Abstract
Objective
Anaplastic thyroid carcinoma (ATC) remains one of the most lethal known human cancers. Targeted molecular therapy with cetuximab, a monoclonal antibody against EGFR, offers new treatment potentials for patient with ATC. Cetuximab has also been reported to have synergistic effects when combined with irinotecan, a topoisomerase inhibitor. Therefore, we hypothesized that cetuximab and irinotecan would be effective in inhibiting the growth and progression of ATC in a murine orthotopic model.
Design
The in vitro anti-proliferative effects of cetuximab and irinotecan on ATC cell line ARO were examined. We also studied the in vivo effects of cetuximab and irinotecan on the growth, invasion, and metastasis of orthotopic ATC tumors in nude mice. The in vivo antitumor efficacy of cetuximab/irinotecan combination was also compared with that of doxorubicin.
Results
Cetuximab alone did not show any anti-proliferative or pro-apoptotic effect on this cell line. However, when combined with irinotecan, cetuximab potentiated the in vitro anti-proliferative and pro-apoptotic effect of irinotecan. Cetuximab, irinotecan, and cetuximab/irinotecan combination resulted in 77%, 79%, and 93% in vivo inhibition of tumor growth, respectively. Incidences of lymph node metastasis, laryngeal invasion, and tumor microvessel density were also significantly decreased in these treatment groups. Furthermore, the cetuximab/irinotecan combination was significantly more effective than doxorubicin in inhibiting the growth of orthotopic ATC xenografts.
Conclusions
Combination therapy with cetuximab/irinotecan inhibits the growth and progression of orthotopic ATC xenografts in nude mice. Given the lack of curative options for patients with ATC, combination therapy with cetuximab and irinotecan treatment warrants further study.
INTRODUCTION
Carcinomas of the thyroid gland account for approximately 1% of all new malignant diseases in the U.S (1). Relatively high cure rates can be achieved in well-differentiated thyroid carcinomas such as papillary and follicular thyroid carcinomas. However, anaplastic thyroid carcinoma (ATC) is one of the most aggressive human malignancies known and carries a grave prognosis. Although ATC accounts for only 1.6% of all thyroid cancers, the median overall survival following diagnosis is only 6 months (2,3).
The treatment of ATC is often multidisciplinary and frequently includes the use of doxorubicin for which single agent response rates range from 5% to 20% (4,5). Regardless, it is clear that no effective treatments exist for ATC. This may be due in part to the rarity of this disease but nevertheless reflects the inadequacy of the available treatment options and suggests an urgent need for development of novel treatment strategies.
It is well established that the Epidermal Growth Factor Receptor (EGFR) is a valid and promising therapeutic target in solid tumors that overexpress this receptor. In particular, multiple preclinical and clinical studies have demonstrated the therapeutic efficacy of EGFR inhibition in lung, head and neck, and colorectal carcinoma (6,7). Inhibition of the EGFR pathway as a therapeutic modality for ATC is a novel approach that has yet to be fully investigated. Ensinger et al examined the expression of EGFR in the largest collection of ATC specimens to date (25 specimens) and found this receptor to be overexpressed in 40% of the specimens (8). ATC cell lines derived from human tumors have also been shown to express variable levels of EGFR (9). Inhibition of EGFR pathway using monoclonal antibodies and small molecule inhibitors has demonstrated anti-proliferative effects on ATC cell lines in vitro (9,10). Schiff et al were the first to report the in vivo effects of EGFR inhibition on ATC xenografts in nude mice (11). In this study, the administration of gefinitib (Iressa), a small molecule inhibitor of the EGFR tyrosine kinase, to nude mice bearing subcutaneous ATC xenografts resulted in significant inhibition of tumor growth. Kim et al also demonstrated that AEE788, a dual inhibitor of EGFR and VEGFR tyrosine kinases, produced significant cytostatic and cytotoxic effects on ATC cell lines in vitro and also inhibited the growth of subcutaneous ATC xenografts in nude mice (12).
Cetuximab (Erbitux, C225), a human-murine chimeric monoclonal antibody to EGFR, has been extensively studied in numerous preclinical and clinical studies (13,14). Preclinical studies have shown that cetuximab is able to inhibit the growth of colon, head and neck, and pancreatic carcinoma xenografts in nude mice (15–17). More importantly, a randomized, phase III clinical trial showed that cetuximab prolongs the survival of patients with untreated head and neck cancers in combination with radiotherapy as compared to treatment with radiotherapy alone (18).
Furthermore, multiple studies have demonstrated synergism between the molecular inhibition of EGFR and DNA damaging agents such as radiation or the camptothecin class of chemotherapeutic agents such as topotecan or irinotecan (19,20). In particular, cetuximab has been approved by the FDA for use with irinotecan (Camptosar, CPT-11) in patients with irinotecan-refractory colorectal carcinoma (21). Irinotecan, an inhibitor of topoisomerase I, acts by preventing the relaxation of DNA supercoiling during DNA replication (22). This FDA approval of combination therapy with cetuximab and irinotecan was based on a randomized clinical trial which showed that patients receiving the combination therapy showed higher response rate and significantly longer time to recurrence (21). Despite encouraging data such as these, there are no studies in the literature that have examined the effects of cetuximab or irinotecan, either as single agent therapy or combined therapy, against ATC. The aim of the present study was to investigate the therapeutic potentials of cetuximab and irinotecan against ATC using an orthotopic model of ATC in nude mice. We show that the co-administration of cetuximab and irinotecan significantly inhibited the growth, invasion, metastasis, and the angiogenesis of orthotopic ATC xenografts in nude mice.
MATERIALS AND METHODS
Reagents
For in vivo administration, irinotecan (Pharmacia and Upjohn Co., Kalamazoo, MI) was diluted in phosphate-buffered saline (PBS) to concentration of 5 mg/mL. Cetuximab (Imclone, New York, NY) was administered undiluted at concentration of 2 mg/mL. For in vitro experiments, both agents were diluted in tissue-culture medium to the appropriate concentration. Propidium iodide (PI) and Tetrazolium (MTT) were both purchased from Sigma-Aldrich Corp (St. Louis, MO).
Animals
Male athymic nude mice, age 8 to 12 weeks, were purchased from the animal production area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the National Institutes of Health. The mice were used in accordance with the Animal Care and Use Guidelines of The University of Texas M.D. Anderson Cancer Center (Houston, TX) under a protocol approved by the Institutional Animal Care Use Committee.
Cell Lines and Culture Conditions
ATC cell line ARO was used. This cell line was obtained from Sai-Ching Yeung, M.D., Ph.D., Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas M.D. Anderson Cancer Center. The ARO cell line has been demonstrated previously to express EGFR and to secrete EGF (11). The cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin, sodium pyruvate, and non-essential amino acids. Adherent monolayer cultures were maintained on plastic and incubated at 37 °C in 5% carbon dioxide and 95% air. The cultures were free of Mycoplasma species.
Measurement of Cell Proliferation
ARO cell line was plated at two thousand cells per well in 96-well tissue culture plates. After 24 hours, the cells were treated with cetuximab (0.5 g/mL) in RPMI1640 medium supplemented with 2% FBS for 72 hours. To determine the effects of combined treatment with irinotecan and cetuximab on the proliferation of ATC cell lines, ARO cell line was plated as described above. After 24 hours, the cells were treated with various concentrations of irinotecan (0 – 6 M) with or without 0.5 g/mL of cetuximab for 72 hours. To measure the number of metabolically active cells after a 3-day incubation period, we used an MTT assay as measured by a 96-well microtiter plate reader (MR-5000; Dynatech Laboratories Inc, Chantilly, VA) at an optical density of 570 nm. These experiments were performed at least in triplicates.
Measurement of Cell Death
To measure cell death, ARO cells were plated at a density of 2×1052 cells/well in 38-mm six-well plates (Costar, Cambridge, MA) and maintained for 24 hours before treatment with irinotecan. After 24 hours, the cells were treated with cetuximab (1 g/mL) in RPMI1640 medium supplemented with 2% FBS. After 48 hours of treatment with cetuximab, the extent of cell death was determined by PI staining of hypodiploid DNA. To determine the effects of combined treatment with irinotecan and cetuximab on the apoptosis of ATC cell lines, ARO cells were plated as described above. It has been shown previously that the cytotoxic effects of cetuximab and topotecan, another member of the camptothecin agents, can be maximized by the sequential treatment of the tumor cell lines first with topotecan followed by cetuximab (15). Therefore, 24 hours after plating, the cells were first treated with various concentrations of irinotecan (0 – 10 M) for 24 hours in RPMI1640 medium with 2% FBS. After 24 hours, cetuximab was then added to the media to a final concentration of 1 g/mL. The cells were then incubated with both irinotecan and cetuximab for another 48 hours. After the 72-hour period, the apoptotic fraction was determined by PI staining of hypodiploid DNA. For PI staining, the treated cells were resuspended in a Nicoletti buffer (50mg/mL PI, 0.1% sodium citrate, 0.1% Triton X-100) for 20 min at 4 °C. Cells were then analyzed by flow cytometry, and the sub-G0/G1 fraction was measured. These experiments were performed at least in triplicates.
Effects of Cetuximab and Irinotecan on the Growth of Orthotopic ATC Xenografts in Nude Mice
Orthotopic xenografts in nude mice were established as described previously (23). Briefly, ARO cells were harvested from subconfluent cultures by trypsinization and washed. 5 ×105 ARO cells in a volume of 5 L were injected into the right thyroid lobe of each mouse. The tumors were allowed to develop during the following 4 days. The mice were then randomized into four groups (14 mice in the control group and 10 mice in each of the treatment group), and the drugs were administered as follows: 1) cetuximab via intraperitoneal (IP) injection, 1mg/injection, twice per week, 2) irinotecan IP injection at 50 mg/kg, once per week, 3) both cetuximab, 1mg/injection, IP, twice per week and irinotecan, 50 mg/kg, IP, once per week, or 4) 500 L of PBS administered intraperitoneally once/week as placebo.
The mice were treated for 4 weeks and the weighed twice per week. Our animal protocol required that the animals be killed if they lost >20% of body weight or if they became moribund. However, none met the criteria for sacrifice before the end of the treatment period. At the end of the 4-week treatment period, the mice were killed by CO2 asphyxiation and necropsy was performed. The cervical lymph nodes, the lungs, and the thyroid tumors were removed. At the time of the necropsy, the tumor sizes were measured in all three dimensions. The volumes of the tumors were determined using the formula V=4/3(π)XYZ, where “X”, “Y”, and “Z” represent the radius of the tumors in each dimension. Percentage of tumor inhibition was calculated according to the formula [1−(T/C)] × 100 where T and C represent the mean tumor volumes of the treatment group and the control group, respectively.
Effects of Cetuximab and Irinotecan on the Survival of Nude Mice Bearing Orthotopic ATC Xenografts
Orthotopic ATC xenografts were established in nude mice as described above. Four day after the tumor cell injection, the mice were randomized into four groups (10 mice in each group) and treated with placebo, cetuximab, irinotecan, or both agents as per the schedules described in the previous section. The mice were weighed twice per week and killed if the animals showed weight loss of >20% or appeared moribund. The mice were treated for 58 days.
Comparison of the Anti-tumor Effects of Cetuximab/Irinotecan Combination with Doxorubicin
Orthotopic ATC xenografts were established in nude mice as described above. Four day after the tumor cell injection, the mice were randomized into three groups (10 mice in each group): control, doxorubicin, and cetuximab/irinotecan combination group. The control mice were given 500 L of PBS via IP injection once per week. Doxorubicin was administered by IP injection at a dose of 5 mg/kg, once every four days. The cetuximab/irinotecan combination group received cetuximab and irinotecan per the schedule described in the previous section. The mice were treated for three weeks and weighed twice a week. At the end of the three-week period, necropsy was performed and the tumor sizes were measured in all three dimensions. The tumor volumes were then calculated as described above.
Immunohistochemical Analysis
For staining with rabbit anti-mouse CD31 (PharMingen, San Diego, CA), rat anti-mouse CD204 (Serotec, Raleigh, NC), and rat anti-mouse F4/80 antibodies (Serotec), frozen tumors were sectioned and mounted on positively charged Superfrost slides (Fisher Scientific, Houston, TX), air dried for 30 min, and fixed in cold acetone for 10 min. Endogenous blocking was performed with 3% hydrogen peroxide followed by protein blocking using 5% horse serum with 1% goat serum (protein-blocking solution). The slides were incubated with primary antibody (1:800, 1:200, and 1:100 dilutions for anti-CD31, F4/80, and CD204 antibodies, respectively) for 18 hours at 4°C. The samples were then washed and blocked with protein-blocking solution for ten minutes and incubated with appropriate secondary antibodies conjugated to horseradish peroxidase. Positive staining was visualized using DAB chromogen and counterstained with Hoechst stain.
To image the DAB stained sections, we utilized a Microphot-FX microscope (Nikon, Melville, NY) equipped with a three-chip-charged couple device (CCD) color video camera (Model DXC990; Sony Corp, Tokyo, Japan). To quantify microvessel density (MVD), the CD31-labeled endothelial cells were counted from four random 0.159 mm2 fields (100x magnification) per slide from total of five slides per study group. In order to quantify F4/80 and CD204 staining, computer-assisted image analysis was performed using Image Pro Plus software (Media Cybernetics, Silver Spring, MD). The image analysis was performed on three to four random 0.159 mm2 fields (100X magnification) per slide from total of five slides per group. The photomontages were prepared using Photoshop software (Adobe Systems Inc., San Jose, CA).
Statistical Analysis
To assess synergy between irinotecan and cetuximab we first define synergy as follows: a combination treatment is said to be synergistic if the combined treatment results in more tumor inhibition than the tumor inhibition associated with the individual treatments alone. If we define e to be the mean tumor inhibition for the cetuximab arm, define i to be the mean tumor inhibition for the irinotecan arm, and define the mean tumor inhibition in the combination arm as c then we would declare the combination treatment to be synergistic if min(e, i) > c. We used a Bayesian modeling approach and calculated the posterior probability Pr(min(e, i) > c |data) (i.e., the posterior probability that the minimum of the two posterior mean tumor sizes for irinotecan alone, i, or cetuximab alone, e, was greater than the mean posterior tumor size for the combination c). For this Bayesian analyses we assumed that the data followed a normal distribution with each treatment group having its own mean and variance. Furthermore, we gave the mean parameters non-informative, half-normal prior distributions (i.e., ~ N(0.0, 1E10)I(0,∞)). Bayesian methods, unlike classical methods, express uncertainty about treatment effects in terms of probability. Thus in this report, we summarized our uncertainty about effects using posterior probabilities (i.e., the probability of synergy given the observed data). Posterior probabilities greater than 0.975 (analogous to a one-sided P value of 0.025 within a classical framework) were considered statistically meaningful to demonstrate synergy.
The average tumor volumes of the control and treatment groups as well as tumor MVD, F4/80, and CD204 staining intensities were compared using the independent sample t-test. The incidences of cervical metastases were compared using chi-square test. The survival data was analyzed by Kaplan-Meier methods and the survival periods compared by the log-rank test.
RESULTS
Cetuximab Enhances the In Vitro Anti-proliferative and Pro-apoptotic Effects of Irinotecan on ATC Cell Line ARO
Treatment of ARO cells with 0.5 g/mL of cetuximab alone for 72 hours did not result in anti-proliferative effects. (Fig. 1A). However, when combined with irinotecan, cetuximab enhanced the anti-proliferative effects of irinotecan (Fig. 1B). The IC50 for the anti-proliferative effect were 5.5 M and 4 M in the absence and presence of cetuximab, respectively. The magnitudes of growth inhibition at the IC50 were approximately 18% and 30%. Likewise, treatment of ARO cells with 1 g/mL of cetuximab did not result in the induction of apoptosis (Fig. 1C). The addition of cetuximab at this concentration to irinotecan, however, increased the pro-apoptotic effect of irinotecan and decreased the IC50 for apoptosis from 3 M to 1.8 M. The overall apoptosis was increased by approximately 30% (Fig. 1D). The in vitro IC50 for antiproliferative and proapoptotic effects of irinotecan was within the plasma concentration achievable in human patients (3–5 M) when given intravenously at dose of 350 mg/mm2 (24,25). Although we used 2% FBS in our media to minimized the effect of exogenous EGF, it may be argued that there are still sufficient EGF or other growth factors in 2% FBS to overcome the effect of the cetuximab. Despite this concern, we elected to use 2% FBS as 0% FBS departs very significantly from the physiologic conditions of human serum. This consideration should be noted in the interpretation of our data.
Fig. 1.
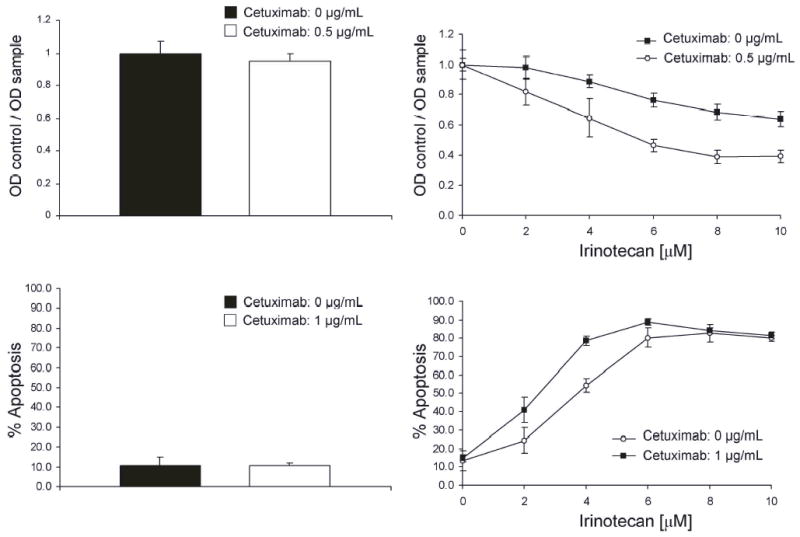
A, Cetuximab does not inhibit the proliferation of ATC cell line ARO. ARO cells were incubated with 0.5 g/mL of cetuximab for 72 hours. The degree of proliferation was then measured with an MTT assay. B, Cetuximab enhances the anti-proliferative effects of irinotecan. ARO cells were incubated with various concentrations of irinotecan with and without 0.5 g/mL of cetuximab for 72 hours. The degree of proliferation was then measured with an MTT assay. C, Cetuximab does not induce the apoptosis of ATC cell line ARO. ARO cells were incubated with 1 g/mL of cetuximab for 48 hours. The degree of apoptosis was then measured using a flow-cytometry based assay on Propidium Iodide stained cells. D, Cetuximab enhances the proapoptotic effects of irinotecan. The ARO cells were treated with various concentrations of irinotecan for 24 hours. Cetuximab was then added to a final concentration of 1 g/mL and the cells were incubated in the presence of both cetuximab and irinotecan for additional 48 hours.
Cetuximab and Irinotecan Inhibit the Growth of Orthotopic ATC Xenografts in Nude Mice
After having demonstrated in vitro activity of cetuximab and irinotecan on ATC cell line ARO, we examined the in vivo activity of the two agents. Both cetuximab and irinotecan produced significant growth inhibition of orthotopic ATC xenografts when used as single-agent therapy. However, the greatest growth inhibition was achieved by the co-administration of cetuximab and irinotecan (Fig. 2A–E). At the end of the 4-week treatment period, cetuximab, irinotecan, and the two agents together showed approximately 77%, 79%, and 93% decreases, respectively, in mean tumor volume compared with tumors from the placebo treated group. All treatment groups produced statistically significant differences in mean tumor volume when compared with the control group (P < 0.05). The differences in mean tumor volume between each single-agent treatment group and the combination treatment group were also statistically significant (P < 0.05).
Fig. 2.
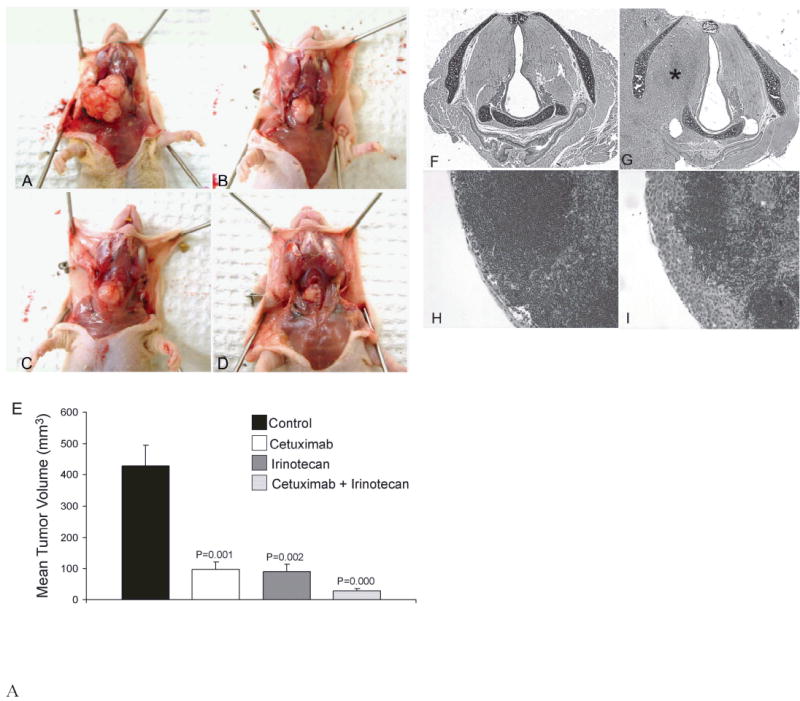
Mice bearing orthotopic ATC xenografts were treated with cetuximab, irinotecan, or both cetuximab and irinotecan as outlined in the Materials and Method section for 4 weeks. Photographs of representative tumors from A, control group, B, cetuximab group, C, irinotecan group, and D, combination treatment group at the end of the treatment period. E, Mean tumor volumes for each group (+ SEM) at the end of the treatment period. F, Normal larynx from the combination treatment group, G, Larynx from control group showing ATC tumor (*) invading the right paraglottic space through the inferior constrictor muscle, H, Normal cervical lymph node, I, Cervical lymph node with subcapsular metastasis (F–I; H+E stain, original magnification X100).
The degree of tumor growth inhibition by the single-agent treatment groups was compared to that of the combination therapy group to assess for possible synergism between cetuximab and irinotecan. For this purpose, we used a Bayesian analysis where synergism between cetuximab and irinotecan was established if the variable Pr(min(e, i) > c |data) (as defined in the Materials and Methods Section) was greater than 0.975. Our analysis showed Pr(min(e, i) > c |data) to be 0.981, or that there were 98.1% chance that the minimum of the two posterior mean tumor sizes for the single agent treatment groups (cetuximab and irinotecan) is greater than the posterior mean tumor size for the combination treatment group. Based on this analysis, we conclude that the co-administration of cetuximab and irinotecan produced synergy in the inhibition of orthotopic ATC tumor growth.
The incidence of laryngeal invasion was greater than 90% in the control group compared to 30%, 50%, and 11% in the cetuximab, irinotecan, and combination treatment group, respectively (P < 0.05) (Table 1). The incidences of laryngeal invasion between the single agent and the combination treatment groups were not statistically significant (P>0.05). The preferential route of laryngeal invasion was through the inferior constrictor musculature posterior to the thyroid cartilage (Fig. 2F,G). Cetuximab and irinotecan also significantly decreased the incidence of lymph node metastasis (Fig 2H–I, Table 1). The incidences of lymph node metastasis in the cetuximab, irinotecan, and combination treatment groups were 10%, 20%, and 10%, respectively, compared to 64% in the control group (P < 0.05). The incidences of lymph node metastasis between the single agent and the combination treatment groups were not statistically significant (P>0.05). None of the mice showed pulmonary metastasis on histological examination of the lungs. Lastly, cetuximab and irinotecan were well tolerated by the animals without significant weight loss and none of the animals required sacrifice prior to the end of the study (data not shown).
Table 1.
Effect of cetuximab and irinotecan on the incidence of lymph node metastasis and laryngeal invasion in nude mice bearing orthotopic ATC xenografts.
| Treatment groups | Incidence of cervical LN metastasis | P values a | Incidence of laryngeal invasion | P values a |
|---|---|---|---|---|
| Control | 9/14 (64%) | 12/13 (92%) b | ||
| Cetuximab | 1/10 (10%) | 0.011 | 3/10 (30%) | 0.003 |
| Irinotecan | 2/10 (20%) | 0.040 | 5/10 (50%) | 0.035 |
| Cetuximab | 1/10 (10%) | 0.011 | 1/9 (11%) b | 0.000 |
Irinotecan
Independent sample t-test
One tissue specimen each from the control group and cetuximab+irinotecan group could not be accurately evaluated for laryngeal invasion due to partial loss of tissue during processing
Cetuximab Decreases the Tumor Microvessel Density and Induces Tumor Infiltration by Macrophages
Although cetuximab alone did not affect the proliferation or the apoptosis of ATC cell lines in vitro, the administration of cetuximab to tumor bearing mice resulted in greater than 70% tumor inhibition. It has been shown in several tumor models that this discrepancy between the in vitro and in vivo effects of cetuximab is due to its ability to inhibit tumor angiogenesis (26). In order to determine the effects of cetuximab and irinotecan on tumor angiogenesis, CD31 staining was used to quantify the degree of tumor microvessel density. Treatment with cetuximab, irinotecan, and cetuximab/irinotecan combination produced 28%, 15%, and 39% decrease in the mean tumor microvessel density compared to the control group (Fig. 3A, B). The decreases in MVD of all three treatment-groups were statistically significant compared with the control group.
Fig. 3.
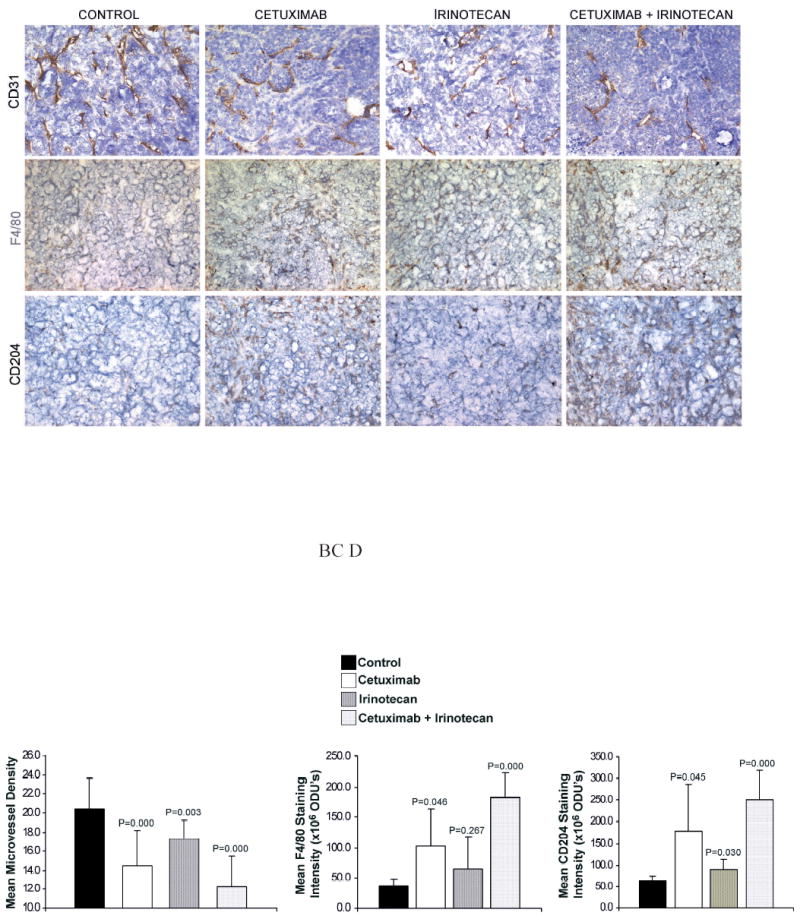
Immunohistochemical Analysis. After 4-week treatment with cetuximab, irinotecan, or both agents, the ARO tumor sections were immunostained for CD31 (to quantify the MVD) as well as F4/80 and CD204 (to assess tumoral macrophage infiltration). A, Representative staining from each group (original magnification X100). Quantification of B, CD31, C, F4/80, and D, CD204 staining intensity. The unit for staining intensity is Optical Density Units (ODU’s). The values are listed as Mean Staining Intensity + SD.
It has also been postulated that therapeutic monoclonal antibodies directed against transmembrane receptors can elicit host-immune responses such as antibody dependent cell cytotoxicity (ADCC) (27). In order to assess for evidence of host-response to the tumor xenografts, immunohistochemical staining was performed against murine macrophage markers F4/80 and CD204. The mean staining intensities of both F4/80 and CD204 were increased by approximately 2-fold and 4-fold in the tumors from the cetuximab and combination treatment groups, respectively (Fig. 3A, C, D). These increases in tumoral macrophage infiltration were statistically significant (P < 0.05) for both F4/80 and CD204.
Comparison of Doxorubicin to Cetuximab/Irinotecan Combination Therapy in Athymic Nude Mice Bearing Orthotopic ATC xenografts
Although no single chemotherapeutic agent has shown significant activity against ATC, the agent used most often in the setting of multimodality therapy include the taxanes and doxorubicin (28,29). In order to compare the tumor inhibitory effects of doxorubicin to that of the combined therapy with cetuximab and irinotecan, nude mice with orthotopically established ATC xenografts were treated with placebo, doxorubicin, or combination of cetuximab and irinotecan. At the end of the three-week treatment period, the mean tumor volume in the doxorubicin group was decreased by only 7.5% compared to the control group (P = 0.739) (Fig. 4A–D). In contrast, the co-administration of cetuximab and irinotecan decreased the mean tumor volume of ATC xenografts by approximately 97% (P = 0.000).
Fig. 4.
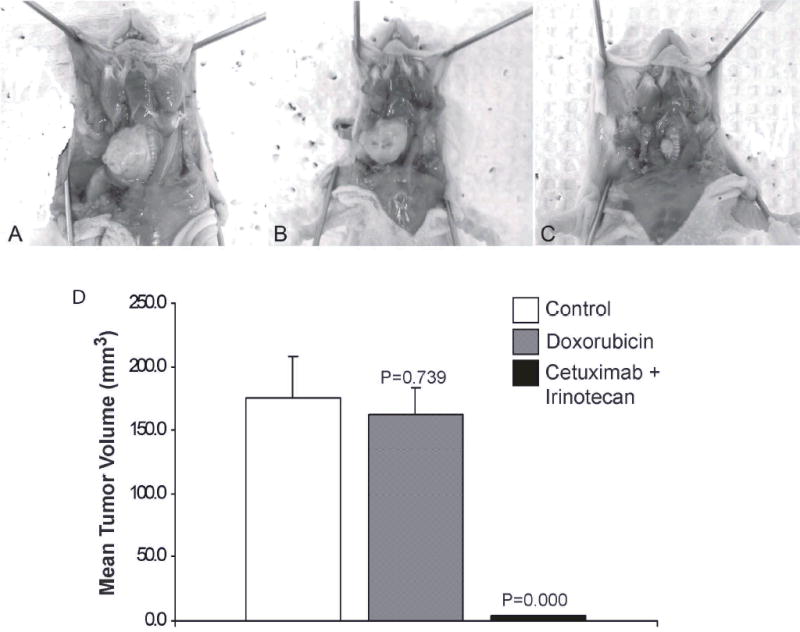
Comparison of the anti-tumor effects of doxorubicin and cetuximab/irinotecan combination therapy on the growth of orthotopic ATC xenografts in nude mice. Photographs of representative tumors from A, control group, B, doxorubicin group, C, cetuximab/irinotecan group at the end of the treatment period. D, Mean tumor volumes (+ SEM) for each group at the end of the treatment period.
Despite its relative ineffectiveness in the inhibition of tumor growth, doxorubicin showed significant toxicity in the test animals as manifested by progressive loss of weight (data no shown). The weight loss exhibited by the doxorubicin group is unlikely to be due to the growth of thyroid tumors as this effect typically occurs after 3 to 4 weeks into the treatment period.
Cetuximab and Irinotecan Improve the Survival of Athymic Nude Mice Bearing Orthotopic ATC xenografts
In the absence of any treatment, all of the control mice in the survival study succumbed to the thyroid tumors by day 50 due to the obstruction of the upper aerodigestive tract. In contrast, all of the mice in the combination treatment group were alive at the end of the survival study (Fig. 5). The median survival period for control, cetuximab, irinotecan, and combination groups were 38, 51, 51, and 58 days, respectively. The differences in survival between the treatment groups compared with the control group were statistically significant when compared by log-rank test (P < 0.001). Furthermore, each single-agent treatment groups also showed statistically significant difference in survival compared to the combination treatment group (P < 0.01).
Fig. 5.
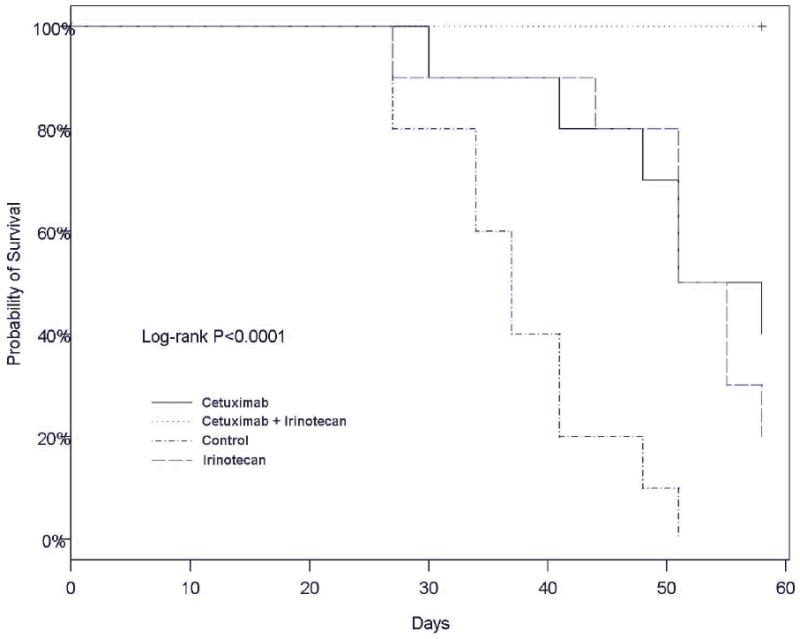
Kaplan-Meier survival curve showing the effects of cetuximab and irinotecan on the survival of nude mice bearing orthotopic ATC xenografts. P values were < 0.05 for all treatment groups when compared to the control group. Both single-agent therapy groups also showed statistically significant differences in survival compared with the combination group.
DISCUSSION
In the present study, the concurrent use of irinotecan and the molecular blockade of the EGFR signaling pathway with cetuximab resulted in the inhibition of the growth, invasion, metastasis, and the angiogenesis of orthotopic ATC xenografts in nude mice. Statistical analysis also showed that cetuximab and irinotecan interacted synergistically to produce their anti-tumor effects. Furthermore, combination therapy with cetuximab and irinotecan was shown to be more effective and yet significantly less toxic in this model than doxorubicin, which is used frequently for the treatment of ATC.
Although cetuximab was able to moderately enhance the in vitro cytostatic and cytotoxic effects of irinotecan, cetuximab alone did not exhibit any effects on the proliferation or the apoptosis of ATC cell line ARO. However, when administered in vivo as single-agent therapy, cetuximab was able to produce significant inhibition of ATC tumor growth (approximately 70%). This discordance between the in vitro and in vivo activity of cetuximab has also been reported in preclinical study of other tumor types such as renal cell carcinoma (30).
A possible explanation for this observation may be the potential for cetuximab to induce ADCC (27). Consistent with this possibility, our study showed that orthotopic ATC xenografts treated with cetuximab undergo a significant increase in tumor infiltration by macrophages. The human portion of cetuximab is of IgG1 subclass and consequently, is able to interact with Fc receptors of effector cells such as natural killer (NK) cells. Although the ability of C225 to induce ADCC has not yet been directly demonstrated, Bleeker et al showed that a human IgG1 anti-EGFR antibody was able to induce efficient ADCC in vitro against A431 cells which overexpress EGFR (31). Another example of the mobilization of ADCC by antibodies engineered to target membrane receptors is rituximab. Rituximab is a chimeric (human-murine) monoclonal antibody to CD20 that has emerged an effective therapy for B-cell malignancies and non-Hodgkin’s lymphoma (32). In the case of rituximab, the induction of ADCC by the Fc portion of rituximab has been shown to be critical in its cytotoxic effects (33).
Another potential explanation for the enhanced in vivo activity of cetuximab may be its anti-angiogenic property. EGFR blockade with cetuximab has been shown to down regulate the production of VEGF by tumor cells and decreased the MVD in nude mice xenografts of transitional cell carcinoma, renal cell carcinoma, and colorectal carcinoma (26, 30, 34). This study showed that cetuximab is also able to inhibit the angiogenesis of orthotopic ATC xenografts demonstrating that the endothelium of thyroid gland is sensitive to the anti-angiogenic effects of cetuximab. Therefore, it is likely that the anti-neoplastic effect of cetuximab is multi-faceted and is the result of combination of effects including the blockade of tumor-expressed EGFR, recruitment of immune responses, and anti-angiogenesis.
Although irinotecan has not yet been evaluated for the treatment of ATC, its role in the treatment of colorectal, ovarian, cervical, small-cell lung, and non small-cell lung carcinoma has been evaluated in several studies (22,35). In particular, two phase III clinical trials have shown that combining irinotecan with 5-fluorouracil in the treatment of colorectal carcinoma had survival benefit in patients with metastatic colorectal carcinoma over treatment with either agent alone (36,37). Given the activity of irinotecan against other adenocarcinomas and the data presented in this study, the role of irinotecan in the treatment of ATC appears promising.
The synergy between EGFR inhibition and DNA damaging agents such as radiation or irinotecan has been reported in multiple studies (16,17). However, the mechanism responsible for this interaction remains to be fully elucidated. It is clear, however, that the molecular blockade of EGFR by cetuximab results in changes in the expression or the activity of several cellular factors including CDK2, cyclin A, cyclin E, and p27KIP1, and that these changes may modify the tumor cell’s response to external stress (38). Huang et al showed that the treatment of cultured SCC cells with cetuximab resulted in the suppression of postradiation DNA damage repair (39). Another interesting observation is that cellular stress such as irinotecan and UV radiation increases the phosphorylation of EGFR, lending further support to the hypothesis that the cellular survival responses depend on the integrity and possibly the upregulation of the EGFR activity (40). Therefore, it is likely that the cellular Kim et al changes induced by the inhibition of EGFR pathway results in the impairment of effective DNA repair and recovery, and thereby amplify the apoptotic and anti-proliferative effects of agents such as irinotecan. Toxicity is a major concern in the treatment planning of any elderly patient or those with compromised medical status. ATC occurs mainly in the elderly with the mean age of 70 years (24,25). Furthermore, these patients often have compromised medical and nutritional status due to the obstruction of the upper aerodigestive tract. A major advantage of cetuximab over other EGFR inhibitors is its safety profile that has been established in several clinical trials. Cetuximab, when administered as monotherapy, results frequently in mild toxic effects most commonly manifesting as a maculopapular rash (19,41). Irinotecan, on the other hand, has been associated with diarrhea and, less frequently, myelosuppresion (21). However, it is possible that the co-administration with cetuximab may allow dose reduction of irinotecan and thereby alleviate its toxic effects in patients with impaired functional status. In our athymic, nude mice model, the prolonged administration of cetuximab/irinotecan combination did not produce weight loss and were better tolerated than doxorubicin. Finally, the limitations of our study should be noted. First, the ARO cell line used in this study has been shown previously to express EGFR and to secrete EGF (11). Although the majority of ATC tumors express EGFR, the level of expression is more variable (8,11). Before cetuximab could be considered for clinical study, its effectiveness against other frequently studied ATC cell lines including those cell lines that express low EGFR level needs to be evaluated. Second, because no single chemotherapeutic agent is active against ATC, several agents have been used in the treatment of this disease. Although we have compared the effectiveness of combined treatment with cetuximab and irinotecan with that of doxorubicin, we have not compared with combined treatment with other commonly used agents such as paclitaxel. Thirdly, the increase in tumor-infiltrating macrophages in cetuximab-treated tumors by itself is only suggestive of ADCC. Further studies are necessary to validate this hypothesis.
In conclusion, we have provided data that support the study of cetuximab and irinotecan in the treatment of ATC. Both agents showed significant activity as single agent therapy as well as synergistic activity when co-administered in inhibiting the growth and progression of orthotopic ATC xenografts in nude mice. The regimen of cetuximab and irinotecan was significantly more effective than doxorubicin, an agent commonly used for this disease. Although our preclinical data require further validation, cetuximab and irinotecan may offer a novel therapeutic approach for this uniformly fatal disease.
Footnotes
This work was supported by the M. D. Anderson Cancer Center SPORE in Head and Neck Cancer Grant P50 CA097007A and the U.T. M.D. Anderson Cancer Center PANTHEON Program.
References
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 2.Pasieka JL. Anaplastic thyroid carcinoma. Curr Opin Oncol. 2003;15:78–83. doi: 10.1097/00001622-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEERS) program 1973–1991. Cancer. 1997;79:564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Shimaoka K, Schoenfeld DA, DeWys WD, et al. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–60. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja S, Ernst H. Chemotherapy of thyroid carcinoma. J Endocrinol Invest. 1987;10:303–10. doi: 10.1007/BF03348135. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Grandis JR. Targeting epidermal growth factor receptor in head and neck cancer. Head Neck. 2003;25:67–73. doi: 10.1002/hed.10224. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Hammond LA. HER-targeted tyrosine-kinase inhibitors. Oncology. 2002;63 (Suppl 1):6–16. doi: 10.1159/000066198. [DOI] [PubMed] [Google Scholar]
- 8.Ensinger C, Spizzo G, Moser P, et al. Epidermal growth factor receptor as a novel therapeutic target in anaplastic thyroid carcinomas. Ann N Y Acad Sci. 2004;1030:69–77. doi: 10.1196/annals.1329.009. [DOI] [PubMed] [Google Scholar]
- 9.Gabler B, Aicher T, Heiss P, Senekowitsch-Schmidtke R. Growth inhibition of human papillary thyroid carcinoma cells and multicellular spheroids by anti-EGF-receptor antibody. Anticancer. 1997;17:3157–9. [PubMed] [Google Scholar]
- 10.Bergstrom JD, Westermark B, Heldin NE. Epidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cells. Exp Cell Res. 2000;259:293–9. doi: 10.1006/excr.2000.4967. [DOI] [PubMed] [Google Scholar]
- 11.Schiff BA, McMurphy AB, Jasser SA, et al. Epidermal growth factor receptor (EGFR) is overexpressed in anaplatic thyroid cancer, and the EGFR inhibitor gefinitib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res. 2004;10:8594–602. doi: 10.1158/1078-0432.CCR-04-0690. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Schiff BA, Yigitbasi OG, et al. Targeted molecular therapy of anaplastic thyroid carcinoma with AEE788 – a dual specific inhibitor of epidermal growth factor receptor and vascular endothelial growth factor receptor tyrosine kinases. Mol Cancer Ther. 2005;4:632–40. doi: 10.1158/1535-7163.MCT-04-0293. [DOI] [PubMed] [Google Scholar]
- 13.Calvo E, Rowinsky EK. Clinical experience with monoclonal antibodies to epidermal growth factor receptor. Curr Oncol Rep. 2005;7:96–103. doi: 10.1007/s11912-005-0034-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Vokes EE, Kies MS. Cetuximab in cancers of the lungs and head + neck. Semin Oncol. 2004;31 (1 suppl 1):61–7. doi: 10.1053/j.seminoncol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Ciardello F, Bianco R, Damiano V, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–16. [PubMed] [Google Scholar]
- 16.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- 17.Buchsbaum DJ, Bonner JA, Grizzle WE, et al. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. Int J Radiat Oncol Biol Phys. 2002;54:1180–93. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JA, Harari PM, Giralt JL. Cetuximab (Erbitux TM) prolongs survival in patients with locally advanced squamous cell carcinoma of the head and neck: a phase III study of high dose radiation therapy with and without cetuximab. Proc Am Clin Oncol 2004 abstract 5507.
- 19.Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004;30:255–68. doi: 10.1016/j.ctrv.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Fuchs CS. Epidermal growth factor receptor inhibitors and colorectal cancer. Oncology (Huntington) 2004;18 (14 suppl 14):35–8. [PubMed] [Google Scholar]
- 21.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–61. [PubMed] [Google Scholar]
- 23.Kim S, Park YW, Schiff BA, et al. An orthotopic model of anaplastic thyroid carcinoma in nude, athymic mouse. Clin Cancer Res. 2005;11:1713–21. doi: 10.1158/1078-0432.CCR-04-1908. [DOI] [PubMed] [Google Scholar]
- 24.Mathijssen RH, van Alphen RJ, Verweij J, et al. Clinical Pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- 25.Raymond E, Bioge V, Faivre S, et al. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol. 2002;20:4303–4312. doi: 10.1200/JCO.2002.03.123. [DOI] [PubMed] [Google Scholar]
- 26.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–65. [PubMed] [Google Scholar]
- 27.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–10. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Giuffida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. 2000;11:1083–9. doi: 10.1023/a:1008322002520. [DOI] [PubMed] [Google Scholar]
- 29.Haigh PI. Anaplastic thyroid carcinoma. Curr Treat Options Oncol. 2000;1:353–7. doi: 10.1007/s11864-000-0051-8. [DOI] [PubMed] [Google Scholar]
- 30.Prewett M, Rothman M, Waksal H, Feldman M, Bander NH, Hicklin DJ. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4:2957–66. [PubMed] [Google Scholar]
- 31.Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, et al. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173:4699–707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- 32.Johnson P, Glennie M. The mechanism of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30 (1 suppl 2):3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- 33.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 34.Ciardiello F, Bianco R, Damiano V, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor recpetor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotides in human GEO colon cancer cells. Clin Can Res. 2000;6:3739–47. [PubMed] [Google Scholar]
- 35.Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003;361:2235–42. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 36.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 37.Saltz LB, Locker PK, Pirotta N, Elfring GL, Miller LL. Weekly irinotecan (CPT-11), leucovorine (LV), and fluorouracil (FU) is superior to daily X5 LV/FU in patients (PTS) with previously untreated metastatic colorectal cancer (CRC) Proc Am Soc Clin Oncol. 1999;18:233a. [Google Scholar]
- 38.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monocloncal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–9. [PubMed] [Google Scholar]
- 39.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–74. [PubMed] [Google Scholar]
- 40.Koizumi F, Kanzawa F, Ueda Y, et al. Synergistic interaction between the EGFR tyrosine kinase inhibitor gefinitib (“Iressa”) and the DNA topoisomerase I inhibitor CPT-11 (irinotecan) in human colorectal cancer cells. Int J Cancer. 2004;108:464–72. doi: 10.1002/ijc.11539. [DOI] [PubMed] [Google Scholar]
- 41.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]


