Abstract
In order to investigate the possible role of Ixodes ricinus as a vector of zoonotic Babesia microti infection in Europe, a European rodent isolate (HK) and a zoonotic American isolate (GI) were studied in transmission experiments. PCR detected B. microti in the blood and spleens of infected gerbils (Meriones unguiculatus) and also in laboratory-induced infections of I. ricinus ticks. B. microti DNA was detected by PCR in all pooled samples of nymphs and the majority of adults that had fed as larvae and nymphs, respectively, on gerbils with acute infection of the European isolate, confirming that I. ricinus could serve as a vector in Europe. The American isolate, GI, proved to be equally infective for larval and nymphal I. ricinus as the HK strain, despite a very different appearance in gerbil erythrocytes. Nymphs infected with the HK and GI strains readily infected gerbils. In contrast to the finding in acute infections, ticks that fed on gerbils with chronic infections of HK and GI did not become infected. It was also found that the HK strain was not transmitted transovarially. The finding that a B. microti strain (GI) from a distant geographical region (United States) can infect and be transmitted by I. ricinus suggests that other European B. microti strains, in addition to the HK strain used here, are probably infective for I. ricinus, supporting the view that infection of humans with European B. microti may be a regular occurrence.
Babesia microti is a tick-transmitted rodent blood parasite that was first reported as a cause of human disease in 1969 in the northeastern United States (23). Several hundred cases are now reported from this region each year (7). The disease is characterized by a gradual onset of malaise with anorexia, fever, headaches, myalgia, and other vague symptoms which may persist for long periods. Occasionally dangerous fulminating infections occur, particularly in immunocompromised or aged individuals. The vector is the tick Ixodes scapularis, and transmission is transstadial, the infection being acquired from mice (e.g., the white-footed mouse, Peromyscus leucopus) by larval or nymphal ticks and transmitted by nymphs or adults (5). Transovarial transmission, which occurs in other Babesia species, such as B. canis and B. bovis, has not been reported in B. microti (19).
Babesia microti is also present throughout Europe, but no verified cases of human disease have been reported. This may be because the rodent tick Ixodes trianguliceps, implicated as the main vector in Europe (5, 7), does not bite humans. However, it has been shown that at least one European strain of B. microti may be transmitted by Ixodes ricinus (21), which is known to be a vector of several zoonotic diseases (4), and seroprevalence studies suggest that infection of humans with B. microti may occur in Europe (6, 9). In the present study, the infectivity for I. ricinus of a European strain and a zoonotic American strain of B. microti was investigated by PCR and by transmission to and from gerbils (Meriones unguiculatus).
MATERIALS AND METHODS
Experimental animals.
The gerbils used in this study were males, 12 to 16 weeks old, and were obtained from Harlan United Kingdom, Bicester, Oxon, United Kingdom. Their care and use complied with European Community Directive 86/609/EC.
B. microti isolates.
The European (HK) strain was originally isolated from a microtine rodent, the bank vole (Clethrionomys glareolus), in the Hannover area of Germany (8). The American (GI) strain of B. microti was isolated from a human case in Nantucket, Mass. (14). Both strains were obtained from the Department of Tropical Public Health, Harvard University, Boston, Mass. (courtesy of S. R. Telford III) as infected fresh hamster blood. The infected blood was inoculated into gerbils and subpassaged at least 10 times in order to obtain gerbil-adapted strains. The initial infections were weak and transient, but by the tenth passage parasitemias in excess of 20% in the case of HK and 50% in the case of GI were regularly obtained. Spontaneous recovery then usually occurred, and the gerbils maintained a carrier status for an indefinite period.
Transmission of B. microti from gerbils to I. ricinus ticks.
Donor gerbils were infected with cryopreserved stabilates of B. microti, and the resulting infections were then inoculated intraperitoneally into experimental animals in doses of 106 infected erythrocytes diluted in phosphate-buffered saline (PBS). Ticks were exposed to infection with B. microti by infesting pairs of infected gerbils. Larvae and nymphs were exposed to rising parasitemias of both B. microti strains, and larvae were also exposed to chronic infections of both strains at low parasitemias, following spontaneous recovery of gerbils.
Larvae from a pathogen-free laboratory culture of I. ricinus were applied by brush (approximately 300 per animal) to gerbils that had been lightly anesthetized by sodium pentobarbitone intraperitoneal injection. Gerbils were infested with nymphs from the same culture by applying 10 to each animal by brush and a further 20 contained in 1-cm-diameter plastic capsules attached with impact adhesive (Evostic; Evode Ltd, Stafford, United Kingdom) under light halothane anesthesia. The animals were maintained over trays of water from which detached engorged ticks were harvested. Engorged ticks were maintained at 20°C and 92% relative humidity and analyzed for infection by PCR approximately 4 weeks after molting.
Transmission of B. microti to gerbils by infected ticks.
Two experiments were performed to demonstrate the infectivity of nymphal ticks that had been exposed to B. microti infection as larvae.
(i) Experiment 1.
Five gerbils were infested with 35 nymphs each that were infected with the HK B. microti strain as larvae approximately 4 months earlier. Twenty of the nymphs were confined in capsules as described above, and 15 were applied by brush.
(ii) Experiment 2.
Three gerbils were infested with 30 nymphs each (20 in capsules) that were infected as larvae with the GI B. microti strain approximately 4 months earlier.
In both experiments, thin blood smears were made daily from day 7 postinfestation and stained with Giemsa stain in order to detect parasites by microscopy. Blood samples were also analyzed by PCR once parasites had been observed in thin blood smears
Transstadial persistence and transovarial transmission studies.
PCR analysis was used to investigate transstadial persistence and transovarial transmission of the HK infection in two experiments in which infected ticks were fed on laboratory rabbits, which are refractory to B. microti. Infection persisting in the absence of reinfection was investigated by feeding 40 infected nymphs on the clipped and shaved back of a 10-week-old New Zealand laboratory rabbit, within a 5-cm capsule attached with latex adhesive (Copydex; Henkel Adhesives Ltd., Winsford, Cheshire, United Kingdom). The adults that developed from the engorged nymphs were analyzed for infection by PCR.
Transovarial transmission was investigated by feeding 20 infected female ticks (accompanied by uninfected males) on a rabbit and examining the resulting larvae by PCR (pools of 100 larvae from each of 10 larval batches) and by xenodiagnosis using 10 gerbils (approximately 300 larvae per gerbil from each of 10 larval batches). The larvae were applied by brush, and thin blood smears were taken daily from day 7 postinfestation for the detection of parasites.
PCR analysis. (i) DNA purification.
DNA was extracted from gerbil whole blood, gerbil spleen, and nymphal and adult I. ricinus ticks by the use of the QIAamp DNA mini kit (Qiagen, Hilden, Germany), applying the blood and fluid and tissue protocols, respectively. Prior to extraction, ticks previously stored in 70% ethanol were placed in 1.5-ml microcentrifuge tubes containing 50 μl of ATL buffer (supplied in the kit) and disintegrated by cutting the ticks with the sharp edges of a disposable needle. The spleen, previously stored in ethanol, was divided into 10-mg portions, placed in microcentrifuge tubes, and cut into small pieces. After the addition of 150 μl and 180 μl of ATL buffer to the tube with the tick and the spleen samples, respectively, the tissue extraction protocol as described by the manufacturer was followed. The purified DNA on the spin column was eluted in 100 μl of AE buffer, and 10 μl of this extract was used as the substrate in the subsequent PCR assay.
(ii) PCR assay.
The PCR was performed in a 50-μl reaction mixture containing 10 μl of purified B. microti DNA, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM each dATP, dCTP, dGTP, and dTTP, 1 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, Calif.), and 0.4 μM each of two B. microti-specific primer pairs: Bab1 (5′-CTTAGTATAAGCTTTTATACAGC-3′) and Bab4 (5′-ATAGGTCAGAAACTTGAATGATACA-3′) designed for the 18S rRNA gene (13).
Amplification was performed in a GeneAMP PCR System 2400 (Applied Biosystems) starting with a pre-PCR heat step of 12 min at 94°C to activate the heat-activated DNA polymerase, followed by a touchdown protocol involving 12 cycles with denaturation at 94°C for 20 s, annealing temperature starting at 67°C for 30 s, and then lowered 1°C every cycle until reaching 55°C, and extension at 72°C for 30 s. The touchdown program was followed by 30 cycles of 94°C for 20 s, 55°C and 72°C for 30 s, and ending at 72°C for 5 min. The 238-bp amplification product was visualized on an ethidium bromide-stained 2% agarose gel and documented in a Gel Doc 2000 (Bio-Rad Laboratories, Hercules, Calif.).
(iii) Sequencing of the PCR product.
The 238-bp PCR product was purified using the spin column technique (Jet Quick Genome Inc.) and sequenced by using Bab1 as the sequencing primer and the Big Dye Terminator Cycle sequencing ready reaction kit (Applied Biosystems) according to the manufacturer's protocol. The products were further purified by ethanol-sodium acetate precipitation before capillary electrophoresis was performed on the ABI Prism 310 genetic analyzer (Applied Biosystems).
RESULTS
Parasite morphology.
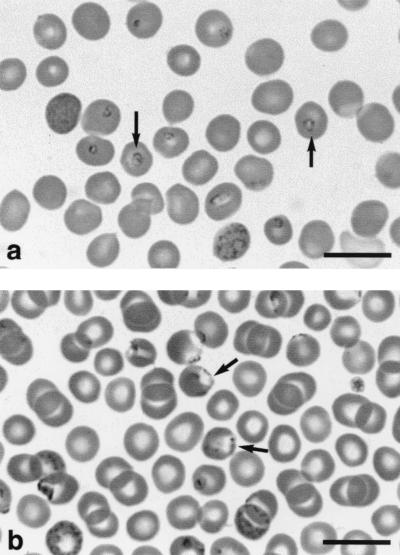
Thin blood smears stained with Giemsa stain showed that although the European and American strains of B. microti are regarded as belonging to the same species, their appearance in gerbil erythrocytes is different (Fig. 1). Whereas the European HK strain occurs mainly centrally in erythrocytes, the zoonotic American GI strain usually occurs on the periphery of erythrocytes and has a blister-like appearance. These characteristics are sufficiently consistent for the two strains to be very readily differentiated in thin blood smears.
FIG. 1.
B. microti in gerbil erythrocytes. (a) HK (European) strain, showing parasites in central position (arrows). (b) GI (American) strain, showing parasites in peripheral position (arrows).
PCR.
Despite the different morphology of the two strains in gerbil erythrocytes (Fig. 1), the PCR system, designed for the 18S rRNA gene of the American strains (13), produced the same product from the European strain. Nucleotide sequencing of the 238-bp PCR product from the European HK strain showed 100% homology to the PCR product of the American GI strain and to the two sequences in this area described in GenBank (accession nos. U09833 and M93660).
Transmission of B. microti to I. ricinus ticks. (i) Exposure of larvae to acute infections.
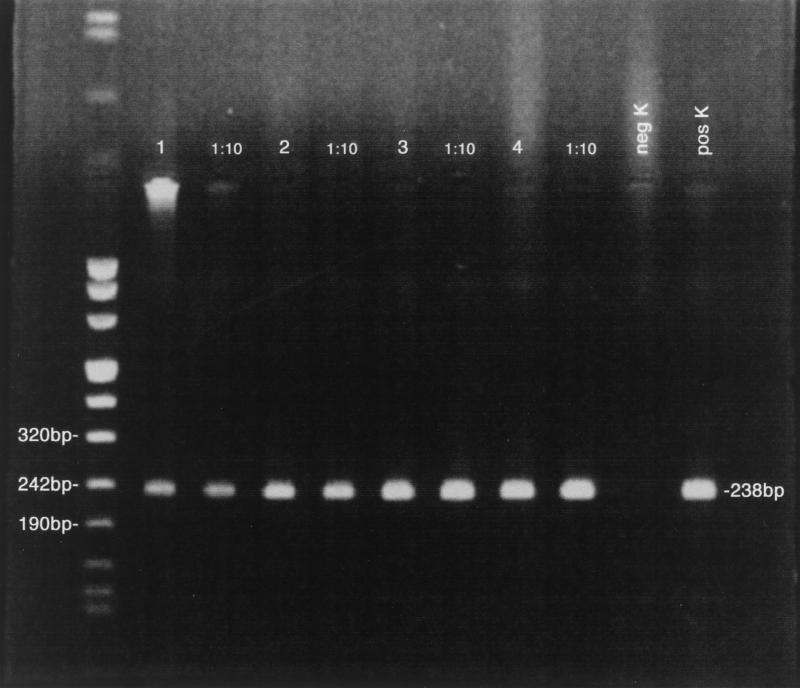
A total of 434 engorged larvae from HK-infected gerbils and 392 from gerbils infected with GI were collected. The parasitemias that the feeding larvae were exposed to ranged from 6 to 19% in the case of HK and 6 to 63% in the case of GI. The larvae were allowed to molt to nymphs, and 4 weeks after the start of the molt, 20 nymphs from each batch were placed in 70% alcohol for storage before being analyzed for infection by PCR in pools of two. For both HK (Fig. 2) and GI (data not shown), all 10 pools were positive for the infection (Table 1). The remaining nymphs were retained for the experiments on transmission to gerbils.
FIG. 2.
Detection by PCR (lanes 1 to 4 at 1:1 and 1:10 dilutions) of European (HK) B. microti (238-bp product) in I. ricinus nymphs exposed to acute infections as larvae. neg K and pos K, negative and positive controls, respectively.
TABLE 1.
Infection of I. ricinus larvae and nymphs by exposure to B. microti HK and GI strains in individual gerbils
| B. microti strain | Tick stage applied | No. of ticks applied | No. of engorged ticks collected | % Parasitemia during tick feeding | No. of ticks PCR positive 4 wk after molting |
|---|---|---|---|---|---|
| HK | Larva | 300 | 170 | 6.1-15.7 | 10/10 pools of 2 |
| 264 | 10.4-19.8 | 10/10 pools of 2 | |||
| Nymph | 30 | 23 | 1.4-13.1 | 4/5 females | |
| 22 | 4.5-15.1 | 3/5 males | |||
| GI | Larva | 300 | 240 | 11.6-52.7 | 10/10 pools of 2 |
| 151 | 5.6-63.3 | 10/10 pools of 2 | |||
| Nymph | 30 | 27 | 1.4-51.1 | 4/4 females | |
| 24 | 6.3-49.3 | 4/6 males |
(ii) Exposure of nymphs to acute infections.
Forty-five engorged nymphs were collected from HK-infected gerbils and 51 from GI-infected gerbils. They were exposed to parasitemias ranging from 5 to 13% and 9 to 51%, respectively. Samples of 10 adult ticks (5 females and 5 males) that developed from these nymphs were analyzed for infection by PCR. For the HK-infected ticks, 7 of 10 were positive, and for the GI-infected ticks, 8 of 10 were positive. In both cases, more females were positive than males (Table 1). Remaining HK adults were retained for the experiment on transovarial transmission.
(iii) Exposure of larvae to chronic infections.
Larvae were fed on gerbils with parasitemias ranging from 0.1 to 0.3% (HK) and 1.0 to 5.0% (GI), approximately 1 month after recovery from acute infections. The nymphs that molted from these larvae were analyzed by PCR. No positives were found in 20 nymphs (10 pools of two) exposed to the HK strain, and only one pool of the GI nymphs was positive out of 16 pools of 5 nymphs each. No positive samples were detected in a second PCR analysis of 22 GI nymphs from the same batch (11 pools of two nymphs each).
Transmission of B. microti to gerbils by infected ticks.
Nymphal I. ricinus infected with the HK and GI strains of B. microti were used to infest gerbils as described above. B. microti was detected in thin blood smears from all the gerbils 2 to 2.5 weeks later, demonstrating the infectivity of ticks infected with the European HK or the American GI B. microti strain (Table 2). Prepatent periods were slightly longer (3 to 5 days) compared with syringe infections (106 infected erythrocytes). HK maximum parasitemias were lower compared with syringe infections, but GI maximum parasitemias were similar. PCR analysis of blood samples confirmed the identity of the parasites as B. microti.
TABLE 2.
Transmission of HK and GI B. microti strains to gerbils by laboratory-infected I. ricinus nymphs
| B. microti strain | No. of unfed nymphs applied | No. of engorged nymphs collected | Prepatent period (days after infestation, >0.1% parasitemia) | Maximum parasitemia (%) |
|---|---|---|---|---|
| HK | 35 | 10 | 13 | 13.6 |
| 17 | 18 | 10.7 | ||
| 7 | 17 | 15.1 | ||
| 10 | 16 | 10.0 | ||
| 16 | 16 | 4.0a | ||
| GI | 30 | 2 | 18 | 55.1 |
| 21 | 14 | 58.4 | ||
| 17 | 14 | 62.7 |
Died on fourth day of patency.
Transstadial persistence and transovarial transmission.
Infected nymphs (exposed as larvae with HK) were fed on a rabbit, which is refractory to infection with B. microti, and 20 of these ticks were subsequently analyzed for infection by PCR after they had molted to adults. None of these adults proved positive by PCR, suggesting that B. microti does not persist in the tick beyond one instar, and infection of the tick must occur at the instar preceding the transmitting stage. Furthermore, the parasites were not transmitted transovarially following infection of nymphs on gerbils and feeding of the resulting adults on a rabbit. None of the 10 gerbils used to feed larvae from 10 batches became parasitemic, and PCR analysis of samples of at least 100 unfed larvae from each batch did not detect B. microti DNA.
DISCUSSION
I. ricinus is the most widespread and abundant ixodid tick in western Europe and frequently bites humans (2). Most human tick bites are caused by nymphs, but larvae and adult females are also involved (10, 11, 16).
I. ricinus is an important vector of zoonotic diseases, including Lyme borreliosis, now recognized as the most prevalent vector-borne disease in the temperate northern hemisphere. Less common diseases transmitted by this tick species include tick-borne encephalitis, ehrlichiosis, and babesiosis caused by B. divergens in immunocompromised patients. B. microti is well known as a cause of human disease in the United States (5), and cases have recently been documented in Japan (17, 22), but despite the presence of this parasite in Europe, no human cases have been confirmed.
The absence of human disease in Europe has been explained by the perception that the rodent tick, I. trianguliceps, is the main vector in Europe (5, 7). However, the present study has demonstrated the vector competence of I. ricinus for a European strain of B. microti, using PCR for detection of the parasite in ticks and gerbils for classical transmission studies, confirming the earlier observations of Walter and Weber (21). In addition, this study has shown that I. ricinus is an efficient vector of the zoonotic GI strain from North America, where it is transmitted by the related tick species I. scapularis.
The observation that I. ricinus transmits a parasite strain from a distant geographical region implies that other strains of B. microti occurring within the geographical range of I. ricinus may also be transmitted by this tick species, despite unpublished studies suggesting that in some areas I. ricinus is not vector competent for B. microti (L. Gern, personal communication). Further support for the suggestion that I. ricinus may be an important vector for B. microti is provided by a recent study concerning PCR detection of Babesia spp. in ticks collected in Slovenia (1). In this study, four gene sequences obtained from 364-bp fragments showed 100% homology with B. microti M93660 and 99.7% homology with B. microti U0833 (GenBank accession nos.).
For both the European HK strain and the American GI strain, transmission to ticks from gerbils only occurred during the acute phase of infections. Although the parasite appears to persist in the gerbil (at low parasitemias), transmission did not occur during this chronic phase. The European HK strain was isolated from the bank vole, Clethrionomys glareolus, and B. microti parasitemias can reach similar or higher levels in this host (15, 21) as in the gerbil. The lack of infectivity of gerbils in the chronic phase of the infection is similar to the situation described in the vole-I. trianguliceps model (15). However, unlike B. microti in voles, syringe transfer of infection in gerbils did not appear to reduce transmission to I. ricinus.
The present study also demonstrated that transstadial transmission (larva to nymph or nymph to adult tick) occurs but that the parasite does not persist beyond more than one molt and transovarial transmission does not occur. Transovarial transmission is probably more likely to occur when engorging female ticks are exposed to infection, but this cannot occur in nature because I. ricinus adult females do not engorge on the small rodent reservoir hosts of B. microti. In the absence of transovarial transmission, larval I. ricinus may be considered to pose no risk of infection with B. microti, unlike larvae infected with B. divergens (3). Nymphal bites of humans are far more frequent than adult female bites (more than 80% in the study by Robertson et al. [16]), and nymphs are therefore likely to pose the greatest risk when infected with zoonotic strains of B. microti.
The similarities and differences between the European and American B. microti strains were interesting. Both the American and the European strains were readily transmitted by I. ricinus, and the PCR primers designed for American B. microti (13) detected the American GI strain and the European HK strain with equal facility. However, the two strains are readily differentiated in gerbil blood smears, and other biological differences, including host specificity, may occur.
B. microti is a well-established entity as a human pathogen in the United States, whereas the zoonotic status of European strains of B. microti is unknown. Although no definite autochthonous human cases caused by European B. microti have been identified, serosurveys suggest that infection of humans may occur (6, 9), and one study has suggested that B. microti infection may underlie cases of Lyme borreliosis that show unusual symptoms and/or resistance to treatment (12).
In summary, our study has shown that I. ricinus can be infected by a European isolate of B. microti but also by a zoonotic American strain when feeding on acutely but not chronically infected gerbils. The transmission studies suggest that infection of humans with European B. microti may be a regular occurrence.
Acknowledgments
This research was supported in part by the Wellcome Trust.
We are grateful to Bernard Kaye and Annetta Zintl for assistance with the photography.
REFERENCES
- 1.Duh, D., M. Petrovec, and T. Avsic-Zupanc. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J. Clin. Microbiol. 39:3395-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estrada-Peña, A., and F. Jongejan. 1999. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23:685-715. [DOI] [PubMed] [Google Scholar]
- 3.Gray, J. S. 1991. The development and seasonal activity of the tick. Ixodes ricinus: a vector of Lyme borreliosis. Rev. Med. Vet. Entomol. 79:323-333. [Google Scholar]
- 4.Gray, J. S., and O. Kahl. 2001. Ticks as vectors of zoonotic pathogens in Europe, p. 547-551. In R. B. Halliday, D. E. Walter, H. C. Proctor, R. A. Norton, and M. J. Colloff (ed.), Acarology: Proceedings of the 10th International Congress. CIRO Publishing, Melbourne, Australia.
- 5.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunfeld, K. P., R. Allwinn, S. Peters, P. Kraiczy, and V. Brade. 1998. Serologic evidence for tick-borne pathogens other than Borrelia burgdorferi (TOBB) in Lyme borreliosis patients from mid-western Germany. Wien. Klin. Wochenschr. 110:901-908. [PubMed] [Google Scholar]
- 7.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 8.Krampitz, H. E. 1979. Babesia microti: morphology, distribution and host relationship in Germany. Zentralbl. Bakteriol. Orig. A 244:411-415. [PubMed] [Google Scholar]
- 9.Krampitz, H. E., H. Buschmann, and P. Münchoff. 1986. Gibt es latente Babesien-infektionen beim Menschen in Süddeutschland? Mitt. Österr. Ges. Tropenmed. Parasitol. 8:233-243. [Google Scholar]
- 10.Liebisch, A., and G. Liebisch. 1996. Hard ticks (Ixodidae) biting humans in Germany and their infection with Borrelia burgdorferi, p. 465-468. In R. Mitchell, D. J. Horn, G. R. Needham, and C. Welbourne (ed.), Acarology IX, vol. 1. The Ohio Biological Survey, Columbus, Ohio.
- 11.Maiwald, M., R. Oehme, O. March, T. N. Petney, P. Kimmig, K. Naser, H. A. Knappe, D. Hassler, and M. von Knebel Doebertitz. 1998. Transmission risk of Borrelia burgdorferi sensu lato from Ixodes ricinus ticks to humans in southwest Germany. Epidemiol. Infect. 121:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meer-Scherrer, L. 1999. Babesia-infectionen nun auch in der Scheiz? Diagnostica 52:3-4. [Google Scholar]
- 13.Persing, D. H., D. Mathiesen, W. F. Marshal, S. R. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30:2097-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piesman, J., S. J. Karakshian, S. Lewengrub, M. A. Rudzinska, and A. Spielman. 1986. Development of Babesia microti sporozoites in adult Ixodes dammini. Int. J. Parasitol. 16:381-385. [DOI] [PubMed] [Google Scholar]
- 15.Randolph, S. E. 1995. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus). Parasitology 110:287-295. [DOI] [PubMed] [Google Scholar]
- 16.Robertson, J. N., J. S. Gray, and P. Stewart. 2000. Tick bite and Lyme borreliosis risk at a recreational site in England. Eur. J. Epidemiol. 16:647-652. [DOI] [PubMed] [Google Scholar]
- 17.Saito-Ito, A., M. Tsuji, Q. Wei, S. He, T. Matsui, M. Kohsaki, S. Arai, T. Kamiyama, K. Hioki, and C. Ishihara. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J. Clin. Microbiol. 38:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebek, Z., B. Rosicky, and W. Sixl. 1977. The occurrence of babesiasis affecting small terrestrial mammals and the importance of this zoonosis in Europe. Folia Parasitol. (Praha) 24:221-228. [PubMed] [Google Scholar]
- 19.Spielman, A., M. L. Wilson, J. F. Levine, and J. Piesman. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu. Rev. Entomol. 30:439-460. [DOI] [PubMed] [Google Scholar]
- 20.Walter, G. 1984. Transmission and course of parasitemia of Babesia microti (Hannover I strain) in the bank vole (Clethrionomys glareolus) and field vole (Microtus agrestis). Acta Trop. 41:259-264. [PubMed] [Google Scholar]
- 21.Walter, G., and G. Weber. 1981. A study on the transmission (transstadial, transovarial) of Babesia microti, strain “Hannover i,” in its tick vector, Ixodes ricinus. Tropenmed. Parasitol. 32:228-230. [PubMed] [Google Scholar]
- 22.Wei, Q., M. Tsuji, A. Zamoto Kohsaki, M. T. Matsui, T. Shiota, S. R. Telford III, and C. Ishihara. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 39:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Western, K. A., G. D. Benson, N. N. Gleason, G. R. Healy, and M. G. Schultz. 1970. Babesiosis in a Massachusetts resident. N. Engl. J. Med. 283:854-856. [DOI] [PubMed] [Google Scholar]