Abstract
One hundred twenty-six urease-negative gastric biopsy specimens were evaluated for the presence of Helicobacter genus-specific 16S ribosomal DNA (rDNA) and H. pylori-specific glmM DNA sequences by PCR. The species specificity of the glmM PCR assay was demonstrated, as H. pylori was the only Helicobacter species that yielded the expected glmM amplicon. Most urease-negative specimens (118 of 126 specimens) lacked Helicobacter DNA. However, 8 of 126 urease-negative specimens contained Helicobacter 16S rDNA. In order to identify the Helicobacter species present in urease-negative gastric biopsy specimens, 16S rDNA amplicons were cloned and sequenced. Sequence comparisons were performed by analyses of the sequences in public sequence databases. Two samples contained 16S rDNA that was identified as H. cinaedi with 100% identity and that spanned approximately 400 bp (398 and 398 bp, respectively). In contrast, multiple differences (97% identity; 390 of 398 bp) were observed with H. pylori 16S rDNA in this region. This finding was verified by sequencing an overlapping 537-bp fragment within the 5′ portion of 16S rDNA. Although the clinical findings were consistent with H. pylori infection (e.g., duodenal ulcer disease), rapid urease testing and DNA sequence analyses suggested the presence of H. cinaedi organisms and the absence of H. pylori in two human antral biopsy specimens. This study represents the first report of an enteric urease-negative helicobacter in the human stomach. Although these organisms were previously associated with extragastric infections, the roles of these organisms in the pathogenesis of chronic gastritis or peptic ulcer disease remain unclear.
The genus Helicobacter consists of 24 formally recognized species, 7 of which have been associated with human disease. The best studied of these is the gastric pathogen Helicobacter pylori. H. pylori infection is commonly diagnosed by testing of gastric biopsy specimens by the rapid urease test (RUT). In addition to urease testing, other methods include smear evaluation, culture, and antigen detection. Molecular methods, such as PCR, have also been used to detect H. pylori but are used only in the research setting (12). The sensitivity of testing by RUT is generally greater than 90% (5) and provides a useful tool for screening of gastric biopsy specimens for the presence of H. pylori. However, the interpretation of results can be difficult when equivocal results are obtained. In a previous study, we found that most RUT-negative specimens were negative by DNA amplification. We detected Helicobacter DNA in several RUT-negative samples (8). Since urease production is characteristic of H. pylori, we wanted to determine if urease-negative, Helicobacter DNA-positive samples contained H. pylori.
Gastric biopsy specimens were obtained from patients by esophagogastroduodenal (EGD) endoscopy and inoculated into RUT kits (CLOtest; Ballard Medical Products, Draper, Utah). One hundred twenty-six gastric biopsy specimens were recovered from RUT-negative specimens. The biopsy specimens were processed and evaluated for the presence of Helicobacter genus-specific 16S ribosomal DNA (rDNA; 422 bp) (forward primer, 5′-GCT ATG ACG GGT ATC C; reverse primer, 5′ -GAT TTT ACC CCT ACA CCA) and H. pylori-specific glmM DNA (forward primer, 5′-AAG CTT ACT TTC TAA CAC TAA CGC; reverse primer, 5′-AAG CTT TTA GGG GTG TTA GGG GTT T) (6) sequences by PCR, as described previously (8).
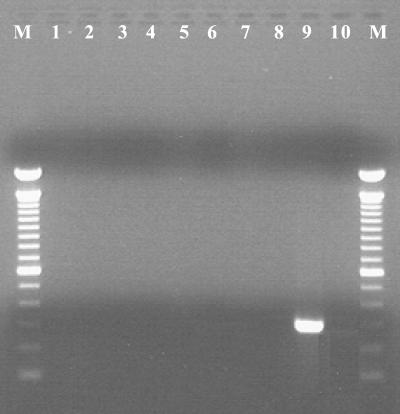
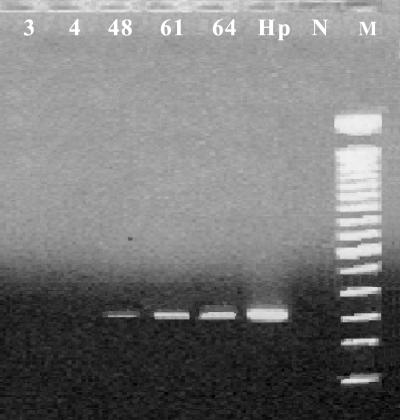
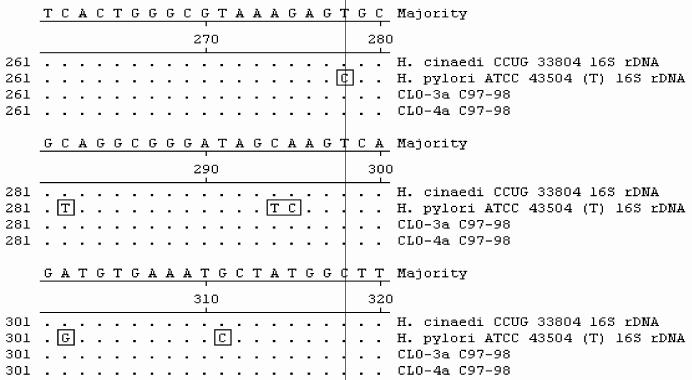
All biopsy specimens positive for the 16S rDNA but negative for glmM amplicons were further analyzed by DNA sequencing. Two overlapping portions of the 16S rDNA were cloned into pCR4-TOPO vectors (Invitrogen, Carlsbad, Calif.) and sequenced. Segments of the 16S rRNA gene were analyzed by PCR amplification and sequencing analysis. PCR conditions were as follows: an initial denaturation (94°C, 5 min), followed by 40 cycles of denaturation (94°C, 30 s), annealing (50°C, 1 min), and extension (72°C, 2 min), with a final extension (72°C, 8 min). Sequencing was performed in an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) by use of ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction kit chemistry, according to the recommendations of the manufacturer. DNA sequence comparisons were performed by analyses of the sequences in public sequence databases (GenBank and Ribosomal Database Project II) with the Blastn and Sequence Match tools. To determine the specificity of glmM sequences as markers for H. pylori, we evaluated the specificity of glmM amplification with various gastric and enteric helicobacters (Fig. 1). Most urease-negative specimens (118 of 126) lacked Helicobacter DNA. Helicobacter 16S rDNA was detected in 8 of 126 (6%) specimens, while they appeared to be urease negative by RUT. Of these eight specimens, three tissue samples tested positive for glmM DNA, indicating the presence of H. pylori, and were not further analyzed. Three of the remaining five gastric biopsy specimens yielded glmM DNA at low levels, while two specimens were negative for glmM amplicons (Fig. 2). Sequence analysis of the bacterial 16S rDNA sequences of glmM DNA-positive specimens 48, 61, and 64 confirmed the presence of H. pylori. Two glmM DNA-negative samples (specimens 3 and 4) contained 16S rDNA sequences identified as H. cinaedi with 100% identity. These sequences spanned nucleotide positions 270 to 668 (398 and 398 bp, respectively). The presence of H. cinaedi in both patients was verified by sequencing an overlapping 537-nucleotide fragment within the 5′ portion of 16S rDNA, representing nucleotides 8 through 544 (660 bp of the 16S rRNA gene was sequenced in total). Gastric biopsy specimens 3 and 4 failed to yield glmM amplicons. In contrast, multiple differences (97% identity; 390 of 398 bp) were observed when Helicobacter-specific sequences were compared with the H. pylori 16S rDNA sequence (Fig. 3). In one patient, the EGD endoscopic impression was consistent with erosive gastritis and a normal duodenum. Upon histopathologic inspection, eosinophilic gastritis with antral pit abscesses and lymphoid aggregates in the fundic mucosa were reported in this patient. In the second patient, duodenal ulcer disease with a normally appearing gastric mucosa was visualized by EGD endoscopy. Interestingly, the second patient had a history of colitis with histopathologic findings consistent with lymphocytic colitis.
FIG. 1.
Species specificity of glmM primers for H. pylori. Lanes: M, 100-bp molecular mass marker; 1, H. bilis; 2, H. canis; 3, H. cholecystus; 4, H. cinaedi; 5, H. hepaticus; 6, H. mustelae; 7, H. muridarum; 8, H. pullorum; 9, H. pylori; 10, negative. Only H. pylori yielded the 294-bp glmM amplicon among the various species of Helicobacter tested.
FIG. 2.
Detection of H. pylori in gastric biopsy samples by glmM PCR. PCR amplification of glmM DNA sequences shows that specimens 3 and 4 lacked H. pylori DNA, while specimens 48, 61, and 64 were faintly positive for H. pylori glmM DNA. Lane Hp, H. pylori Sydney strain control; lane N, negative control; lane M, 100-bp molecular mass marker.
FIG. 3.
Sequence alignment of 16S rRNA gene fragments from biopsy specimens and GenBank sequences. Sequences from biopsy samples (CLO-3a and -4a) yielded 100% identity with the sequence of H. cinaedi CCUG 33804, while they had less than 97% identity with the sequence of H. pylori. Multiple-nucleotide differences between H. pylori and the H. cinaedi sequences are boxed.
H. cinaedi-like organisms were detected in human gastric tissue by molecular methods. Although the clinical findings were consistent with H. pylori infection (e.g., gastritis and duodenal ulcer disease), RUT, PCR, and DNA sequence analysis confirmed the presence of Helicobacter organisms other than H. pylori. The presence of H. cinaedi organisms in antral biopsy specimens from at least two patients was substantiated by molecular studies.
H. cinaedi causes gastroenteritis (9) and extraintestinal infections, especially in immunocompromised patients, as reviewed elsewhere (1). H. cinaedi bacteremia has been associated with multifocal cellulitis and monoarticular arthritis. This organism has not previously been documented in the gastric antrum. Gastric helicobacters other than H. pylori have been associated with gastritis and peptic ulcer disease in humans, including “H. heilmannii” (2), while H. felis was detected in a gastric biopsy specimen of a Melanesian patient with dyspepsia (3). “H. heilmannii” has been associated with gastritis (4) but has rarely been recovered by culture. Due to the special requirements for culture and isolation of gastric and enteric helicobacters (12), molecular biology-based diagnostic methods facilitate the detection and identification of these organisms in gastric biopsy specimens. An earlier study reported on the molecular biology-based detection of urease-negative H. pylori (10) in chronic gastritis patients, but that organism may have been misidentified. Using several parameters for diagnosis of H. pylori, Ren et al. (10) determined that several chronic gastritis patients were infected with H. pylori, even though they were negative by the CLOtest, culture, and glmM PCR, because biopsy samples were positive for ahpC and histopathologic inspection showed helical bacterial forms. The 26-kDa protein encoded by ahpC, originally considered a specific marker for H. pylori, was subsequently shown to be elaborated by a number of helicobacters, including H. cinaedi (7). It may be possible that these reported urease-negative organisms were not strains of H. pylori but urease-negative helicobacters such as H. cinaedi. A recent study underlines the importance of accurate interpretation of 16S rRNA sequence information for helicobacters, including H. cinaedi (11). Our findings suggest that enterohepatic helicobacters such as H. cinaedi may be considered potential etiologic agents of human gastroduodenal disease. Further investigations of the role of H. cinaedi in gastroduodenal disease are warranted.
This study represents the first report of a urease-negative, enterohepatic helicobacter in the human stomach. Although these organisms were previously associated with extragastric infections, the role of these organisms in the pathogenesis of chronic gastritis or peptic ulcer disease remains unclear.
Acknowledgments
We acknowledge support from the Crohn's & Colitis Foundation First Award (to J.V.) and National Institutes of Health grant awards K08 DK02705 (to J.V.), R01 CA67529 (to J.G.F.), and R01 DK52413 (to J.G.F.).
REFERENCES
- 1.Burman, W., D. Cohn, R. Reves, and M. Wilson. 1995. Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin. Infect. Dis. 20:564-570. [DOI] [PubMed] [Google Scholar]
- 2.Debongnie, J., M. Donnay, J. Mairesse, V. Jamy, X. Dekoninck, and B. Ramdani. 1998. Gastric ulcers and Helicobacter heilmannii. Eur. J. Gastroenterol. Hepatol. 10:251-254. [DOI] [PubMed] [Google Scholar]
- 3.Germani, Y., C. Dauga, P. Duval, M. Huerre, M. Levy, G. Pialoux, P. Sansonetti, and P. Grimont. 1997. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H. felis in a human gastric biopsy. Res. Microbiol. 148:315-326. [DOI] [PubMed] [Google Scholar]
- 4.Heilmann, K., and F. Borchard. 1991. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological and ultrastructural findings. Gut 32:137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laheij, R., W. de Boer, J. Jansen, H. van Lier, P. Sneeberger, and A. Verbeek. 2000. Diagnostic performance of biopsy-based methods for determination of Helicobacter pylori infection without a reference standard. J. Clin. Epidemiol. 53:742-746. [DOI] [PubMed] [Google Scholar]
- 6.Lu, J.-J., C.-L. Perng, R.-Y. Shyu, C.-H. Chen, Q. Lou, S.-K. Chong, and C.-H. Lee. 1999. Comparison for five PCR methods for detection of Helicobacter pylori in gastric tissues. J. Clin. Microbiol. 37:772-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundstrom, A., V. Sundaeus, and I. Bolin. 2001. The 26-kilodalton, AhpC homologue, of Helicobacter pylori is also produced by other Helicobacter species. Helicobacter 6:44-54. [DOI] [PubMed] [Google Scholar]
- 8.Peña, J., J. Fox, M. Ferraro, and J. Versalovic. 2001. Molecular resistance testing of Helicobacter pylori in gastric biopsies. Arch. Pathol. Lab. Med. 125:493-497. [DOI] [PubMed] [Google Scholar]
- 9.Quinn, T., S. Goodell, C. Fennell, S. Wang, M. Schuffler, K. Holmes, and W. Stamm. 1984. Infections with Campylobacter jejuni and Campylobacter-like organisms in homosexual men. Ann. Intern. Med. 101:187-192. [DOI] [PubMed] [Google Scholar]
- 10.Ren, Z., G. Pang, R. Batey, D. Rougley, A. Russel, M. Musicka, M. Dunkley, K. Beagley, and R. Clancy. 2000. Non-urease producing Helicobacter pylori in chronic gastritis. Aust. N. Z. J. Med. 30:578-584. [DOI] [PubMed] [Google Scholar]
- 11.Shen, Z., Y. Feng, F. Dewhirst, and J. Fox. 2001. Coinfection of enteric Helicobacter spp. and Campylobacter spp. in cats. J. Clin. Microbiol. 39:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Versalovic, J., and J. Fox. 1999. Helicobacter, p. 727-738. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.