Abstract
Interpretation of the molecular epidemiological data of Mycobacterium tuberculosis is dependent on the validity of the assumptions that have been made. It is assumed that the IS6110 banding pattern is sufficiently stable to define epidemiological events representing ongoing transmission. However, molecular epidemiological data also support the observation that the IS6110 banding pattern may change over time. Factors affecting this rate may include the nature and duration of disease in a host and the opportunity to experience different host environments during the transmission cycle. To estimate the rate of IS6110 change occurring during the process of transmission, M. tuberculosis isolates from epidemiologically linked patients were genotypically characterized by restriction fragment length polymorphism (RFLP) analysis. The identification of IS6110 banding pattern changes during ongoing transmission suggested that a rate could be estimated. IS6110 change was significantly associated with strains with >5 IS6110 elements (P = 0.013) and was not observed in low-copy-number isolates. The minimum rate of appearance of variant strains was calculated to be 0.14 variant cases per source-case per year. This data suggest that clustering of isolates based on identical RFLP patterns is expected to underestimate transmission in patients infected with high-copy-number isolates. A model based on the rate of appearance of both variant and invariant strains demonstrates that the genotypically defined population structure may change by 18.6% during the study period of approximately 6.5 years. The implications for the use of RFLP data for epidemiologic study are discussed.
The most extensively researched repeat sequence in the genome of Mycobacterium tuberculosis is the transposon element IS6110, a member of the IS 3 transposon family (13, 17). This element may be repeated up to 25 times per genome, and the distribution and stability of these elements in the chromosome of M. tuberculosis have led to the development of an internationally standardized DNA fingerprinting protocol to determine the genotypes of clinical isolates (18, 21). Interpretation of DNA fingerprinting data is based on the assumption that the IS6110 banding pattern is sufficiently stable to allow the grouping of strains with identical IS6110 genotypes as recent epidemiological events (termed clusters), while sufficiently variable to allow the classification of strains with unrelated IS6110 banding patterns (isolates with unique banding patterns) as unrelated epidemiological events (1, 16). Furthermore, it is assumed that clustered isolates represent ongoing transmission between epidemiologically linked patients (5). This methodology has been used in numerous settings to quantify the relative proportion of recent epidemiological events and thereby estimate the extent of recently transmitted disease (1, 16, 23).
While the methodology of generating restriction fragment length polymorphism (RFLP) data is highly standardized, the interpretation varies widely depending on the epidemiological question and assumptions made about the rate of change of RFLP patterns. For instance, in ascribing a case to be a false-positive diagnosis due to laboratory cross-contamination, it is usually assumed that the RFLP pattern should be exactly identical to another in the database, because it is unlikely that the organism will evolve a different pattern in the diagnostic laboratory (22). However, in well-defined outbreak settings, occasional cases are observed in which the RFLP pattern is closely related, but differs by one or two insertion elements (6). The latter data suggest that, as a strain spreads through the community, strain variants will evolve that are progeny of the same epidemic clone, but manifest subtle differences in RFLP patterns (4). The rate of RFLP pattern change in a community is largely unknown, yet is an essential element in setting criteria for reporting isolates to be matched or unrelated.
As a first step towards understanding the evolution of RFLP patterns, genotypic analysis of serial isolates collected from patients with persistent disease has been performed to estimate rates of IS6110 banding pattern change over time (7, 14, 15, 26). The frequency of banding pattern changes reported varied considerably between the different studies, and it was suggested that this might reflect (unspecified) differences in the epidemiology of disease in the different geographical regions (14). Assuming that the IS6110 banding pattern changes at an equal rate in different strains, de Boer et al. (7) calculated an evolution rate by analyzing serial isolates from 544 patients with persistent disease. Twenty-five of these manifested altered banding patterns over time, permitting the extrapolation of a banding pattern half-life of 3.2 years. When this methodology was applied to data from San Francisco, a half-life of about 2 years was calculated (7).
From these results, it was concluded that the rate of evolution of the IS6110 banding pattern was sufficiently slow to allow molecular epidemiological calculations (7). However, the rate of IS6110 banding pattern change as a bacterium travels through a community is largely unknown and is expected to reflect both changes within a host during persistent disease and changes during the transmission cycle, such as when the organism encounters a new host. One study from Germany, looking at cases with epidemiological links, found that pattern alterations in contacts who developed tuberculosis were rare (15). This study, however, had small numbers of transmission events and did not permit the analysis of chains of transmission, as occurs in a high-incidence setting. In this study, we investigated the rate of IS6110 banding pattern change in a high-incidence community of the Western Cape Province of South Africa by studying RFLP patterns in patients who reside in the same or neighboring households. These results are discussed in the context of the rate of appearance of variant strains and the influence of variant strains on the genotypic bacterial population structure as a function of time.
MATERIALS AND METHODS
Study setting.
During the period from mid-1992 to December 1998, M. tuberculosis isolates were collected from patients attending health care clinics within two adjacent suburbs in Cape Town, South Africa (3). This community experiences an extremely high incidence of tuberculosis, with approximately 251 new bacteriologically confirmed adult cases of infection per 100,000 of population per year (19). Before 1995, the National TB Program depended on sputum cultures for the diagnosis of tuberculosis with follow-up sputum samples requested by the attending physician on clinical grounds. Since 1995, the South African National TB Program has been conducted according to the World Health Organization directly observed therapy (short course) (DOTS) strategy with sputum taken for Ziehl-Neelsen smear at presentation for diagnosis, at 2 months for sputum conversion, and again at 6 months after initiation of therapy to monitor response to therapy. As part of the research project, all Ziehl-Neelsen-positive samples are sent for culture. Clinical information, including the residential address, of each patient was recorded at the time of diagnosis, and these data were stored in a Microsoft Access database.
RFLP generation and GelCompar analysis.
All isolates were classified according to the internationally standardized DNA fingerprinting protocol by using the IS-3′ probe (18, 21). The Southern blot autoradiographs were normalized, and the IS-3′ bands were assigned by using GelCompar 4.1 software. The assignments were visually checked independently by two people, and only bands with an intensity of >20% of the average band intensity were scored as representing IS6110-mediated evolutionary events (8). Replicative transposition was identified when the evolved strain showed an additional IS6110-hybridizing band, while deletion of an IS6110 element was identified when an IS6110-hybridizing band was absent from the evolved strain. Variation in the electrophoretic mobility of an IS6110-hybridizing band was classified as a band shift representing a mutational event in the chromosomal domain flanking the IS6110 element. Banding pattern changes suspected of being the result of partial digestion of methylated restriction sites (20) were excluded. All band changes were confirmed by repeating the digestion at least once. Cluster analysis was done by the unweighted pair group method with arithmetic mean (UPGMA) and the Dice coefficient (12). Each IS-3′ banding pattern was assigned a cluster number and tabulated in a Microsoft Access table to enable linking of the DNA fingerprinting data to the clinical data.
Study population. (i) Isolates collected from epidemiologically related patients.
All patients residing in the same household (including the households next door [n ± 2] on the same side of the street) and infected with either identical strains or genotypically related strains (IS6110 banding pattern differing by up to four hybridizing bands) were identified from the databases. It is assumed that patients residing in these households are epidemiologically linked and that their genotypically related or identical M. tuberculosis isolates represent ongoing transmission. These households were divided into two groups: (i) households where the strain genotype was invariant during the course of sampling and (ii) households where the strain genotypes included a variant identified during the course of sampling. To avoid a possible bias in the number of patients included in the variant patient group, strains with more than four changes in the IS6110 banding pattern were considered to reflect reinfection rather than evolution and were excluded from the study. In addition, if the variant strain was found to be present in the community prior to appearance in the patient, these patients were also excluded. In such cases, it was suspected that the patient might have been reinfected by a community source-case. This stringent exclusion criterion excludes the possibility that the IS6110 banding pattern may revert to a previous evolutionary state, thereby conceivably underestimating the extent of IS6110 change.
The appearance time interval was calculated as the time (days) from the date when the first isolate was collected in each household (source-case) to the date when the first isolate was collected from each secondary case.
(ii) Rate calculation.
Assuming that the first case in each household was the source-case (2) and that this case infected all other persons in the household who subsequently developed disease, the rate at which variant isolates appeared (RV) (variant cases per source-case per year) as function of ongoing transmission (in the different households) can be described by the equation RV = CV/Nt0/tav, where CV is the number of cases with variant isolates (excluding subsequent transmission of the variant strain), Nt0 is the total number of source-cases, and tav is the average appearance time interval (years) where tav = ti/T, ti is the sum of the appearance time intervals (years), and T is the number of transmission events.
The rate at which invariant isolates appeared (RIV) (invariant cases per source-case per year) as a function of ongoing transmission is calculated as RIV = CIV − Nt0/Nt0/tav, where CIV is the total number of cases with invariant isolates.
Statistical analysis.
Fisher's exact test was used to test whether changes in the IS6110 banding pattern were associated with the number of IS6110 insertions present in the evolving strain. In addition, Fisher's test was used to establish whether the appearance of variant isolates was related to a previous history of disease. The Mann-Whitney test was used to determine whether the appearance of variant isolates was related to the median sample collection period, thereby demonstrating a correlation between appearance and time.
RESULTS
Study population.
During the period from mid-1992 to the end of 1998, M. tuberculosis isolates were cultured from 865 patients (representing a recovery of approximately 70% of all culture-positive patients) resident and attending the health care clinics in a suburb of Cape Town, South Africa (Fig. 1) (3, 23). Forty-six of these patients (5%) were excluded from the study because their cultures were either contaminated or lost viability. Of the remaining patients, 817 (99.7%) had an M. tuberculosis isolate genotypically classified by IS-3′ DNA fingerprinting (Fig. 1) (18, 21).
FIG. 1.
Flow chart showing patients included in the study.
M. tuberculosis isolates collected from epidemiologically related patients.
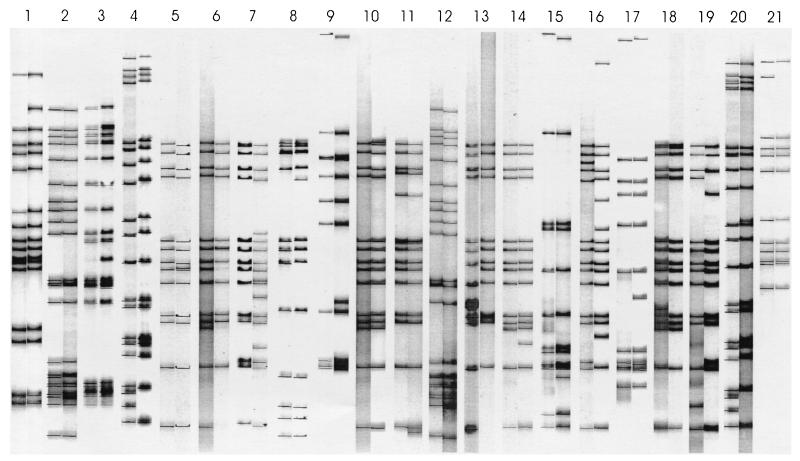
To investigate the relationship between transmission and the appearance of strain variants, all patients who were epidemiologically linked (because they reside in the same household or adjacent households) were identified. A total of 248 patients from 105 households met the inclusion criteria. In 83 households, transmission of an invariant strain from 83 source-cases to 107 secondary cases was observed (Fig. 1). In the remaining 22 households, the appearance of a variant strain or strains was observed during ongoing transmission. Four of the households representing patients infected with a variant strain were excluded (four source-cases and four secondary cases), because it was suspected that these patients might have been reinfected by a community contact. In the remaining 18 households, 21 patients were infected with a variant M. tuberculosis isolate originating from 18 source-cases (11 patients infected with an invariant isolate were also identified in these 18 households) (Fig. 1). Analysis of the IS6110 banding pattern changes (Fig. 2) showed that 13 (62%) of the variant strains evolved by replicative transposition, while 6 (29%) evolved by deletion of one or more IS6110 elements. Only 1 (4.5%) strain evolved by band shifts, while another strain (4.5%) evolved by a combination of replicative transposition and band shifts. This suggests that replicative transposition is the predominant evolutionary mechanism.
FIG. 2.
IS6110 RFLPs of M. tuberculosis isolates collected from epidemiologically linked patients. Each pair of lanes shows the IS6110 banding pattern of the source case (left) and the variant secondary case (right) resident in the same or neighboring household.
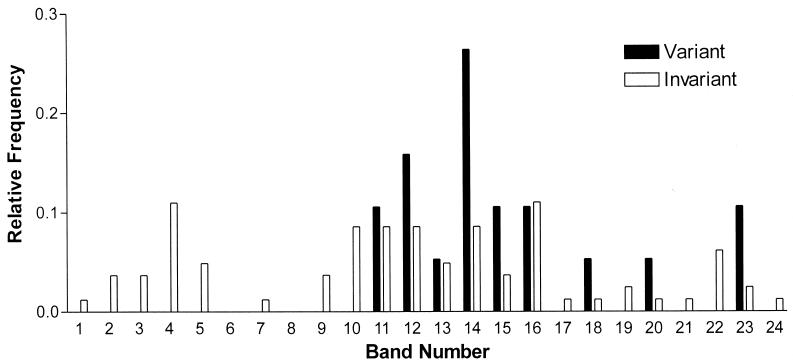
Figure 3 shows a plot of the frequency of observed banding pattern changes as a function of IS6110 copy number. Two major strain groups representing either low- or high-copy-number IS6110 strains were analyzed, because it has previously been suggested that these groups represent different evolutionary lineages that evolve independently (10, 25). The absence of IS6110 banding pattern changes in strains with <6 IS6110 insertions is significant (Fisher's exact test, P = 0.013), demonstrating that the low-copy-number IS6110 strains evolve at a different rate from the high-copy-number IS6110 strains. For this reason, the low-copy-number strains have been excluded from any rate calculations (see below).
FIG. 3.
Relative frequency of source-case strains generating either variant or invariant secondary cases in epidemiologically related episodes as a function of the number of IS6110 insertions.
Comparison of patients who were infected by a variant strain and those infected by an invariant strain did not identify significant demographic differences between the two patient groups (Table 1). Therefore, it is unlikely that the variant strains arose due to patient-specific macro factors, which could stimulate IS6110-mediated genome variation. Review of the clinical records of variant secondary cases showed that in 19 cases (90.5%), the observed banding pattern changes occurred prior to the initiation of antituberculosis therapy. Therefore, it is unlikely that tuberculosis therapy influences genomic evolution. The appearance of variant isolates was not associated with a previous episode of disease (Fisher's exact test, P = 0.62). However, there is a weakly significant difference (Mann-Whitney test, P = 0.043) in the median time interval (between the first isolate of the source-case and the first isolate of the secondary case) for the patients with variant and invariant strains. This could suggest that the longer the time interval between infection and the development of disease, the greater the chance of observing change.
TABLE 1.
Demographics of patients recently infected within a householda
| Strain type | No. of patients | No. (%) male | Mean age (yr) | No. (%) with previous episode of tuberculosis | No. (%) with pulmonary tuberculosis | Median time (days) |
|---|---|---|---|---|---|---|
| Variant | 21 | 14 (67) | 34 ± 11 | 10 (48)b | 19 (90) | 739c |
| Invariant | 92 | 50 (54) | 33 ± 12 | 36 (39) | 91 (99) | 392 |
Shown are results for patients recently infected (excluding the source-case) in which the infecting M. tuberculosis strain has >5 IS6110-hybridizing elements and is either variant or invariant from the source-case strain.
P = 0.62 (Fisher's exact test).
P = 0.043 (Mann-Whitney test).
Given the epidemiological links for patients residing in the households studied, it is likely that any changes in the IS6110 banding pattern must have occurred during the interval between the collection of the first isolate (source-case) and the first variant isolate. Based on the assumption that the first patient to present with tuberculosis in each household infected all of the patients who subsequently presented with disease with a similar genotype (2), a rate of appearance of IS6110 variants can be expressed as a function of both time and number of infectious sources. By using the rate calculation RV = CV/Nt0/tav, the minimum average rate for which variant high-copy-number strains appear as tuberculosis cases is RV = 0.140 variant cases/source-case/year. The rate of appearance of invariant isolates is RIV = 0.614 invariant cases/source-case/year.
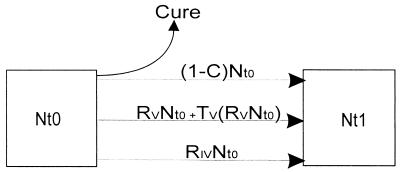
To determine the influence of genotypic variation on the bacterial population structure as defined by IS-3′ RFLP analysis, it is assumed that each household represents a subset of the study population. Within each household, strains can be broadly categorized as those that transmit and cause disease in a secondary case and those that do not cause disease in a secondary case within the study period. Because genotypic variation could not be identified in the latter, calculations have focused on determining a rate of change for strains which were transmitted and had the propensity to change (only high-copy-number strains with an IS6110 copy number of >5). We propose a simple model to describe the total number of variant and invariant strains (Nt1) appearing in the household population as a function of both transmission and evolution within a defined time interval (Fig. 4). From this model, the rate of genotypic change is calculated as Nt1 = [RV × Nt0 + TV(RV × Nt0) + RIV × Nt0] × t1 +(1 − C)Nt0.
FIG. 4.
Model describing genotypic change in the bacterial population. At the onset of the study time interval (t0), the number of source-cases will be Nt0. During the following time interval (t1), these cases will transmit disease to contacts within their respective households. The rate at which the contacts develop disease with a variant strain will be RV × Nt0. The rate of appearance of an invariant strain will be RIV × Nt0, while the rate of transmission of the variant strains will be TV(RV × Nt0), where TV is a transmission rate. It is assumed that a proportion (C) of the source-cases will be cured. The number of infected patients at time t1 will be Nt1.
Applying this formula to the data (Table 2), 82 source-cases would yield 113 secondary cases (assuming that all the source-cases were cured during this time interval). Twenty-one of these cases will be expected to be newly evolved strain variants. This suggests that the genotypic population structure in this patient group will change by 18.6% within the study period of approximately 6.5 years.
TABLE 2.
Characteristics of M. tuberculosis isolates with >5 IS6110-hybridizing elements transmitted within the context of different households
| Characteristic | Abbreviation of variable | Result |
|---|---|---|
| Sum of appearance intervals (yr) | ti | 206.5 |
| Total no. of transmission events | T | 113 |
| Avg appearance interval (yr) | tav = ti/T | 1.83 |
| No. of source-cases | Nt0 | 82 |
| No. of variant cases | CV | 21 |
| No. of invariant cases | CIV − Nt0 | 92 |
Cluster analysis of the DNA fingerprint database showed that the variant source-cases subsequently infected five patients. This result confirms that variant strains are amplified by secondary transmission and thereby will alter the genotypic bacterial population structure as a function of time.
DISCUSSION
The advent of molecular epidemiology has permitted great advances in the study of tuberculosis and other infectious diseases. While the techniques needed for molecular epidemiological studies have become largely standardized, the interpretation of molecular epidemiological data remains dependent on a number of assumptions. One such assumption is that genotypic identity is a measure of recent epidemiological events, allowing an estimation of the degree to which disease is due to recent or ongoing transmission (1, 11, 16). However, in the molecular epidemiological analysis of M. tuberculosis, this criterion excludes any change in the IS6110 banding patterns associated with transmission, and therefore such isolates may be incorrectly classified as representing remote epidemiological events. The data presented in this study suggest that pattern changes will occur in strains with a high IS6110 copy number and that the impact of these changes on epidemiological analysis should be considered.
A number of practical and theoretical reasons exist for excluding strains differing by one or two bands from RFLP-defined clusters. Foremost among these are the simplicity of dichotomously classifying isolates as either having the exact match or not. While the imposition of identical matching may not always be appropriate, the alternative of accepting subtle alterations in patterns is problematic, because isolates from chains of transmission will develop increasingly divergent patterns. Determining at what point the pattern is no longer similar enough then becomes an arbitrary process. In theory, this process may be facilitated through understanding the evolution of pattern change. However, previously published observations demonstrate that a single DNA fingerprinting probe is not able to accurately define evolutionary direction (9). Thus, a database may contain an RFLP pattern, A, for which there are two similar patterns (B and C), but it is usually not possible to determine if both B and C derive from A (at which point, all three patterns should belong to the same cluster) or whether B derived from A, which itself derived at some prior time from C (in which case, the epidemiologically relevant cluster may include only A and B). Further complicating the interpretation is that a molecular clock of the IS6110 banding pattern during ongoing transmission is required to suggest rules by which variant strains should be included in or excluded from the clusters. As DNA fingerprinting databases become larger, representing longer time intervals and isolates from wider geographic regions, these questions will become more and more important. This is particularly the case in geographical regions where there is a high incidence of disease and large populations of genotypically related strains are found (24).
In an effort to address some of these questions, this study analyzed the stability of the IS6110 banding pattern during the transmission of a strain from a source-case to a close contact. Because transmission involves the passage of a strain from a person with disease to a contact (who subsequently developed disease), it is conceivable that evolutionary change will occur at any point within this time interval. Preliminary analysis of serial isolates of M. tuberculosis showed that evolutionary events are more likely to have occurred prior to sampling (data not shown); therefore, to study change, it is more appropriate to identify the original source-case strain for comparison. In this study, a significant number of IS6110 band pattern changes were observed during transmission of M. tuberculosis isolates with >5 copies of IS6110. This ability to change was not found to be associated with either the patient demographics or the exposure to antituberculosis therapy. This implies that antituberculosis drugs in their present form do not significantly influence molecular strain identity, and therefore the observed evolutionary changes reflect a natural genetic flux. The identification of chromosomal alterations permitted the calculation of a rate of appearance of 0.14 variant cases per source-case per year. The calculated rate implies that while the IS6110 banding pattern remains stable in the majority of epidemiologically linked events, changes in a subset of transmission events are expected to alter the genotypes observed by 18.6% over the course of a 6.5-year study (2.9% per year). Because the patients included in this study are a subset of the tuberculosis patients in the community, this rate of change applies to the broader tuberculosis patient population and suggests that RFLP variants will also occur during transmission events that do not occur within households. The use of the absolute genotypic identity of strains with >5 IS6110 copies to estimate transmission in a community is therefore expected to result in an underestimate of transmission.
The strong association between the ability to change and the number of IS6110 insertions demonstrates that mutation mediated by IS6110 in low-copy strains is significantly slower than that detected in the high-copy strains. Furthermore, no correlation between the number of IS6110 elements per genome and evolutionary rate could be identified. Therefore, it would appear more prudent for molecular epidemiological calculations to treat these strain groupings separately rather than assuming that characteristics identified in one group of strains can be extrapolated to another group of strains. For this reason, a rate of change was calculated with only the transmission data from patients infected with high-copy-number strains.
The calculations presented herein also highlight the importance of the rate of appearance of invariant strains. The absence of evolution within this group suggests that while genotypes are expected to evolve during an epidemiological study, certain genotypes will also persist for extended periods within a study population, representing endemic strains. The observation of such a genotype more than once during a study period may therefore represent either a new transmission event or independent reactivation events. The interpretation of such observations may be aided by further epidemiological information, including the pretest likelihood of ongoing transmission suggested by additional knowledge about tuberculosis transmission in that community. For these numerous reasons, the use of identical genotypes to estimate recent epidemiological events requires due consideration.
Acknowledgments
This work was made possible grants from the GlaxoSmithKline Action TB Initiative, the Sequella Global Tuberculosis Foundation and the National Research Foundation (THRP).
E. Engelke, S. Carlini, and M. De Kock are thanked for technical assistance.
REFERENCES
- 1.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Drucker, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., S. A. Warren, H. Salamon, P. C. Hopewell, A. Ponce de Leon, C. L. Daley, and P. M. Small. 1999. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444-449. [DOI] [PubMed] [Google Scholar]
- 3.Beyers, N., R. P. Gie, H. L. Zietsman, M. Kunneke, J. Hauman, M. Tatley, and P. R. Donald. 1996. The use of a geographical information system (GIS) to evaluate the distribution of tuberculosis in a high-incidence community. S. Afr. Med. J. 86:40-41, 44. [PubMed] [Google Scholar]
- 4.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275:452-457. [PubMed] [Google Scholar]
- 5.Classen, C. N., R. Warren, M. Richardson, J. H. Hauman, R. P. Gie, J. H. Ellis, P. D. van Helden, and N. Beyers. 1999. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax 54:136-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daley, C. L., P. M. Small, G. F. Schecter, G. K. Schoolnik, R. A. McAdam, W. R. Jacobs, Jr., and P. C. Hopewell. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N. Engl. J. Med. 326:231-235. [DOI] [PubMed] [Google Scholar]
- 7.de Boer, A. S., M. W. Borgdorff, P. E. de Haas, N. J. Nagelkerke, J. D. van Embden, and D. van Soolingen. 1999. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J. Infect. Dis. 180:1238-1244. [DOI] [PubMed] [Google Scholar]
- 8.de Boer, A. S., K. Kremer, M. W. Borgdorff, P. E. W. de Haas, H. F. Heersma, and D. van Soolingen. 2000. Genetic heterogeneity in Mycobacterium tuberculosis isolates reflected in IS6110 restriction fragment length polymorphism patterns as low-intensity bands. J. Clin. Microbiol. 38:4478-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang, Z., N. Morrison, B. Watt, C. Doig, and K. J. Forbes. 1998. IS6110 transposition and evolutionary scenario of the direct repeat locus in a group of closely related Mycobacterium tuberculosis strains. J. Bacteriol. 180:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fomukong, N., M. Beggs, H. el Hajj, G. Templeton, K. Eisenach, and M. D. Cave. 1997. Differences in the prevalence of IS6110 insertion sites in Mycobacterium tuberculosis strains: low and high copy number of IS6110. Tuber. Lung Dis. 78:109-116. [DOI] [PubMed] [Google Scholar]
- 11.Glynn, J. R., J. Bauer, A. S. de Boer, M. W. Borgdorff, P. E. Fine, P. Godfrey-Faussett, and E. Vynnycky. 1999. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int. J. Tuberc. Lung Dis. 3:1055-1060. [PubMed] [Google Scholar]
- 12.Hermans, P. W., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, and D. Frommel. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 13.McAdam, R. A., P. W. Hermans, D. van Soolingen, Z. F. Zainuddin, D. Catty, J. D. van Embden, and J. W. Dale. 1990. Characterization of a Mycobacterium tuberculosis insertion sequence belonging to the IS3 family. Mol. Microbiol. 4:1607-1613. [DOI] [PubMed] [Google Scholar]
- 14.Niemann, S., E. Richter, and S. Rüsch-Gerdes. 1999. Stability of Mycobacterium tuberculosis IS6110 restriction fragment length polymorphism patterns and spoligotypes determined by analyzing serial isolates from patients with drug-resistant tuberculosis. J. Clin. Microbiol. 37:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemann, S., S. Rüsch-Gerdes, E. Richter, H. Thielen, H. Heykes-Uden, and R. Diel. 2000. Stability of IS6110 restriction fragment length polymorphism patterns of Mycobacterium tuberculosis strains in actual chains of transmission. J. Clin. Microbiol. 38:2563-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 17.Thierry, D., M. D. Cave, K. D. Eisenach, J. T. Crawford, J. H. Bates, B. Gicquel, and J. L. Guesdon. 1990. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 18:188.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, D. A. Enarson, N. Beyers, and P. D. van Helden. 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341:1174-1179. [DOI] [PubMed] [Google Scholar]
- 20.van Soolingen, D., P. E. W. de Haas, R. M. Blumenthal, K. Kremer, M. Sluijter, J. E. M. Pijnenburg, L. M. Schouls, J. E. R. Thole, M. W. G. Dessens-Kroon, J. D. A. van Embden, and P. W. M. Hermans. 1996. Host-mediated modification of PvuII restriction in Mycobacterium tuberculosis. J. Bacteriol. 178:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Soolingen, D., P. E. de Haas, P. W. Hermans, and J. D. van Embden. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235:196-205. [DOI] [PubMed] [Google Scholar]
- 22.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren, R., J. Hauman, N. Beyers, M. Richardson, H. S. Schaaf, P. Donald, and P. van Helden. 1996. Unexpectedly high strain diversity of Mycobacterium tuberculosis in a high-incidence community. S. Afr. Med. J. 86:45-49. [PubMed] [Google Scholar]
- 24.Warren, R., M. Richardson, S. G. D. van der Spuy, T. Victor, S. Sampson, N. Beyers, and P. van Helden. 1999. DNA fingerprinting and molecular epidemiology of tuberculosis: use and interpretation in an epidemic setting. Electrophoresis 20:1807-1812. [DOI] [PubMed] [Google Scholar]
- 25.Warren, R. M., S. L. Sampson, M. Richardson, G. D. van der Spuy, C. J. Lombard, T. C. Victor, and P. D. van Helden. 2000. Mapping of IS6110 flanking regions in clinical isolates of M. tuberculosis demonstrates genome plasticity. Mol. Microbiol. 37:1405-1416. [DOI] [PubMed] [Google Scholar]
- 26.Yeh, R. W., A. Ponce de Leon, C. B. Agasino, J. A. Hahn, C. L. Daley, P. C. Hopewell, and P. M. Small. 1998. Stability of Mycobacterium tuberculosis DNA genotypes. J. Infect. Dis. 177:1107-1111. [DOI] [PubMed] [Google Scholar]