as inducer of both HO-1 and VEGF. Accordingly, inhibition of HO-1 activity by SnPPIX reversed CoPPIX-induced VEGF production. On the other hand, CoCl2 which also potently induces HO-1 upregulates production of VEGF in an HO-1 independent manner.
Our results clearly demonstrated that CoCl2 induces VEGF synthesis by increased VEGF transcription through HRE activation and this mechanism is completely different from the action of CoPPIX. We showed that although full VEGF promoter was activated by CoPPIX, HRE element was not affected by this compound. In turn, lack of involvement of HO-1 in CoCl2-induced VEGF expression may result from the very fast stabilization of HIF-1 and immediate induction of VEGF, which may preclude the significant influence of HO-1 pathway, requiring more time for activation.
Recent studies suggest that changes in the level of ROS provide a redox signal for HIF-1 induction by hypoxia (for a review see 36). Also under normoxic conditions ROS can mediate HIF-1α protein accumulation and HIF-1 -dependent transcription by hormones, growth factors, and transition metals (9, 14, 18,28). Moreover, ROS mediated induction of HIF-1 has been reported to regulate VEGF expression in different cell types like smooth muscle cells (4,28), human ovarian cancer cells (9) or fibroblasts (14). Accordingly, data presented here indicate that CoCl2-induced HIF-1α activation is dependent on ROS generation.
In this study we have also examined the effect of CoPPIX and CoCl2 on IL-8 synthesis in HMEC-1. IL-8, the CXC chemokine is not only a potent chemoattractant for neutrophils and T lymphocytes and regulator of leukocyte survival but is also involved in the regulation of angiogenesis (23). It is produced by many cell types, including endothelial cells, after stimulation with the inflammatory cytokines, such as tumor necrosis factor-α and interleukin-1β (12). Same papers show that this production can be also connected with the activities of VEGF, which can induce IL-8 synthesis (22,27).
Our results show that two agents containing cobalt ion, namely CoPPIX and CoCl2, regulate IL-8 expression in a completely different way. CoPPIX induces IL-8 mRNA, promoter activity and protein synthesis, whereas CoCl2 does not influence IL-8 generation. Importantly, the action of CoPPIX on IL-8 synthesis seems to be HO-1-independent. This mechanism is different from VEGF regulation, which is reliant on HO-1 pathway. Our data are partially opposite to those obtained by Pae and coworkers (26). They have proposed that HO-1 induced VEGF acts as an IL-8 regulator. In their experiments induction in IL-8 was dependent on HO-1 as they have observed a decrease in IL-8 protein synthesis after transfection of siRNA against HO-1 (26). Those differences might be caused by cell specific effect as HUVEC were used in that study (26) while HMEC-1 in ours.
It is well established that NO can induce both HO-1 (11,31) and VEGF (for a review see: 7) expression in various cell types, including endothelial cells, probably via induction of oxidative stress but in our experiments we did not observe upregulation of nitric oxide synthase expression after CoPPIX treatment (data not shown). Moreover, we have checked if CoPPIX can cause oxidative stress and in that way induce IL-8 expression. However, we did not observe increase in free radical formation.
Taken together, CoPPIX induces both VEGF and IL-8 productions but the mechanisms of these inductions seem to be completely different; VEGF expression is augmented in HO-1 dependent manner, whereas IL-8 production is HO-1 independent. In contrast, CoCl2 induces VEGF through HIF-1 activation, regardless the HO-1 expression. Moreover, it does not up-regulate IL-8 synthesis. Our data strongly suggest that although both CoPPIX and CoCl2 induce HO-1 they have also other specific activities. Further studies are underway to elucidate the mechanism(s) mediating the induction of VEGF and IL-8 by HO-1 activators.
Acknowledgments
This work was partially supported by grants from Ministry for Science and Informatics Technology (PBZ-KBN-107/P04/2004, 2 PO4B 016 26). We thank Mr. Slawomir Golda for help in measuring ROS generation. Dr Jacek Miedzobrodzki is acknowledged for access to the EG Berthold chemoluminometer. A. Jozkowicz is the recipient of the Wellcome Trust International Senior Research Fellowship.
Abbreviations
- CO
carbon monoxide
- HIF-1
hypoxia inducible factor-1
- HMEC-1
human microvascular endothelial cell line
- HO
heme oxygenase
- HRE
hypoxia responsive element
- IL-8
interleukin 8
- NAC
N-acetylcysteine
- NO
nitric oxide
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
REFERENCES
- 1.Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, Walsh K, Aird WC. Vascular endothelial growth factor activates PBK/Akt/forkhead signaling in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 2.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J. Clin. Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azenshtein E, Meshel T, Shina S, Barak N, Keydar I, Ben-Baruch A. The angiogenic factors CXCL8 and VEGF in breast cancer: regulation by an array of pro-malignancy factors. Cancer Lett. 2005;217:73–86. doi: 10.1016/j.canlet.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 4.BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. J. Biol. Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 5.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 6.Christova TY, Gomeva GA, Taxirov SI, Duridanova DB, Setchenska MS. Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma-bearing mice: protective role of heme oxygenase. Toxicol. Lett. 2003;138:235–242. doi: 10.1016/s0378-4274(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 7.Dulak J, Jozkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid. Redox. Signal. 2003;5:123–132. doi: 10.1089/152308603321223612. [DOI] [PubMed] [Google Scholar]
- 8.Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, Pachinger O, Weidinger F, Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox. Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 9.Duyndam MC, Hulscher TM, Fontijn D, Pinedo HM, Boven E. Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1alpha protein by the oxidative stressor arsenite. J. Biol. Chem. 2001;276:48066–48076. doi: 10.1074/jbc.M106282200. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrin. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic. Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- 12.Fujishima S, Hoffman AR, Vu T, Kim KJ, Zheng H, Daniel D, Kim Y, Wallace EF, Larrick JW, Raffin TA. Regulation of neutrophil interleukin 8 gene expression and protein secretion by LPS, TNF-alpha, and IL-1 beta. J. Cell. Physiol. 1993;154:478–485. doi: 10.1002/jcp.1041540305. [DOI] [PubMed] [Google Scholar]
- 13.Gerbitz A, Ewing P, Wilke A, Schubert T, Eissner G, Dietl B, Andreesen R, Cooke KR, Holler E. Induction of heme oxygenase-1 before conditioning results in improved survival and reduced graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 2004;10:461–472. doi: 10.1016/j.bbmt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Grzenkowicz-Wydra J, Cisowski J, Nakonieczna J, Zarebski A, Udilova N, Nohl H, Jozkowicz A, Podhajska A, Dulak J. Gene transfer of CuZn superoxide dismutase enhances the synthesis of vascular endothelial growth factor. Mol. Cell. Biochem. 2004;264:169–181. doi: 10.1023/b:mcbi.0000044386.45054.70. [DOI] [PubMed] [Google Scholar]
- 15.Jozkowicz A, Dulak J. Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim. Pol. 2003;50:69–79. [PubMed] [Google Scholar]
- 16.Jozkowicz A, Huk I, Nigisch A, Weigel G, Weidinger F, Dulak J. Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxid. Redox. Signal. 2002;4:577–585. doi: 10.1089/15230860260220076. [DOI] [PubMed] [Google Scholar]
- 17.Kaliman PA, Nikitchenko IV, Sokol OA, Strel'chenko EV. Regulation of heme oxygenase activity in rat liver during oxidative stress induced by cobalt chloride and mercury chloride. Biochemistry. 2001;66:77–82. doi: 10.1023/a:1002889814723. [DOI] [PubMed] [Google Scholar]
- 18.Kim HH, Lee SE, Chung WJ, Choi Y, Kwack K, Kim SW, Kim MS, Park H, Lee ZH. Stabilization of hypoxia-inducible factor-1alpha is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine. 2002;17:14–27. doi: 10.1006/cyto.2001.0985. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Weisz A, Ogura T, Hitomi Y, Kurashima Y, Hashimoto K, D'Acquisto F, Makuuchi M, Esumi H. Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J. Biol. Chem. 2001;276:2292–2298. doi: 10.1074/jbc.M008398200. [DOI] [PubMed] [Google Scholar]
- 20.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin. Sci. 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 21.Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, Abraham N, Dennery PA. Heme oxygenase-1 modulates fetal growth in the rat. Lab. Invest. 2002;82:687–692. doi: 10.1097/01.lab.0000017167.26718.f2. [DOI] [PubMed] [Google Scholar]
- 22.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J. Biol. Chem. 2002;277:10445–10451. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 24.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 26.Pae HO, Oh GS, Choi BM, Kim YM, Chung HT. A molecular cascade showing nitric oxide-heme oxygenase-1-vascular endothelial growth factor-interleukin-8 sequence in human endothelial cells. Endocrinology. 2005;146:2229–2238. doi: 10.1210/en.2004-1431. [DOI] [PubMed] [Google Scholar]
- 27.Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, Geehan CS, Luster AD, Sayegh MH, Briscoe DM. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J. Biol. Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Iso-o N, Takeshita S, Tsukamoto K, Mori I, Sato T, Ohno M, Nagai R, Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem. Biophys. Res. Commun. 2003;302:138–143. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 30.Taketani S, Kohno H, Yoshinaga T, Tokunaga R. Induction of heme oxygenase in rat hepatoma cells by exposure to heavy metals and hyperthermia. Biochem. Int. 1988;17:665–672. [PubMed] [Google Scholar]
- 31.Villarete LH, Remick DG. Nitric oxide regulation of IL-8 expression in human endothelial cells. Biochem. Biophys. Res. Commun. 1995;211:671–676. doi: 10.1006/bbrc.1995.1864. [DOI] [PubMed] [Google Scholar]
- 32.Wagner M, Cadetg P, Ruf R, Mazzucchelli L, Ferrari P, Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003;63:1564–1573. doi: 10.1046/j.1523-1755.2003.00897.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 34.Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, Berliner JA, Reddy ST. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001;21:1585–1591. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz A, Kliche S, Mayr-Beyrle U, Fellbrich G, Waltenberger J. p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem. Biophys. Res. Commun. 2003;306:730–736. doi: 10.1016/s0006-291x(03)01064-7. [DOI] [PubMed] [Google Scholar]
- 36.Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim. Pol. 2004;51:563–585. [PubMed] [Google Scholar]


