Abstract
Fifteen Enterobacter clinical isolates (11 Enterobacter cloacae isolates, 3 Enterobacter aerogenes isolates, and 1 Enterobacter gergoviae isolate), representing 0.4% of all Enterobacter isolates recovered in our hospital from 1989 to 2000, were suspected of harboring an extended-spectrum β-lactamase (ESBL). These isolates were recovered from 14 different patients. ESBLs were transferred by conjugation into an Escherichia coli recipient strain. Pulsed-field gel electrophoresis (PFGE) revealed a single clone of E. aerogenes and six different clones of E. cloacae. Four of these E. cloacae clonal types were represented by only one isolate each, but the other two were represented by three and four isolates, respectively. Isoelectric focusing, susceptibility phenotyping, PCR analysis, and sequencing demonstrated the presence of three different ESBLs. The most frequent was the recently characterized CTX-M-10 ESBL, which was found in the E. gergoviae isolate and in all but one of the E. cloacae isolates. The remaining E. cloacae isolate harbored a TEM-27 ESBL, and the three E. aerogenes isolates harbored a TEM-24 ESBL. PFGE revealed that our E. aerogenes strain was indistinguishable from the French TEM-24-producing E. aerogenes endemic clone. Although a low prevalence of ESBL-producing Enterobacter isolates was found in our institution over a 12-year period, a diversity of nonepidemic E. cloacae clones was detected, as was the persistence of the CTX-M-10 β-lactamase. The presence of the TEM-24-producing E. aerogenes French clone in our institution also demonstrates the intercountry dissemination of ESBL-producing isolates.
Plasmid-mediated extended-spectrum β-lactamases (ESBLs) were first reported in the mid-1980s (14, 15). Since then they have been responsible for several outbreaks caused by expanded-spectrum cephalosporin-resistant enterobacteria. Klebsiella pneumoniae and Escherichia coli are the most frequently involved organisms, and TEM-1- and SHV-1-derived β-lactamases are the most common ESBLs found in these species (25). These enzymes have also been reported at a much lower frequency among inducible AmpC β-lactamase-producing members of the family Enterobacteriaceae, such as Enterobacter spp., Citrobacter spp., Morganella morganii, and Serratia spp. (6, 10, 17, 23, 27, 28, 38). The most common ESBLs found in these species also belong to the TEM- and SHV-derived β-lactamases.
Enterobacter species have been found to be one of the most important causes of nosocomial infections during the last few years (9, 30). These organisms have been associated with several outbreaks generally involving derepressed mutants overproducing their chromosomal β-lactamase or, more infrequently, expressing ESBL (7, 10, 23, 30).
The aims of this study were to establish the epidemiological relationship among all ESBL-producing Enterobacter isolates recovered in our institution over a decade, as well as to characterize the ESBLs expressed in these isolates and the plasmids harboring ESBL genes. In addition, we investigated if our ESBL-producing Enterobacter aerogenes isolates were related to those found in large outbreaks in other European countries (7, 10, 19).
MATERIALS AND METHODS
Bacterial strains.
All Enterobacter clinical isolates which were suspected of harboring an ESBL on the basis of their resistance phenotypes and the results of the double-disk synergy test (DDST) (12, 16, 23, 37, 38) were studied. These isolates, 11 Enterobacter cloacae isolates, 3 E. aerogenes isolates, and 1 Enterobacter gergoviae isolate, were recovered from 14 different patients attending the Hospital Ramón y Cajal, a 1,200-bed teaching institution, between 1989 and 2000. Bacterial identification and initial antibiotic susceptibility testing were performed by using the semiautomatic PASCO system (Difco, Detroit, Mich.) or the semiautomatic WIDER system (Fco. Soria Melguizo, Madrid, Spain). For clonal comparison, three ESBL-producing E. aerogenes isolates belonging to an endemic clone widely spread throughout France and Belgium were included (7, 10).
DDST and antibiotic susceptibility testing.
DDST was performed with conventional amoxicillin-clavulanate, cefotaxime, ceftazidime, cefepime, and aztreonam disks that were applied 20 and 30 mm apart (12). The MICs of amoxicillin, ticarcillin, piperacillin, piperacillin-tazobactam, cefazolin, cefuroxime, cefotaxime, ceftazidime, cefepime, aztreonam, and imipenem were determined by agar dilution according to NCCLS guidelines (21). The respective manufacturers provided all antibiotics as powders. Determination of susceptibilities to aminoglycosides, quinolones, and trimethoprim-sulfamethoxazole was performed by using the semiautomatic PASCO system or the semiautomatic WIDER system.
Conjugation experiments and frequency of resistance transfer.
Mating experiments were performed with E. coli BM21 (nalidixic acid resistant, lactose fermentation positive, and plasmid free) (2) or a rifampin-resistant mutant of that strain (BM21R) as the recipient. Overnight cultures of recipient and donor strains grown on brain heart infusion (BHI) broth (Difco) at 37°C were inoculated at a 1:2 ratio (donor to recipient) into fresh BHI broth and were then incubated overnight at 37°C. Samples (0.1 ml) of this mixture were spread onto the surfaces of Mueller-Hinton agar plates with and without 64 μg of nalidixic acid per ml or 100 μg of rifampin per ml and 2 μg of cefotaxime per ml or 1 μg of ceftazidime per ml. Samples from donors and recipients were used as controls. Colonies growing on the selection plates were subjected to DDST to confirm the presence of ESBL transconjugants. The frequency of transfer was expressed relative to the number of donor cells.
IEF.
Bacteria exponentially growing at 37°C in Luria-Bertani medium were harvested, and cell-free lysates were prepared by sonication. Isoelectric focusing (IEF) was performed by applying the crude sonic extract to Phast gels (pH gradient, 3 to 9) in a PhastSystem apparatus (Pharmacia AB, Uppsala, Sweden) (11). β-Lactamases with known pIs (TEM-1, pI 5.4; TEM-4, pI 5.9; TEM-3, pI 6.3; TEM-24, pI 6.5, SHV-2, pI 7.6; SHV-4, pI 7.8; CTX-M-10, pI 8.1; and SHV-5, pI 8.2) were focused in parallel as controls. Gels were stained with 500 μg of nitrocefin (Oxoid, Basingstoke, United Kingdom) per ml to identify β-lactamase bands.
PCR amplification of ESBLs and sequencing.
ESBL amplification was performed with genomic DNA from wild-type isolates and plasmid DNA from the corresponding transconjugants (High Pure Plasmid Isolation kit; Roche Diagnostics GmbH, Mannheim, Germany) with the appropriate primers and cycling conditions for the TEM and SHV β-lactamases, as described previously (18, 29). For CTX-M-10, PCR amplification was performed by using the following primers: CTX-M-F8 (5′-CCGCGCTACACTTTGTGG C-3′) and CTX-M-R3 (5′-TTACAAACCGTTGGTGAC G-3′). Cycling conditions were as follows: 35 cycles of 94°C for 60 s, 56°C for 60 s, and 72 for 2 min, with a final period of extension at 72°C for 10 min. For CTX-M-9, PCR amplification was performed with primers 5′-GTGACAAAGAGAGTGCAA CGG-3′ and 5′-ATGATTCTCGCCGCTGAAGCC-3′ and cycling conditions of 35 cycles of 94°C for 45 s, 62°C for 45 s, and 72 for 45 s, with a final period of extension at 72°C for 10 min (33). The PCR products were separated in 0.8% agarose gels and visualized under UV light after staining with ethidium bromide. The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced on an ABI PRISM 377 automated sequencer (Applied Biosystems, Foster City, Calif.).
Pulsed-field gel electrophoresis (PFGE).
Bacterial DNA was prepared as described previously (13), and XbaI (Roche) was used as the restriction enzyme. Plugs were loaded onto a 1.2% agarose gel (Molecular Biology Certified Agarose; Bio-Rad, Hemel Hempstead, United Kingdom). DNA separation was performed in a CHEF-DRIII apparatus (Bio-Rad, La Jolla, Calif.) with the following conditions: 200 V for 26 h with pulse times of 1 to 15 s for 7 h and 15 to 35 s for 19 h. DNA macrorestriction patterns were visually compared and interpreted according to the criteria established by Tenover et al. (35).
Plasmid analysis.
Plasmid DNA from E. coli transconjugants corresponding to representative isolates of different E. cloacae clones was obtained as described previously (36). Plasmid sizes were estimated from standard curves of the logarithm of the molecular sizes of the plasmids from E. coli V517 (eight plasmids ranging from 2.1 to 54.2 kb) and E. coli NCTC 50192 (four plasmids of 7, 36.2, 63.8, and 148.5 kb, respectively) against the relative mobilities of the isolates tested. For plasmid fingerprinting, DNA was obtained with a QIAgen Plasmid Midi kit (Qiagen), digested with EcoRI, and subjected to electrophoresis in a 1% agarose gel at 100 V for 3 h.
RESULTS
A total of 15 Enterobacter isolates recovered from January 1989 to October 2000 in our hospital (0.4% of all Enterobacter isolates tested) were recognized as harboring an ESBL. The characteristics of the different Enterobacter isolates, including the sample origin and the date and units of isolation, are summarized in Table 1. The isolates were recovered from 14 patients: 4 (28.6%) from intensive care units, 4 (28.6%) from surgical wards, and 6 (42.8%) from medical wards. All Enterobacter isolates were positive by DDST, and resistance to expanded-spectrum cephalosporins was transferred into E. coli BM21 by conjugation at frequencies ranging from 10−5 to 10−8. IEF of sonic extracts of these E. coli tranconjugants revealed that all but one of the E. cloacae isolates and the E. gergoviae isolate transferred a pI 8.1 β-lactamase. There was no transfer of the following bands: a pI 7.6 β-lactamase band, probably related to an SHV-type enzyme; an unidentified pI 7.8 band; and the pI >8.5 band, most likely the AmpC protein. PCR amplification was positive for the CTX-M-10 ESBLs and negative for the CTX-M-9 ESBLs for all these isolates and the corresponding transconjugants harboring this pI 8.1 β-lactamase. Sequencing of the blaCTX-M PCR products from the E. gergoviae isolate and two representative E. cloacae isolates confirmed the Ala27Val and Arg38Gln substitutions within the sequence of the CTX-M-3 β-lactamase, which correspond to the recently described CTX-M-10 β-lactamase (24).
TABLE 1.
Characteristics of ESBL-producing Enterobacter isolates
| Isolate | PFGE type | β-Lactamase isoelectric focusinga | ESBL | Approx. plasmid sizeb | Isolation date | Unitc | Sample origind | Patient no. |
|---|---|---|---|---|---|---|---|---|
| E. cloacae RYC39737/89 | ECL1 | 5.9 + >8.5 | TEM-27 | 59 (4.0 × 10−7) | June 1989 | CP-ICU | Catheter | 1 |
| E. gergoviae RYC63027/91 | NDc | 5.4 + 7.6 + 8.1 | CTX-M-10 | 55 (2.0 × 10−6) | November 1991 | Pneumology | BAS | 2 |
| E. cloacae RYC95983/97 | ECL2 | 7.8 + 8.1 + >8.5 | CTX-M-10 | 61 (4.0 × 10−7) | October 1997 | Rheumatology | Urine | 3 |
| E. cloacae RYC40274/97 | ECL3 | 7.8 + 8.1 + >8.5 | CTX-M-10 | 51 (3.2 × 10−5) | December 1997 | CV-ICU | Blood | 4 |
| E. cloacae ECL105522/97 | ECL3 | 7.8 + 8.1 + >8.5 | CTX-M-10 | December 1997 | CV-ICU | BAS | 4 | |
| E. cloacae RYC91922/99 | ECL3 | 7.8 + 8.1 + >8.5 | CTX-M-10 | October 1999 | Internal medicine | Sputum | 5 | |
| E. cloacae RYC66281/00 | ECL3 | 7.8 + 8.1 + >8.5 | CTX-M-10 | April 2000 | Urology | Urine | 6 | |
| E. cloacae RYC43823/99 | ECL4 | 8.1 + >8.5 | CTX-M-10 | 44 (4.0 × 10−7) | January 1999 | Infectious diseases | Sputum | 7 |
| E. cloacae RYC71078/99 | ECL5 | 8.1 + >8.5 | CTX-M-10 | 90 (3.6 × 10−5) | June 1999 | Pneumology | BAS | 8 |
| E. cloacae RYC84009/89 | ECL6 | 8.1 + >8.5 | CTX-M-10 | 56 (2.2 × 10−6) | September 1999 | Traumatology | Wound | 9 |
| E. cloacae RYC47537/00 | ECL6 | 8.1 + >8.5 | CTX-M-10 | January 2000 | Traumatology | Urine | 10 | |
| E. cloacae RYC48677/00 | ECL6 | 8.1 + >8.5 | CTX-M-10 | January 2000 | Traumatology | Urine | 11 | |
| E. aerogenes RYC102643/98 | EA1 | 6.5 + 7.6 + >8.5 | TEM-24 | NDe (3.8 × 10−4) | November 1998 | Internal medicine-ICU | Catheter | 12 |
| E. aerogenes RYC91506/99 | EA1a | 6.5 + 7.6 + >8.5 | TEM-24 | October 1999 | CV-ICU | Wound | 13 | |
| E. aerogenes RYC102675/99 | EA1a | 6.5 + 7.6 + >8.5 | TEM-24 | December 1999 | Pneumology | BAS | 14 |
IEF from wild-type Enterobacter sp. sonic extract. The β-lactamases produced by transconjugants are underlined.
Values are in kilobases. Values in parentheses are conjugation frequencies.
CP-ICU, cardiopediatric intensive care unit; CV-ICU, cardiovascular surgery intensive care unit.
BAS, bronchial aspirate.
ND, not done.
Only one E. cloacae isolate transferred a pI 5.9 β-lactamase (Table 1). In this case, PCR was positive for the TEM β-lactamase and negative for the SHV, CTX-M-9, and CTX-M-10 β-lactamases. This strain was isolated from an intravascular catheter culture from which a Salmonella enterica isolate was also obtained (20). This S. enterica isolate was responsible for a nosocomial outbreak involving an epidemic plasmid encoding a TEM-27 ESBL (20). The TEM-type β-lactamase of our Enterobacter isolate had a pI identical to that of TEM-27 and conferred a pattern of resistance to extended-spectrum cephalosporins identical to that conferred by TEM-27. These results suggested the presence of the TEM-27 enzyme in this E. cloacae isolate, in addition to the AmpC β-lactamase.
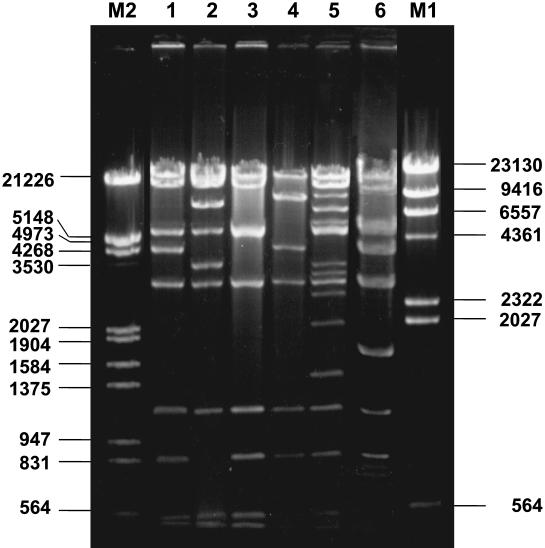
PFGE analysis revealed six different clonal types (Fig. 1) among our 11 E. cloacae isolates. Four of these clones were represented by a single isolate each (isolate RYC39737/89, clone ECL1; isolate RYC95983/97, clone ECL2; isolate RYC43823/99, clone ECL4; and isolate RYC71078/99, clone ECL5). The other two clones were represented by four (clone ECL3) and three (clone ECL6) isolates, respectively (Table 1). Clone ECL3 was detected in strains from three different patients with no apparent temporal or spatial relationships. In contrast, the three isolates of clone ECL6 were found in three patients hospitalized in the same ward within a few months, showing that an undetected limited outbreak of ESBL-producing E. cloacae had occurred in this unit.
FIG. 1.
PFGE of XbaI-digested genomic DNA of E. cloacae isolates. Lane M, bacteriophage lambda ladder PFGE marker (New England Biolabs); lanes 1 and 2, E. aerogenes RYC102675/99 and RYC91506/99 (pattern EA1a), respectively; lane 3, E. aerogenes RYC102643/98 (pattern EA1); lanes 4, 5, 12, and 13, E. cloacae RYC40274/97, RYC105522/97, RYC91922/99, and RYC66281/00, respectively (pattern ECL3); lanes 6, 7, and 8, E. cloacae RYC84009/99, RYC47537/00, and RYC48677/00, respectively (pattern ECL6); lane 9, E. cloacae RYC71078/99 (pattern ECL5); lane 10, E. cloacae RYC43823/99 (pattern ECL4); lane 11, E. cloacae RYC95983/97 (pattern ECL2); lane 14, E. cloacae RYC39737/89 (pattern ECL1). Numbers on the left are molecular sizes (in kilobases).
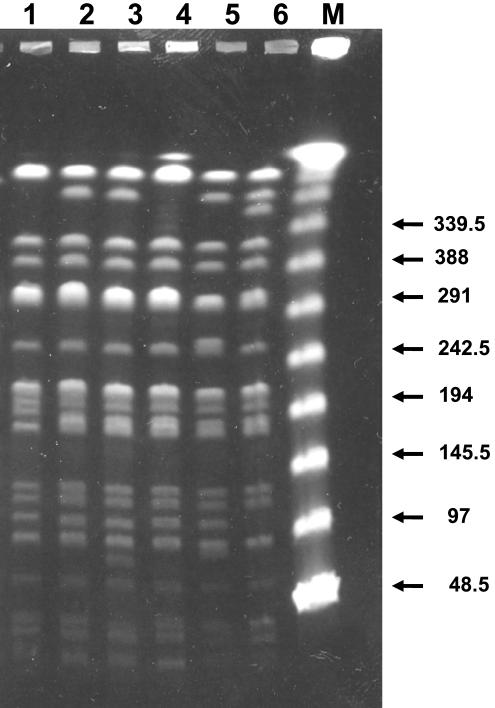
All transconjugants harboring the CTX-M-10 β-lactamase had only one plasmid (data not shown). The results of plasmid fingerprinting of six E. coli transconjugants representing all six Enterobacter clones harboring this β-lactamase are shown in Fig. 2. Estimated plasmid sizes ranged from 44 kb (clone ECL4) to 90 kb (clone ECL5). Although none of the plasmids were found to be identical, they showed bands in common. For instance, the band patterns for plasmids from the E. gergoviae transconjugant and from the transconjugant of clone ECL2 (Fig. 2, lanes 1 and 3, respectively) differed by only one band.
FIG. 2.
EcoRI restriction patterns of plasmids obtained from transconjugants of CTX-M-10-producing Enterobacter isolates. Lanes M1 and M2, DNA molecular size markers II and III, respectively (Roche Diagnostics); lane 1, R + E. gergoviae RYC63027; lane 2, R + E. cloacae RYC95983/97 (clone ECL2); lane 3, R + E. cloacae RYC40274/97 (clone ECL3); lane 4, R + E. cloacae RYC43823/99 (clone ECL4); lane 5, R + E. cloacae RYC71078/99 (clone ECL5); lane 6, R + E. cloacae RYC84009/99 (clone ECL6). Numbers on the left and the right are molecular sizes (in kilobases). R +, E. coli transconjugants of ESBL-producing Enterobacter isolates.
On the other hand, all three E. aerogenes isolates belonged to the same clone (clone EA1). They were isolated from different patients hospitalized in unrelated wards. The three E. aerogenes isolates were found to harbor a pI 6.5 β-lactamase that could be transferred into E. coli BM21 by conjugation (Table 1) and amplified with TEM-specific primers. On the contrary, the pI 7.6 band and the pI >8.5 band were not observed in the transconjugants. Sequencing of the blaTEM PCR product showed the Gln39Lys, Glu104Lys, Arg164Ser, Ala237Thr, and Glu240Lys substitutions which had previously been found in TEM-24 ESBL (4). It is worth noting that our E. aerogenes clone (clone EA1) was indistinguishable (Fig. 3) from the TEM-24-producing E. aerogenes endemic clone found in French hospitals (3, 10). This observation was confirmed by running in parallel our digested E. aerogenes genomic DNA with the genomic DNAs of the isolates described by Galdbart et al. (10). Isolate RYC102643/98 (clone EA1) was clonally related to French subtypes, whereas isolates RYC91506/99 and RYC102675/99 (clone EA1a) showed a PFGE profile identical to that of subtype 1c (Fig. 3).
FIG. 3.
Comparative PFGE of XbaI-digested genomic DNA of E. aerogenes isolates from this study (lanes 1, 2, and 3, respectively) and three isolates belonging to the French TEM-24-producing E. aerogenes endemic clone (9) (lanes 4, 5, and 6, respectively). Lane M, bacteriophage lambda ladder PFGE marker (New England Biolabs). Lane 1, E. aerogenes RYC102643/98 isolate (pattern EA1); lanes 2 and 3, E. aerogenes RYC102675/99 and RYC91506/99, respectively (pattern EA1a); lanes 4, 5, and 6, subtypes 1d, 1c, and 1f of the French E. aerogenes endemic clone, respectively. Numbers on the right are molecular sizes (in kilobases).
The MICs for all Enterobacter clinical isolates together with those for their E. coli transconjugants are shown in Table 2. The MICs of cefotaxime or cefepime were higher than those of ceftazidime for E. cloacae isolates and the corresponding transconjugants harboring the CTX-M-10 β-lactamase. On the contrary, the MIC of ceftazidime was higher than that of cefotaxime for the E. cloacae isolate harboring TEM-27 β-lactamase. All E. cloacae and E. aerogenes clinical isolates were susceptible to the combination of piperacillin-tazobactam, which denotes the nonhyperproduction of their AmpC β-lactamases. The E. gergoviae and E. cloacae isolates harboring the CTX-M-10 β-lactamase were susceptible to aminoglycosides, whereas the E. aerogenes and the E. cloacae isolates with the TEM-24 and the TEM-27 β-lactamase, respectively, showed aminoglycoside resistance. It is remarkable that the E. aerogenes isolates were highly resistant to tobramycin but susceptible to gentamicin, whereas the E. cloacae isolate harboring the TEM-27 ESBL was resistant to gentamicin, intermediate to tobramycin, and susceptible to amikacin. These phenotypes were also expressed in the corresponding E. coli transconjugants. All E. aerogenes isolates, four E. cloacae isolates, and the E. gergoviae isolate were resistant to nalidixic acid (Table 2). Moreover, only E. aerogenes isolates were resistant to co-trimoxazole.
TABLE 2.
β-Lactam MICs for ESBL-producing Enterobacter isolates and their corresponding transconjugants
| Antibiotic | MIC or MIC range (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| pI 5.9 (TEM-27)
|
pI 8.1 (CTX-M-10)
|
pI 8.1 (CTX-M-10)
|
pI 6.5 (TEM-24)
|
|||||
| E. cloacae (n = 1) | E. coli transconjugant (n = 1) | E. gergoviae (n = 1) | E. coli transconjugant (n = 1) | E. cloacae (n = 10) | E. coli transconjugant (n = 10) | E. aerogenes (n = 3) | E. coli transconjugant (n = 3) | |
| Amoxicillin | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 |
| Ticarcillin | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 |
| Piperacilin | >1,024 | >1,024 | 128 | 128 | 128-1,024 | 64-1,024 | 64-128 | 64-128 |
| Piperacillin-tazobactama | 4/4 | 2/4 | 4/4 | 2/4 | 0.5/4-4/4 | 1/4-4/4 | 8/4-32/4 | 4/4-32/4 |
| Cefazolin | >64 | 32 | >256 | >256 | >256 | >256 | >256 | >256 |
| Cefuroxime | 32 | 8 | 512 | 256 | >256 | >256 | 32-256 | 64-256 |
| Cefotaxime | 1 | 0.5 | 64 | 64 | 32-64 | 8-64 | 4-16 | 4-8 |
| Ceftazidime | 128 | 64 | 1 | 0.5 | 0.2-8 | 0.5-2 | 256-512 | 256 |
| Cefepime | 4 | 1 | 1 | 0.5 | 0.5-32 | 0.5-8 | 0.5-1 | 0.5-2 |
| Aztreonam | 128 | 32 | 4 | 4 | 0.5-32 | 0.5-8 | 16-64 | 16-32 |
| Imipenem | 0.2 | 0.1 | 0.2 | 0.2 | 0.1-0.5 | 0.1-0.2 | 0.1-0.2 | 0.1-0.2 |
| Gentamicin | >8 | >8 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| Tobramycin | >8 | 8 | ≤1 | ≤1 | ≤1 | ≤1 | >8 | >8 |
| Amikacin | ≤4 | 0.5 | ≤4 | ≤4 | ≤4 | ≤4 | 8-16 | 8-16 |
| Nalidixic acid | ≤4 | NDb | >16 | ND | ≤4->16 | ND | >16 | ND |
| Ciprofloxacin | ≤0.1 | ND | ≤0.1 | ND | ≤0.1-1 | ND | >4 | ND |
| Co-trimoxazole | ≤2/38 | ≤2/38 | ≤2/38 | ≤2/38 | ≤2/38 | ≤2/38 | >4/76 | >4/76 |
Tazobactam was used at a fixed concentration of 4 μg/ml.
ND, not done.
DISCUSSION
Resistance to expanded-spectrum cephalosporins in most Enterobacter species as well as in other natural AmpC β-lactamase-producing members of the family Enterobacteriaceae is mainly produced by a constitutive overexpression of their chromosomal β-lactamases (8). Plasmid-mediated ESBLs have also been described in these species and have been responsible for several outbreaks (6, 10, 19, 23). Although less common than AmpC hyperproduction, ESBLs among these species are a problem of great concern due to the potential transmission of resistance to other bacterial species and because ESBLs are usually encoded by plasmids that also harbor genes for resistance to non-β-lactam antibiotics such as aminoglycosides (23, 27).
The prevalence of ESBL-producing Enterobacter isolates in our institution (0.4% of all Enterobacter isolates) was similar to that of ESBL-producing E. coli isolates (0.3%) (unpublished data) but was lower than that of ESBL-producing K. pneumoniae isolates (4.8%) (5) or that of Enterobacter spp. with the constitutive phenotype of AmpC hyperproduction (close to 20%). The prevalence of our ESBL-producing Enterobacter spp. was similar to that observed previously (6, 32) but lower than that found by Tzelepi et al. (37). Given the difficulties of detection of ESBL among AmpC-producing members of the family Enterobacteriaceae (37), the real prevalence of ESBL-producing Enterobacter isolates might have been underestimated. The initial suspicion of ESBL in our isolates was mainly based on (i) decreased rates of resistance to piperacillin or ticarcillin in association with tazobactam or clavulanate in commercial microdilution panels; (ii) decreased susceptibility or resistance to ceftazidime and/or cefotaxime; and (iii) the results of DDST with cefepime and clavulanic acid, in which DDST was performed as recommended previously (10, 16, 37, 38).
Six different clonal types among the 11 ESBL-producing E. cloacae isolates were identified by PFGE. IEF and PCR results suggested that 10 of the 11 E. cloacae isolates harbored the same ESBL, which corresponds to the newly characterized CTX-M-10 enzyme in an E. coli isolate from our hospital (24). This β-lactamase has also been found in 40 and 10% of the ESBL-producing K. pneumoniae and E. coli clones recovered in different units from our hospital, respectively, suggesting that this β-lactamase is widespread in our institution (5). In fact, the sequence of the ESBL from the E. gergoviae isolate was found to be identical to that of CTX-M-10. Interestingly, the E. gergoviae isolate was recovered in 1991 (Table 1), showing that the ESBL from that isolate has been present in our institution for at least 10 years. As stated above, due to difficulties in detecting ESBLs among Enterobacter isolates, these organisms may act as a reservoir for transmission to other members of the family Enterobacteriaceae, including E. coli and K. pneumoniae. The presence of CTX-M-10 in different Enterobacter clones over this period was not apparently linked to a unique plasmid element (Fig. 2). However, as all of the plasmids showed bands in common, we can consider the possibility of a single plasmid undergoing an evolutionary process (1). Moreover, the maintenance of the CTX-M-10 enzyme can be related to the permanence in our environment of a common transferable element (such as an integron or a transposon) circulating among different plasmids. Sequencing of the flanked regions of blaCTX-M-10, which is in progress, seems to support this hypothesis (A. Oliver et al., unpublished data).
On the other hand, the spread of ESBL-producing strains among different countries has previously been suspected but has rarely been demonstrated (31, 34). It is remarkable that all three isolates of ESBL-producing E. aerogenes isolates belonged to the same clone (clone EA1), even though they were isolated from nonrelated patients admitted to different units. Interestingly, PFGE and β-lactamase sequencing revealed that our E. aerogenes clone harboring the TEM-24 ESBL was indistinguishable from the clone extensively found in French hospitals and more recently in Belgium (3, 6, 7, 10). Moreover, our isolates had an aminoglycoside resistance phenotype similar to that of the previously described French clone (10), suggesting the production of the same aminoglycoside-modifying enzyme, AAC(6′)-I. Although we cannot rule out the opposite possibility, all these results suggest the importation to our country of the E. aerogenes French clone harboring the TEM-24 ESBL rather than the new emergence of this enzyme in our institution.
From an evolutionary point of view, the acquisition of an ESBL as a mechanism of resistance to the actions of expanded-spectrum cephalosporins may be less frequently found among Enterobacter isolates than among E. coli and K. pneumoniae isolates. All Enterobacter species except E. gergoviae, Pantoea agglomerans (formerly Enterobacter agglomerans), and some Enterobacter sakazakii strains can rapidly adapt to such a challenge by chromosomal AmpC induction or derepression (26) without an ESBL. Interestingly, our first Enterobacter isolate with an ESBL was an E. gergoviae strain isolated in 1991, and a similar finding was reported in China (4). As AmpC-related resistance is not expected to occur in this species, ESBLs may constitute the main strategy of resistance to expanded-spectrum cephalosporins. It is known that hyperproduction of a chromosomal β-lactamase as a result of a mutation may be associated with a significant biological cost to the producer organism (22). If an ESBL plasmid is available in the microbial environment and its expression is less costly than the hyperproduction of the chromosomal enzyme, then there is a clear opportunity for acquisition of a plasmid carrying an ESBL. Such an event may provide extra benefits by allowing the acquisition of resistance to associated antibiotics. For this reason we cannot exclude the possibility that the emergence of ESBLs in E. aerogenes and E. cloacae may be due to the acquisition of a plasmid(s) encoding the ESBL under conditions of challenge with a non-beta-lactam antibiotic.
In conclusion, a low prevalence of ESBL-producing Enterobacter isolates was found in our institution over a 12-year period. Despite the low number of isolates, a diversity of clonal types was identified but the persistence of the same ESBL was detected, as the majority of isolates harbored the CTX-M-10 enzyme. It is worth noting the European intercountry dissemination of the E. aerogenes French clone harboring the TEM-24 enzyme to our hospital, which is in contrast to the scarce presence of other epidemic clones in our institution.
Acknowledgments
This work was supported by research grants from the Consejería de Educación y Cultura, Comunidad de Madrid (grant 08.2/0017/2000), from the Fondo de Investigaciones Sanilanas (grant F15 01/412), and from the Microbial Science Foundation, Madrid, Spain.
We thank Catherine Branger for supplying isolates corresponding to the TEM-24-producing E. aerogenes French clone.
REFERENCES
- 1.Arlet, G., M. Rouveau, I. Casin, P. J. M. Bouvet, P. H. Lagrange, and A. Philippon. 1994. Molecular epidemiology of Klebsiella pneumoniae strains that produce SHV-4 β-lactamase and which were isolated in 14 French hospitals. J. Clin. Microbiol. 32:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baquero, F., D. Bouanchaud, M. C. Martínez-Pírez, and C. Fernández. 1978. Microcin plasmids: a group of extrachromosomal elements coding for low molecular weight antibiotics in Escherichia coli. J. Bacteriol. 135:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosi, C., A. Davin-Regli, C. Bornet, M. Mallea, J. M. Pages, and C. Bollet. 1999. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J. Clin. Microbiol. 37:2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, Y., and M. Chan. 1994. Extended-spectrum beta-lactamases in clinical isolates of Enterobacter gergoviae and Escherichia coli in China. Antimicrob. Agents Chemother. 38:2838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coque, T. M., A. Oliver, J. C. Pérez-Díaz, F. Baquero, and R. Cantón. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Champs, C., D. Sirot, C. Chanal, R. Bonnet, A. J. Sirot, and The French Study Group. 2000. A 1998 survey of extended-spectrum- β-lactamases in Enterobacteriaceae in France. Antimicrob. Agents Chemother. 44:3177-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Gheldre, Y., M. J. Struelens, Y. Glupczynski, P. De Mol, N. Maes, C. Nonhoff, H. Chetoui, C. Sion, O. Ronveaux, M. Vaneechoutte, and le Groupement Pour Le Dépistage, L'Etude et la Prevencion de Infections Hospitalières (GDEPIH-GOSPIZ). 2001. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J. Clin. Microbiol. 39:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrhardt, A. F., and C. C. Sanders. 1993. Beta-lactam resistance amongst Enterobacter species. J. Antimicrob. Chemother. 32(Suppl. B):1-11. [DOI] [PubMed] [Google Scholar]
- 9.Fridkin, S. K., and R. P. Gaynes. 1999. Antimicrobial resistance in intensive care units. Clin. Chest Med. 20:303-316. [DOI] [PubMed] [Google Scholar]
- 10.Galdbart, J. O., F. Lémann, D. Ainouz, P. Féron, N. Lambert-Zechovsky, and C. Branger. 2000. TEM-24 extended-spectrum β-lactamase-producing Enterobacter aerogenes: long-term clonal dissemination in French hospitals. Clin. Microbiol. Infect. 6:316-323. [DOI] [PubMed] [Google Scholar]
- 11.Huovinen, S. 1988. Rapid isolectric focusing of plasmid-mediated β-lactamases with Pharmacia PhastSyst. Antimicrob. Agents Chemother. 32:1730-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann, M. E. 1998. Pulsed-field gel electrophoresis. Methods Mol. Med. 15:17-31. [DOI] [PubMed] [Google Scholar]
- 14.Kliebe, C., B. A. Nies, J. F. Meyer, R. M. Neutzling, and B. Wiedemann. 1985. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 28:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knothe, H., P. Shah, V. Kromery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 16.Livermore, D. M., T. G. Winstanley, and K. P. Shannon. 2001. Interpretive reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J. Antimicrob. Chemother. 48(Suppl. S1):87-102. [DOI] [PubMed] [Google Scholar]
- 17.Luzzaro, F., M. Perilli, R. Migliavacca, G. Lombardi, P. Micheletti, A. Agodi, S. Stefani, G. Amicosante, and L. Pagani. 1998. Repeated epidemics caused by extended-spectrum beta-lactamase-producing Serratia marcescens strains. Eur. J. Clin. Microbiol. Infect. Dis. 17:629-636. [DOI] [PubMed] [Google Scholar]
- 18.Mabilat, C., and S. Goussard. 1995. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-557. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 19.Mammeri, H., G. Laurans, M. Eveillard, S. Castelain, and F. Eb. 2001. Coexistence of SHV-4- and TEM-24-producing Enterobacter aerogenes strains before a large outbreak of TEM-24-producing strains in French hospitals. J. Clin. Microbiol. 39:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morosini, M. I., J. Blázquez, M. C. Negri, R. Cantón, E. Loza, and F. Baquero. 1996. Characterization of a nosocomial outbreak involving an epidemic plasmid encoding for TEM-27 in Salmonella enterica subspecies enterica serotype Othmarschen. J. Infect. Dis. 174:1015-1020. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Negri, M. C., and F. Baquero. 1998. In-vitro selective concentrations of cefepime and ceftazidime for ampC beta-lactamase hyperproducer Enterobacter cloacae variants. Clin. Microbiol. Infect. 4:585-588. [DOI] [PubMed] [Google Scholar]
- 23.Neuwirth, C., E. Siebor, J. López, A. Pechinot, and A. Kazmierczak. 1996. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum β-lactamase to other members of the family Enterobacteriaceae. J. Clin. Microbiol. 34:76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver, A., J. C. Pérez-Díaz, T. M. Coque, F. Baquero, and R. Cantón. 2001. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-10) isolated in Spain. Antimicrob. Agents Chemother. 45:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippon, A., G. Arlet, and P. H. Lagrange. 1994. Origin and impact of plasmid-mediated extended spectrum β-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 13(Suppl. 1):S17-S29. [DOI] [PubMed] [Google Scholar]
- 26.Pitout, J. D., E. S. Moland, C. C. Sanders, H. S. Thomson, and S. R. Fitzsimmons. 1997. β-Lactamases and detection of β-lactam resistance in Enterobacter spp. Antimicrob. Agents Chemother. 41:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitout, J. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, P. Coudron, and C. C. Sanders. 1998. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter strains. Antimicrob. Agents Chemother. 42:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power, P., M. Radice, C. Barberis, C. de Mier, M. Mollerach, M. Maltagliatti, C. Vay, A. Famiglietti, and G. Gutkind. 1999. Cefotaxime-hydrolysing β-lactamases in Morganella morganii. Eur. J. Clin. Microbiol. Infect. Dis. 18:743-747. [DOI] [PubMed] [Google Scholar]
- 29.Rasheed, J. K., C. Jay, B. Metchock, F. Berkowitz, L. Weigel, J. Crellin, C. Steward, B. Hill, A. A. Medeiros, and F. C. Tenover. 1997. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob. Agents Chemother. 41:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders, W. E., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon, K. P., A. King, I. Phillips, M. H. Nicolas, and A. Philippon. 1990. Importation of organisms producing broad-spectrum SHV-group β-lactamases into the United Kingdom. J. Clin. Microbiol. 25:343-351. [DOI] [PubMed] [Google Scholar]
- 32.Silva, J., C. Aguilar, Z. Becerra, F. López-Antunano, and R. García. 1999. Extended-spectrum beta-lactamases in clinical isolates of enterobacteria in Mexico. Microb. Drug Resist. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 33.Simarro, E., F. Navarro, J. Ruíz, E. Miró, J. Gómez, and B. Mirelis. 2000. Salmonella enterica serovar Virchow with CTX-M-like β-lactamase in Spain. J. Clin. Microbiol. 38:4676-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tassios, P. T., M. Gazouli, E. Tzelepi, H. Milch, N. Kozlova, S. Sidorenko, N. J. Legakis, and L. S. Tzouvelekis. 1999. Spread of a Salmonella typhimurium clone resistant to expanded-spectrum cephalosporins in three European countries. J. Clin. Microbiol. 37:3774-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Threlfall, E. J., and N. Woodford. 1995. Plasmid profile typing and plasmid fingerprinting. Methods Mol. Biol. 46:225-236. [DOI] [PubMed] [Google Scholar]
- 37.Tzelepi, E., P. Giakkoupi, D. Sofianou, V. Loukova, A. Kemeroglou, and A. Tsakris. 2000. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varela, C., A. Oliver, T. M. Coque, F. Baquero, and R. Cantón. 2001. Prevalence of extended-spectrum β-lactamases in group-1 β-lactamase-producing isolates. Clin. Microbiol. Infect. 7:278-282. [DOI] [PubMed] [Google Scholar]