Abstract
In the present study we developed an enzyme-linked immunosorbent assay (ELISA) to measure immunoglobulin M (IgM) specific for glycoinositolphospholipids (GIPL) derived from tachyzoite membrane (IgM-GIPL ELISA). The sensitivity and specificity of the assay were compared with those of commercially available Toxoplasma-specific IgM serological tests, namely, immunofluorescence assay (IFA) with fixed tachyzoites and capture ELISA employing tachyzoite extracts. Our results show that all patients with acute toxoplasmosis, as determined by clinical data and conventional serological tests, were also positive by the IgM-GIPL ELISA. Interestingly, many patients that were classified as indeterminate, who had IgG with high avidity but positive results in the IgM-specific IFA and capture ELISA, were negative by the IgM-GIPL ELISA. Finally, we tested the sera from patients with rheumatoid arthritis and various parasitic infections and found no evidence of false positives in the IgM-GIPL ELISA.
Toxoplasma gondii is an intracellular apicomplexan protozoan that infects a large variety of domestic and wild mammals, including humans, as well as birds and is widespread all over the world (6). It is estimated that toxoplasmosis exists in a chronic asymptomatic form in 5 hundred million to 1 billion of the world human population (6). Although infection with T. gondii is usually asymptomatic in most individuals, it causes serious morbidity and mortality in immunocompromised individuals (11). In fact, T. gondii is the major infectious cause of encephalitis in AIDS patients (21, 27), being among the opportunistic pathogens more often encountered in AIDS-defining illness (2, 22).
Further, primary T. gondii infection during the first two trimesters of pregnancy (11) may result in severe tissue damage in the fetus, leading to malformation, newborn death, and abortion. Importantly, most of the effects of T. gondii infection in pregnant women and fetus can be prevented by chemotherapy (24). Therefore, the diagnosis of recent toxoplasmosis in gravid women is of great importance. The identification of most acute cases of toxoplasmosis in humans is based on detection and measurement of parasite-specific immunoglobulin M (IgM) in sera from the patients by indirect immunofluorescence assay (IFA) and immunosorbent capture enzyme-linked immunosorbent assay (capture ELISA) (4, 5, 16, 19, 20, 28, 29). The IFAs have high specificity but lower sensitivity, leading to false-negative results (19). More recently, with the development of the capture ELISA, the sensitivity of the T. gondii-specific IgM test has been improved; however, this increased sensitivity is associated with persistent positive results even 1 year or more after the primary infection with T. gondii (7, 25).
All the serological tests mentioned above employ paraformaldehyde-fixed tachyzoites or whole-tachyzoite extracts rather than purified molecules that are preferentially recognized by human IgM. Different studies suggest that the main targets for T. gondii-specific IgM, present in sera from patients with acute toxoplasmosis, are carbohydrate branches from glycosylinositolphospholipids (GIPL) on the tachyzoite surface (9, 26). An easy method of enriching or purifying the GIPLs derived from the tachyzoite stage of T. gondii was recently described (9). In the present study, we developed a new ELISA employing GIPL-enriched fractions derived from tachyzoites to detect and quantify the levels of parasite-specific IgM in sera from patients with acute toxoplasmosis.
MATERIALS AND METHODS
Serum samples.
In the first part of this study we used sera from individuals that were not monitored clinically but whose sera were analyzed by commercially available tests to detect IgM and IgG antibodies specific for Toxoplasma. Serum samples from 40 IgG- and IgM-negative controls and 16 chronically infected (IgG-positive and IgM-negative) and 127 acutely infected (IgG-positive and IgM-positive) individuals were used to determine the correlation of results obtained in the IgM-capture ELISA (Abbott Co., Chicago, Ill.) and the IgM-GIPL ELISA.
A second group of patients evaluated in this study was characterized both serologically and clinically for infections with Leishmania spp., Plasmodium spp., Schistosoma mansoni, T. gondii, and Trypanosoma cruzi. Serology specific for Toxoplasma was performed by different methods, i.e., IFA, capture ELISA for IgM and IgG, and an IgG avidity test. In addition, IFA was performed to detect IgG antibodies specific for Leishmania spp., Plasmodium spp., S. mansoni, and T. cruzi. The groups of patients were defined as follows: 10 serologically negative and asymptomatic healthy controls; 20 individuals with acute toxoplasmosis as determined by serology and clinical symptoms; 21 individuals considered indeterminate because of persistent positive results in the T. gondii-specific IgM tests, anti-T. gondii IgG antibodies with high avidity, and no clinical history or recent symptoms indicating newly acquired Toxoplasma infection; 16 asymptomatic individuals with chronic toxoplasmosis; 30 patients with malaria; 40 individuals infected with T. cruzi; 40 individuals infected with S. mansoni; 80 individuals with cutaneous or visceral leishmaniasis; and 16 individuals with rheumatoid arthritis. Details of the clinical characterization and T. gondii-specific serological tests for these individuals are presented in Table 1.
TABLE 1.
Sera employed in the comparison between the IgM-GIPL ELISA, IFA, and capture ELISA
| Group | n | Description |
|---|---|---|
| Control | 10 | Human serum samples collected from healthy individuals without clinical symptoms or positive serological tests for infection with T. gondii, T. cruzi. S. mansoni, Plasmodium spp., or Leishmania spp. All patients in this group were negative for Toxoplasma-specific IgM and by the IgG IFA |
| Acute toxoplasmosis | 20 | Human serum samples collected from patients with typical clinical symptoms of infectious mononucleosis-like syndrome, with fever, fatigue, and enlargement of the cervical lymph nodes. The sera from these patients exhibited specific IgM antibodies (titer of ≥64), IgG antibodies (titer of ≥256) by IFA, and low avidity of T. gondii-specific IgG |
| Chronic toxoplasmosis | 14 | Human serum samples collected from immunocompetent individuals with asymptomatic toxoplasmosis. These serum samples exhibited specific IgG antibodies (titer of ≥64), negative results for T. gondii specific IgM (titer of <16) in the IFA, and high avidity of T. gondii-specific IgG. |
| Indeterminate | 21 | Serum samples collected from patients presenting asymptomatic infections. However, the results of the laboratory assays were unclear for these patients. A high level of discordant results was seen in this group, with IgM antibodies (titer of ≥64) being persistently positive for a long period of time, associated with positive results for IgG (titer of ≥64) in the IFA and high avidity of T. gondii-specific IgG. |
| Malaria | 30 | Serum samples collected from patients from areas of malarial endemicity. These samples had specific IgG antibodies for Plasmodium spp. as analyzed in the IFA. From these sera, 14 were negative (titer of <16) and 16 were positive (titer of >64) for Toxoplasma-specific IgG in the IFA. |
| Chagas' disease | 40 | Human serum samples collected from patients with specific IgG antibodies to T. cruzi. The patients presented clinic symptoms of the cardiac, digestive, or asymptomatic form of Chagas' disease. Of these sera, 22 were negative (titer of <16) and 18 were positive (titer of ≥64) for Toxoplasma-specific IgG in the IFA. |
| Schistosomiasis | 40 | Human serum samples collected from patients in areas of endemic S. mansoni infection. Digestive and hepatosplenic symptoms were the clinical forms presented by this group. The samples had specific IgG antibodies to S. mansoni by the IFA. Of these sera, 23 were negative (titer of <16) and 17 were positive (titer of >64) for Toxoplasma-specific IgG in the IFA. |
| Leishmaniasis | 80 | Human serum samples collected from patients from areas of leishmaniasis endemicity. 50% of the patients presented the cutaneous or mucocutaneous form of leishmania infection; the other patients had clinical symptoms of visceral leishmania. All serum samples exhibited specific IgG antibodies to Leishmania spp. in the IFA. Of these sera, 52 were negative (titer of <16) and 28 were positive (titer of >64) for Toxoplasma-specific IgG in the IFA. |
| Arthritis | 16 | Serum samples collected from patients with arthritis. All patients had polyarticular or systemic symptoms of rheumatoid arthritis. All samples were positive for IgM rheumatoid factors. All patients from this group were negative for Toxoplasma-specific IgM and IgG in the IFA. |
Sequential organic solvent extraction and purification of GIPLs derived from tachyzoites.
Tachyzoites of RH strains of T. gondii were maintained by in vitro passage in human foreskin fibroblasts at 37°C (17) and harvested at 4 to 5 days postinfection, centrifuged at 70 × g for 10 min to remove cell debris, and then pelleted at 590 × g for 10 min. The parasite pellet was washed twice by resuspension in cold phosphate-buffered saline (PBS) and centrifugation, lyophilized, and stored at −70°C. Briefly, the tachyzoite pellet was subjected to extraction with chloroform-methanol-water (5/10/4, vol/vol/vol) (1, 9). Ten volumes of chloroform-methanol-water were added to the parasite pellet and sonicated for 15 min, followed by centrifugation at 5,000 × g for 15 min at 10°C. The resulting pellet was subjected to the same protocol twice more, and the supernatants were pooled, dried in a speed vacuum (Savant Instruments Inc., Farmingdale, N.Y.), and subjected to a butan-1-ol-water (1/1, vol/vol) partition. The aqueous phase generated by the butan-1-ol-water partition was designated F2 and used in our assay as the fraction enriched for GIPLs.
Frozen F2 fractions were resuspended in 100 mM of ammonium acetate containing 5% propan-l-ol and subjected to hydrophobic interaction chromatography using octyl-Sepharose resin (Pharmacia Biotech, Uppsala, Sweden) following elution with a propan-l-ol (5-to-60%) gradient. The eluted fractions were resolved by gel electrophoresis (12) and tested in a dot blot and Western blot analysis for reactivity with IgM present in sera from patients with acute toxoplasmosis as previously described (9).
IgM-GIPL ELISA.
Immulon-2 plates (Dynatech Laboratories, Chantilly, Va.) were coated with 100 μl of F2 (GIPL-enriched fraction) or purified GIPL diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Plates were incubated overnight at 4°C, blocked with 2% casein for 2 h at 37°C and then washed four times with 0.15 M PBS (pH 7.2)-0.05% Tween 20 (Sigma Chemical Co., St. Louis, Mo.) (PBS-T). One hundred microliters of test sera at 1:200 dilution in PBS-T-1% bovine serum albumin (Biobras, Montes Claros, Brazil) was added and incubated for 1 h at 37°C. Plates were then washed and incubated with biotinylated-conjugated anti-human IgM (Sigma) at 1:20,000 in PBS-T for 1 h at 37°C. After additional washing with PBS-T, the streptavidin-peroxidase conjugate (Sigma) at a 1:2,000 dilution was added and incubated for 30 min at 37°C. Plates were then washed with PBS-T and developed with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] as the substrate. The reaction was blocked by the addition of 50 μl of 1% sodium dodecyl sulfate solution, and results were read at 405 nm.
Immunofluorescence.
The IFA was used to detect IgG and IgM antibodies to T. gondii as previously described (5), with some modifications. Sera were tested at dilutions from 1:16 to 1:32,000, and the secondary antibodies consisted of fluorescein isothiocyanate-labeled rabbit IgG, directed to γ or μ human heavy chains (Sigma). The working dilutions of the conjugates were determined by block titration with positive and negative reference serum controls. The slides were examined with an epifluorescent microscope (model BH2; Olympus, Tokyo, Japan).
Capture ELISA and avidity ELISA.
The commercial ELISA for detection of Toxoplasma IgG and IgM antibodies was performed basically as previously described (5). Serum samples were tested at 1:16 to 1:32,000 dilutions in duplicate. Positive and negative reference serum controls were included in each assay. Cutoff titers were determined as the mean absorbance values of the negative controls plus 3 standard deviations. Two types of commercially available assays for detection of IgG or IgM, based on indirect or capture tests, respectively, were carried out according to the instructions of the manufacturer (Abbott Co.).
Avidity of T. gondii-specific IgG antibodies was determined as previously described (13). Microtiter plates previously coated with T. gondii tachyzoites were washed three times with PBS-T. The serum samples, in serial twofold dilutions from 1:16 to 1:2,048 were added in duplicate on separate plates. After incubation for 45 min at 37°C, the plates were subjected to differential washing as follows: one plate was washed with a 6 M urea solution in PBS for 5 min, while the other plate was washed only with PBS-T for 5 min. Both plates were washed twice with PBS-T for 5 min. The residual antigen-bound IgG was detected with a rabbit anti-human IgG conjugated to horseradish peroxidase (Sigma) at 1:3,000 and incubated for 45 min at 37°C. After new washes with PBS-T, the reaction was revealed with substrate solution consisting of orthophenylenediamine at 0.5 mg/ml in 0.1 M citrate-phosphate buffer (pH 5.0) and 0.012% H2O2. After incubation for 15 min at room temperature, the reaction was stopped with 2 N H2SO4. The absorbances were measured at 492 nm with a plate reader system (Titertek Multiskan Plus; Huntsville, Ala.). The avidity index was calculated as the ratio between the absorbance obtained for the plate washed with urea and that for the plate without urea and expressed as a percentage.
Statistical analysis.
Antigen concentration and serum dilution were defined by analysis of variance for 10 samples from individuals with either acute or chronic toxoplasmosis. The positive-negative borderline was calculated by Z distribution for IgM assays with 10 unreactive, 20 acute-phase, and 14 chronic-phase samples. Analyses of correlation between commercial tests and the IgM-GIPL ELISA (employing either F2 or purified GIPL) was performed with Statistic software (version 4.5).
RESULTS
Positive correlation between the results obtained in the IgM-GIPL ELISA and commercial test to measure the IgM levels in sera from patients infected with T. gondii.
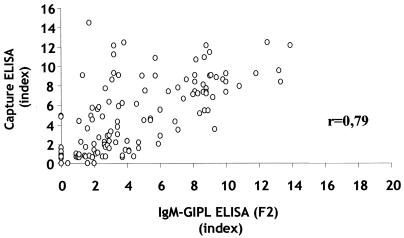
We have recently developed a IgM-GIPL ELISA employing the GIPL-enriched extract (F2) or purified GIPLs from T. gondii tachyzoites to detect anti-T. gondii IgM antibodies (9). Here we compared the IgM-GIPL ELISA with a commercial IgM capture ELISA (Abbott Co.). The two tests were performed in parallel at Fleury Laboratory by M.S., P.L., and M.E.C. using the same sera and not knowing whether the sera were derived from IgM-positive or IgM-negative individuals. The data presented in Fig. 1 as indexes show a high direct correlation between the two tests. A correlation ratio of 0.79 was reached. In addition, only 23 (12.5%) of 183 sera gave discordant results between the two tests. Of those sera, 17 were negative for the commercial test and positive for IgM-GIPL ELISA and 6 were positive for the commercial test and negative for the IgM-GIPL ELISA. These results indicate that the IgM-GIPL ELISA is more sensitive than the commercial capture ELISA test. This finding led us to question if the higher sensitivity of the IgM-GIPL ELISA would result in detection of patients with low but continuous positive IgM results, more likely to be at the early chronic rather than the acute phase of toxoplasmosis.
FIG. 1.
Comparison between the results of GIPL ELISA employing the enriched GIPL fraction (F2) and the commercial IgM-capture ELISA (Abbott Co.) employing tachyzoite extracts to measure the levels of anti-Toxoplasma IgM in serum. r, correlation index for the two tests. Assays were performed without knowledge of the patient's status regarding levels of T. gondii-specific IgM or IgG in serum. Indexes for capture ELISA and IgM-GIPL ELISA were calculated as the absorbance of the tested serum divided by the average of absorbances of negative control sera. Sera were considered positive if the index was >1.6.
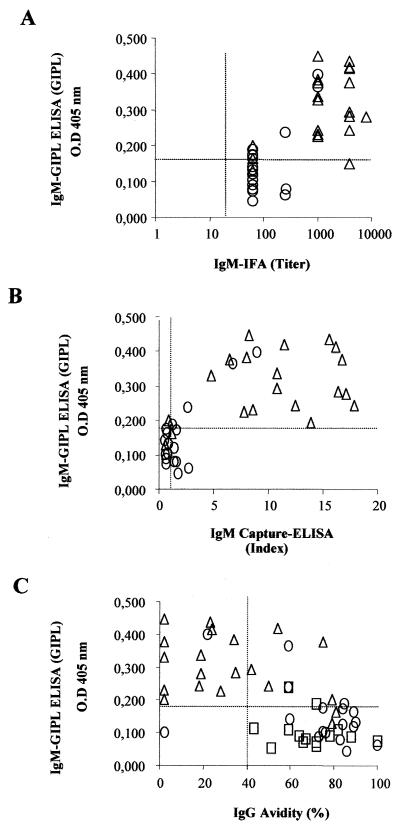
Patients classified as indeterminate with persistent low levels of T. gondii-specific IgM and high-avidity IgG results were negative in the IgM-GIPL ELISA.
In order to further investigate the basis of the discordant results between the IgM-GIPL ELISA and the commercial tests, we used a panel of sera obtained from patients monitored by clinical exams and previously subjected to different T. gondii-specific IgG and IgM serological tests (Table 1). We used sera from 10 seronegative healthy individuals, 20 acutely infected individuals with clinical symptoms and positive IgG and IgM serological tests, and 14 asymptomatic chronically infected individuals that yielded IgG-positive but IgM-negative results. We also tested 21 sera from patients who were classified as indeterminate because of discordant results between the IgM and the IgG avidity tests. In general, sera from indeterminate patients exhibited persistent levels of T. gondii-specific IgM and high-avidity T. gondii-specific IgG. In addition, in terms of clinical evaluation, the indeterminate patients did not fall into the category of acute toxoplasmosis.
The data presented in Fig. 2 correlate the results from IgM-GIPL ELISA employing GIPL with commercial tests. As commercial tests we used IFA and capture ELISA (Abbott Co.) to detect T. gondii-specific IgM, as well the avidity test for T. gondii-specific IgG. Figure 2A and B show that the results obtained with the IgM-GIPL ELISA employing GIPL for the acute-phase sera have a greater uniformity than IFA or commercial ELISA results. It can be also seen that only 3 of the 21 indeterminate sera were positive in the IgM-GIPL ELISA. Importantly, as shown in Fig. 2C, the same three sera presented relatively low avidity values (20, 60, and 60%). In contrast, in the commercial tests, 100 and 48% of the indeterminate sera were IgM positive in the IFA and capture ELISA, respectively, despite their high scores in the avidity test. The sera from patients with chronic disease had high scores in the avidity test and were negative in the IgM-GIPL ELISA (Fig. 2), IFA, and capture ELISA (not shown). Results obtained with the GIPL-enriched fraction (F2) as the antigen were similar to those obtained with purified GIPLs (Fig. 2).
FIG. 2.
Correlation between the results of IgM-GIPL ELISA employing purified GIPL and other tests to detect T. gondii-specific IgM or avidity of T. gondii-specific IgG. All tests were specific for T. gondii antigens. The experiments were performed in a blinded fashion using sera from acute-phase patients (▵; n = 20), indeterminate patients (○; n = 23), and chronic-phase patients (□; n = 14), and the sera were decoded afterwards.
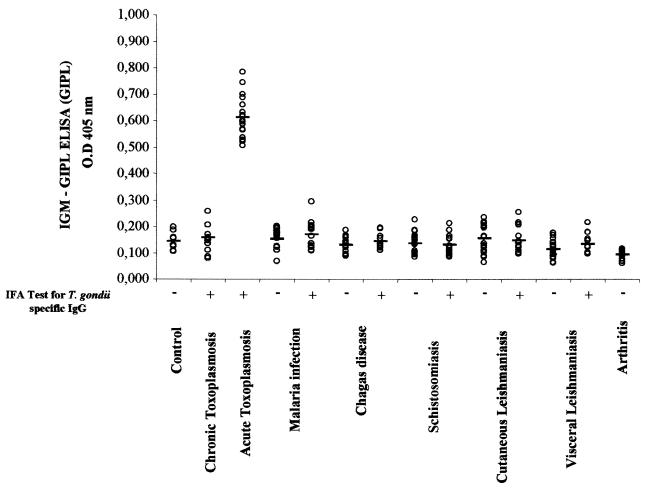
Low cross-reactivity between patients infected with T. gondii or other parasitic infections as measured by the IgM-GIPL ELISA.
Figure 3 shows the results for the IgM-GIPL ELISA, employing purified GIPL and sera from patients infected with various protozoan and helminthic parasites. When we performed the IgM-GIPL ELISA, we observed low levels of cross-reactivity among sera from patients with the different parasitic infections studied. Similar results were obtained when F2 was the antigen. However, 2 out of 30 patients with malaria had high T. gondii-specific IgM levels in the GIPL-ELISA employing F2 (data not shown) but not in the test with purified GIPL (Fig. 3). Nonetheless, the difference in the results obtained with malaria patients and individuals acutely infected with T. gondii was statistically significant (P < 0.01), as measured by IgM-GIPL ELISA employing either F2 (data not shown) or purified GIPL (Fig. 3). Finally, the sera from patients with rheumatoid arthritis, a common cause of false-positive results in the commercial capture ELISA, were all negative in the IgM-GIPL ELISA with either F2 (data not shown) or purified GIPL (Fig. 3).
FIG. 3.
IgM-GIPL ELISA employing purified GIPL was used to distinguish between sera from patients with acute toxoplasmosis and sera from patients with different parasitic diseases (with or without chronic toxoplasmosis) or rheumatoid arthritis. All the sera were tested for anti-T. gondii IgM and IgG by IFA using whole tachyzoites. The experiments were performed in a blinded fashion, and the sera decoded afterwards. O.D, optical density.
DISCUSSION
The immunodiagnosis of the infection with the protozoan parasite T. gondii has been matter of study of various research groups around the world. For anti-T. gondii IgG detection, the “gold standard” method used until now has been the Sabin-Feldman test (10). In addition, a large variety of methods, such as commercially available IFA and ELISA, employing whole parasites or extracts derived from tachyzoites have being used to detect IgM and IgG antibodies against T. gondii. However, there are problems with the detection of parasite-specific IgM and the precise identification of the acute toxoplasmosis; specifically, sensitivity may be too weak to detect low IgM antibody levels, or the assay may detect IgM antibodies that persist for a long period after the primary infection (4). This is a serious matter, since women that acquired infection early before pregnancy can be diagnosed as primarily infected during gestation, resulting in unnecessary chemotherapy (7, 11).
It has already been reported that the main targets for T. gondii-specific IgM in sera from patients with acute toxoplasmosis are carbohydrate branches (16) from GIPL present on the tachyzoite surface (9, 26). Therefore, a practical and simple method was previously developed to enrich or purify these membrane compounds and test them as antigens in an indirect ELISA (9). But in these preliminary tests, this method was not correlated with commercially available tests, and possible cross-reactions of T. gondii glycoconjugates with other protozoan parasites were not considered. So, in the present study, we sought to correlate the previously mentioned ELISA (IgM-GIPL ELISA) with commercially available tests in order to determine its accuracy in the diagnosis of patients clinically defined as having acute toxoplasmosis.
First of all, there was a high correlation index (0.79) between our IgM-GIPL ELISA and a commercially available IgM-capture ELISA (Abbott Co.) that employs sonicated parasites. We considered this level of correlation very promising, since we used F2, which is a GIPL-enriched fraction, and not purified GIPL. In order to further investigate the detection of IgM in sera from patients with toxoplasmosis, we used our IgM-GIPL ELISA and compared it to IgM-specific IFA and capture ELISA as well as the avidity test for anti-T. gondii IgG antibodies. For this investigation, we also used patients that had been well characterized in terms of clinical exams. We observed that, in general, the acute-phase patients positive by the IgM-GIPL ELISA had lower IgG avidity scores, while the avidity scores were higher for anti-T. gondii IgG in chronic-phase patients with negative IgM results. More importantly, we used a group of patients with indeterminate status in terms of acute versus chronic toxoplasmosis. These patients were asymptomatic, possessing persistent levels of anti-T. gondii IgM in serum and high scores in the IgG avidity test. In most cases, the indeterminate patients were negative in our IgM-GIPL ELISA but positive in the commercial IgM tests (IFA and capture ELISA). While the use of low-affinity IgG as being indicative of acute infection with T. gondii is a matter of debate (13, 15), different studies indicate that, consistent with our findings, high-avidity IgG antibodies are good indicators of an infection that was acquired in the past (3, 4, 5, 13).
We concluded that the IgM-GIPL ELISA is more specific and able to discriminate between the acute phase of infection with T. gondii and persistent anti-T. gondii IgM, thus eliminating false-positive results commonly obtained with the commercially available IgM tests. The basis of this difference is uncertain; however, considering that the IgM-GIPL ELISA measures mainly anticarbohydrate antibodies, we speculate that in the late acute phase or in the initial chronic phase, most of the T. gondii-specific IgM antibodies are directed to parasite membrane proteins, such as SAG-1 (18, 19). Therefore, it is possible that the decay of T. gondii-specific IgM is delayed in sera from patients with recent toxoplasmosis, resulting in differences in results obtained with total parasites or parasite extract compared to purified GIPL.
The GIPL structure has been described and analyzed in various species of protozoan parasites, such as T. cruzi, Leishmania major, and Plasmodium falciparum, among others (1, 8, 14, 18, 30, 31). Although, the glycan core and basic structure are conserved, the lipid tails and carbohydrate branches are highly variable among different species and developmental stages of various parasites (23, 30). The possible cross-reactivity between GIPL derived from T. gondii and other parasites was investigated by using sera from patients infected with different parasites (i.e., S. mansoni, T. cruzi, Leishmania spp., and Plasmodium spp.), which are common causes of disease in tropical areas. Our results confirm the findings of other researchers (26) and show no evidence of cross-reactivity.
For future application of our work to the immunodiagnosis of acute toxoplasmosis and production of large-scale ELISA kits, we envisage the need to better define the epitope recognized by the human anti-GIPL IgM and to synthesize such compounds.
Acknowledgments
We thank Antoniana Ursine Krettli, Reginaldo Brazil, and Rodrigo Correa-Oliveira from CPqRR/FIOCRUZ (Belo Horizonte, MG, Brazil) for providing human sera from malaria, schistosomiasis, Chagas' disease, and leishmaniasis patients.
This work was supported in part by CNPq/PADCT SBIO (62.0106/95-6) and FAPEMIG. R.T.G. and J.R.M. are research fellows of the CNPq. M.G. and R.W.D.P. are graduate students with scholarships from COLCIENCIAS and FIOCRUZ, respectively.
REFERENCES
- 1.Almeida, I. C., M. A. J. Ferguson, S. Schenkman, and L. R. Travassos. 1994. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 304:793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammassari, A., R. Murri, A. Cingolani, A. DeLuca, and A. Antinori. 1996. AIDS-associated cerebral toxoplasmosis: an update on diagnosis and treatment. Curr. Top. Microbiol. Immunol. 219:209-222. [DOI] [PubMed] [Google Scholar]
- 3.Bertozzi, L. C., L. A. Suzuki, and C. L. Rossi. 1999. Serological diagnosis of toxoplasmosis: usefulness of IgA detection and IgG avidity determination in a patient with persistent IgM antibody response to Toxoplasma gondii. Rev. Inst. Med. Trop. Sao Paulo 41:175-177. [DOI] [PubMed] [Google Scholar]
- 4.Camargo, M. E., and P. G. Leser. 1976. Diagnostic information from serological tests in human toxoplasmosis. Rev. Inst. Med. Trop. Sao Paulo 18:227-238. [PubMed] [Google Scholar]
- 5.Camargo, M. E., A. W. Ferreira, J. R. Mineo, C. K. Takiguti, and O. S. Nakahara. 1978. Immunoglobulin G and immunoglobulin M enzyme-linked immunosorbent assays and defined toxoplasmosis serological patterns. Infect. Immun. 21:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey, J. P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28:1019-1024. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, K. T., P. J. Wharton, J. D. Johnson, L. New, and R. E. Holliman. 1989. Assessment of immunoglobulin-M immunosorbent agglutination assay (ISAGA) for detecting toxoplasma specific IgM. J. Clin. Pathol. 42:1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson, M. A. J. 1992. Chemical and enzymatic analysis of glycosylphosphatidylinositol anchors, p. 196-230. In N. M. Hooper and A. J. Turner (ed.), The chemical and enzymatic analysis of GPI fine structure. Lipid modification of proteins: a practical approach. IRL Press, Oxford, United Kingdom.
- 9.Giraldo, M., H. Cannizarro, M. A. Ferguson, I. C. Almeida, and R. T. Gazzinelli. 2000. Fractionation of membrane components from tachyzoite forms of Toxoplasma gondii: differential recognition by immunoglobulin M (IgM) and IgG present in sera from patients with acute or chronic toxoplasmosis. J. Clin. Microbiol. 38:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadman, E., J. W. Goding, and J. S. Remington. 1980. Detection and characterization of membrane antigens of Toxoplasma gondii. J. Immunol. 124:2578-2583. [PubMed] [Google Scholar]
- 11.Joiner, K. A., and J. F. Dubremetz. 1993. Toxoplasma gondii: a parasite for the nineties. Infect. Immun. 61:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Marcolino, P. T., D. A. Silva, P. G. Leser, M. E. Camargo, and J. R. Mineo. 2000. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin. Diagn. Lab. Immunol. 7:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConville, M. J., and J. M. Blackwell. 1991. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J. Biol. Chem. 266:15170-15179. [PubMed] [Google Scholar]
- 15.Mechain, B., J. Garin, F. Robert-Gangneux, J. Dupouy-Camet, and F. Derouin. 2000. Lack of utility of specific immunoglobulin G antibody avidity for serodiagnosis of reactivated toxoplasmosis in immunocompromised patients. Clin. Diagn. Lab. Immunol. 7:703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineo, J. R., M. E. Camargo, and A. W. Ferreira. 1980. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect. Immun. 27:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagel, S. D., and J. C. Boothroyd. 1988. The alpha and beta tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol. Biochem. Parasitol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 18.Nagel, S. D., and J. C. Boothroyd. 1989. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J. Biol. Chem. 264:5569-5574. [PubMed] [Google Scholar]
- 19.Noat, Y., D. R. Guptill, J. Mullenax, and J. S. Remington. 1983. Characterization of Toxoplasma gondii antigens that react with human immunoglobulin M and immunoglobulin G antibodies. Infect. Immun. 41:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noat, Y., and J. S. Remington. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies of Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J. Infect. Dis. 142:757-766. [DOI] [PubMed] [Google Scholar]
- 21.Porter, S. B., and M. A. Sande. 1992. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N. Engl. J. Med. 327:1643-1648. [DOI] [PubMed] [Google Scholar]
- 22.Savva, D. 1992. Toxoplasma, p. 163-185. In S. Myint and A. Cann (ed.), Molecular and cell biology of opportunistic infections in AIDS—1992. Chapman & Hall, London, United Kingdom.
- 23.Schwarz, R. T., and S. Tomavo. 1993. The current status of the glycobiology of Toxoplasma gondii: glycosylphosphatidylinositols, N- and O-linked glycans. Res. Immunol. 144:24-31. [DOI] [PubMed] [Google Scholar]
- 24.Sharma, S. D. 1990. Immunology of toxoplasmosis, p. 184-199. In D. J. Wyler (ed.), Modern parasite biology cellular, immunological and molecular aspects—1990. W. H. Freeman & Co., New York, N.Y.
- 25.Sharma, S. D., J. Mullenax, F. G. Araujo, H. A. Erlich, and J. S. Remington. 1983. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131:977-983. [PubMed] [Google Scholar]
- 26.Striepen, B., C. F. Zinecker, J. B. L. Damm, P. A. T. Melgers, G. J. Gerwig, M. Koolen, J. F. G. Vliegenthart, J. F. Dubremetz, and R. T. Schwarz. 1997. Molecular structure of the “low molecular weight antigen” of Toxoplasma gondii: a glucose α1-4 N-acetylgalactosamine makes free glycosyl-phosphatidylinositols highly immunogenic. J. Mol. Biol. 266:797-813. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, Y., and J. S. Remington. 1993. Toxoplasmic encephalitis in AIDS patients and experimental models for study of the disease and its treatment. Res. Immunol. 144:66-67. [DOI] [PubMed] [Google Scholar]
- 28.Tenter, A. M., and A. M. Johnson. 1991. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol. Res. 77:197-203. [DOI] [PubMed] [Google Scholar]
- 29.Tomavo, S., G. Couvreur, M. A. Leriche, A. Sadak, A. Achbarou, B. Fortier, and J. F. Dubremetz. 1994. Immunolocalization and characterization of the low molecular weight antigen (4-5 kDa) of Toxoplasma gondii that elicits an early IgM response upon primary infection. Parasitology 108:139-145. [DOI] [PubMed] [Google Scholar]
- 30.Tomavo, S., J. F. Dubremetz, and R. T. Schwarz. 1992. A family of glycolipids from Toxoplasma gondii. Identification of candidate glycolipid precursor(s) for Toxoplasma gondii glycosylphosphatidylinositol membrane anchors. J. Biol. Chem. 267:11721-11728. [PubMed] [Google Scholar]
- 31.Tomavo, S., R. T. Schwarz, and J. F. Dubremetz. 1989. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol. Cell. Biol. 9:4576-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]