Rapid-cycle real-time PCR by the LightCycler (Roche Molecular Biochemicals, Auckland, New Zealand) provides a very quick, one-step means of genotyping herpes simplex virus (HSV) from clinical samples and a number of assays involving this application have been reported (1, 2, 3).
However, we—among others—have found that a commonly used protocol (1) provides poor genotyping results when the published reagent concentrations are used. The PCR resulted in the “hook effect” phenomenon (Fig. 1) that is the sign of a very efficient PCR (Technical note no. LC 8/99, Roche Molecular Biochemicals, 1999). Simply, in later cycles of the PCR, the amplified strands reanneal before the probes can bind to generate fluorescence. For quantification, this is not an issue, since the crossing point (the cycle number where the reaction enters exponential phase) is of interest. However, subsequent genotyping by melting-curve analysis may be more difficult.
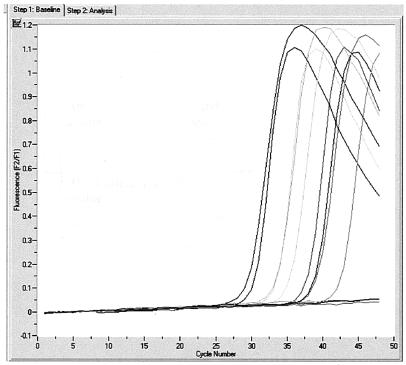
FIG. 1.
Amplification of HSV type 1 (HSV-1) and HSV-2 samples with published reagent concentrations. The drop in fluorescence at later cycle numbers caused by the hook effect is clearly visible.
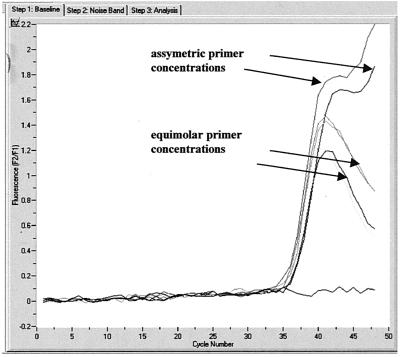
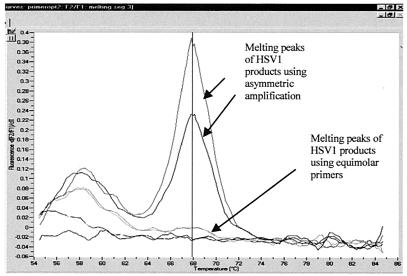
We overcame this phenomenon by the use of asymmetric primer concentrations whereby a higher concentration of the reverse primer (compared to that of the forward primer) was used in the reaction. This resulted in more of the strand complementary to the probes being amplified and allowed more signal to be generated (Fig. 2). This in turn resulted in the easy genotyping of the HSV samples via melting-curve analysis (Fig. 3).
FIG. 2.
Amplification of HSV-1 samples.
FIG. 3.
Melting-curve analysis of HSV-1 samples.
We suggest that the use of asymmetric primer concentrations be considered when new LightCycler genotyping assays that result in the hook effect on the quantitation screen are being optimized.
ADDENDUM IN PROOF
Since the submission of this manuscript, Burggraf et al. have described the benefits of asymmetric primer concentrations for genotyping the M129V polymorphism associated with human prion disease (S. Burggraf et al., Clin. Chem. 48:199-201, 2002). However, equimolar primer concentrations in this assay result in a less-pronounced hook effect than that seen with the HSV assay used here.
REFERENCES
- 1.Espy, M. J., J. R. Uhl, P. S. Mitchell, J. N. Thorvilson, K. A. Svien, A. D. Wold, and T. F. Smith. 2000. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J. Clin. Microbiol. 38:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler, H. H., G. Muhlbauer, B. Rinner, E. Stelzl, A. Berger, H.-W. Dorr, B. Santner, E. Marth, and H. Rabenau. 2000. Detection of herpes simplex virus DNA by real-time PCR. J. Clin. Microbiol. 38:2638-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schalasta, G., A. Arents, M. Schmid, R. W. Braun, and G. Enders. 2000. Fast and type-specific analysis of herpes simplex virus types 1 and 2 by rapid PCR and fluorescence melting-curve-analysis. Infection 28: 85-91. [DOI] [PubMed] [Google Scholar]