Abstract
NO SINGLE NONINVASIVE TEST for pulmonary embolism is both sensitive and specific. Some tests are good for “ruling in” pulmonary embolism (e.g., helical CT) and some tests are good for “ruling out” pulmonary embolism (e.g., D-dimer); others are able to do both but are often nondiagnostic (e.g., ventilation–perfusion lung scanning). For optimal efficiency, choice of the initial diagnostic test should be guided by clinical assessment of the probability of pulmonary embolism and by patient characteristics that may influence test accuracy. This selective approach to testing enables pulmonary embolism to be diagnosed or excluded in a minimum number of steps. However, even with the appropriate use of combinations of noninvasive tests, it is often not possible to definitively diagnose or exclude pulmonary embolism at initial presentation. Most of these patients can be managed safely without treatment or pulmonary angiography by repeating ultrasound testing of the proximal veins after one and 2 weeks to detect evolving deep vein thrombosis. Helical CT and MRI are rapidly improving as diagnostic tests for pulmonary embolism and are expected to become central to its evaluation.
Objective testing for pulmonary embolism is crucial, because clinical assessment alone is unreliable and the consequences of misdiagnosis are serious. Failure to diagnose pulmonary embolism is associated with high mortality,1,2 and incorrect diagnosis of the condition unnecessarily exposes patients to the risks of anticoagulant therapy. This review will outline approaches to the diagnosis of pulmonary embolism that minimize the use of pulmonary angiography, based on 2 guiding principles. In order for a test, or combination of tests, to be considered accurate enough to diagnose the presence of pulmonary embolism, it should have a positive predictive value of 85%. To exclude the presence of pulmonary embolism, such a test should have a negative predictive value of 95%, as compared with pulmonary angiography, or be associated with no more than a 2% frequency of venous thromboembolism during follow-up if it is the basis for withholding treatment.
Aspects of the epidemiology and natural history of venous thromboembolism that are relevant to the optimal selection and interpretation of diagnostic tests for pulmonary embolism are listed in Box 1.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 The presence of risk factors identifies patients in whom it is appropriate to have a low threshold for the investigation of pulmonary embolism.51,52 Some risk factors, such as previous venous thromboembolism,53,54 recent surgery,53,54 malignant disease53 and advanced age,54 help to discriminate between those with, and without, pulmonary embolism.
Box 1.

The sequential course of venous thromboembolism, with progression from the stages of deep vein thrombosis in the calf to proximal deep vein thrombosis and subsequently to pulmonary embolism,2,52 has a number of important diagnostic and management implications. First, identifying asymptomatic deep vein thrombosis can, indirectly, establish the diagnosis of pulmonary embolism; this is very helpful when initial tests for pulmonary embolism are nondiagnostic.20,55 Second, if proximal deep vein thrombosis can be excluded, there is a low short-term risk of pulmonary embolism among patients with nondiagnostic tests at presentation.56,57,58 Third, if proximal deep vein thrombosis is excluded at presentation and does not develop within 2 weeks, patients with nondiagnostic tests for pulmonary embolism have a low long-term risk of subsequent venous thromboembolism.56,57,58
Clinical assessment
Clinical assessment is considered here within the framework of diagnostic tests that influence the probability of pulmonary embolism.59,60,61 Approaches to clinical assessment of pulmonary embolism have fallen into 2 categories: (1) empirical (nonstandardized)59,60,61,62 and, more recently, (2) standardized clinical models or prediction rules.53,54,57
Empirical clinical assessment
In the PIOPED and McMaster studies,59,60 which assessed the accuracy of ventilation–perfusion lung scanning, the clinical probability of pulmonary embolism was categorized as either low, intermediate or high, based on history, physical examination, chest radiograph, electrocardiogram, and either impedance plethysmography of the legs60 or arterial blood gases.59 The prevalence of pulmonary embolism in each of these clinical probability categories, established by pulmonary angiography in patients with abnormal perfusion scans, was 15%, 38% and 79% in the McMaster study60 and 9%, 30% and 68% in the PIOPED study.59 In the Pisa-PED study, which was similar to the McMaster and PIOPED studies but assessed the accuracy of perfusion scanning alone and performed pulmonary angiography less consistently, the prevalence of pulmonary embolism was reported as 9%, 47% and 91% for the low, intermediate and high clinical probability categories.61 More recently, using mostly noninvasive tests as the criterion standard, Perrier and colleagues recorded prevalences of pulmonary embolism of 8%, 36% and 67% based on empirical clinical assessment.62
Standardized clinical assessment
Three research groups have recently published explicit prediction rules for determining the clinical probability of pulmonary embolism.53,54,57,63 Wells and colleagues used an assessment of symptoms and signs, the presence of an alternative diagnosis to account for the patient's presentation and the presence of risk factors for venous thromboembolism to categorize a patient as having low, intermediate or high probability of pulmonary embolism.57 A simplified version of their original model (Box 2) yielded a prevalence of pulmonary embolism of 2% in low-probability (40% of patients), 19% in intermediate-probability (52% of patients) and 50% in high-probability (8% of patients) categories.53 This clinical model has been prospectively validated by its use, in conjunction with other tests, to manage outpatients with suspected pulmonary embolism successfully (see later).58 The Pisa-PED group used an assessment of symptoms and chest radiograph and electrocardiogram findings to divide patients into either high-probability (92% prevalence of pulmonary embolism) or low-probability (11% prevalence of pulmonary embolism) categories.63 These investigators then proposed a 3-category clinical model, based on these criteria and the presence of an alternative diagnosis, to account for the patient's symptoms.63 They subsequently reported that this model yielded prevalences of pulmonary embolism in the low-probability, intermediate-probability and high-probability groups of 2%, 50% and 100%.64 Based on data from 2 prospective studies, Perrier and colleagues65,66 derived a continuous scoring system for the probability of pulmonary embolism that included 8 clinical, blood gas or chest radiograph variables.54 When scores were partitioned into 3 probability categories, the associated prevalences of pulmonary embolism were 10%, 38% and 81%, similar to the results that these investigators obtained using empirical assessment.54 It is important to note that standardized clinical models for the probability of pulmonary embolism may have lower predictive values when used in a setting other than that in which they were derived.67 Differences among centres in the mix of patients who are referred for diagnostic testing may influence the discriminatory value of clinical variables and partly account for this.54,67,68
Box 2.
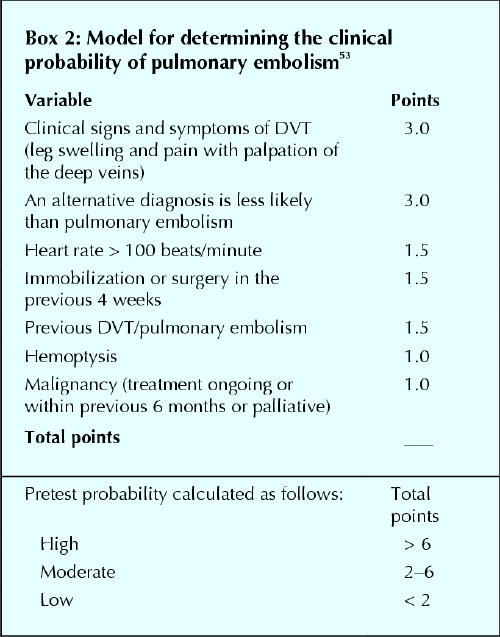
In summary, there is good evidence that clinical assessment, either empirical or standardized, can stratify patients' probability of having pulmonary embolism. The prevalence of pulmonary embolism is expected to be ≤ 10% in patients with a low clinical probability, about 25% in the intermediate-probability group and ≥ 60% in the high clinical probability group.
D-dimer blood testing
D-dimer is formed when cross-linked fibrin is lysed by plasmin, and elevated levels usually occur with pulmonary embolism.69 However, because elevations of D-dimer are nonspecific (e.g., increased by aging, inflammation, cancer), an abnormal result has a low positive predictive value.69 The value of D-dimer is that a negative result can help to exclude pulmonary embolism. There are a wide variety of D-dimer assays, some of which are not suitable as diagnostic tests for pulmonary embolism because they have such poor operating characteristics (i.e., they are inaccurate).69 D-dimer assays that have been validated as tests for pulmonary embolism vary in their sensitivity and specificity, partly because of differences in their accuracy and partly because of the cutoff value they use to define normality (i.e., trade-off between sensitivity and specificity). In practice, depending largely on their sensitivity and associated negative likelihood ratio, D-dimer assays that are valid diagnostic tests for pulmonary embolism can be divided into 2 categories.
Very highly sensitive D-dimer tests
These D-dimer assays have a sensitivity for venous thromboembolism of about 98% or higher.66,69 Their negative likelihood ratio is high enough to “rule out” pulmonary embolism in all patients and, consequently, these assays can be used as a “stand-alone” test for the exclusion of pulmonary embolism.66 However, these assays generally have a low specificity (about 40%) and a high frequency of false-positive results (e.g., 53%),66 which reduces their clinical usefulness. Many conventional enzyme-linked immunosorbent assay (ELISA) D-dimer assays (cut-off of about 500 fibrinogen-equivalent units/mL) fall into this category, but they are not suitable as diagnostic tests because they have a slow turnaround time and require batch analysis.69 “Rapid” ELISA D-dimer assays have recently been developed.69 Perrier and colleagues have shown that one such assay (Vidas DD, bioMérieux, Marcy l'Étoile, France), the results of which were normal in 36% of consecutive outpatients with suspected pulmonary embolism, had a negative predictive value of 100% for subsequent symptomatic venous thromboembolism.66
Moderate-to-highly sensitive D-dimer tests
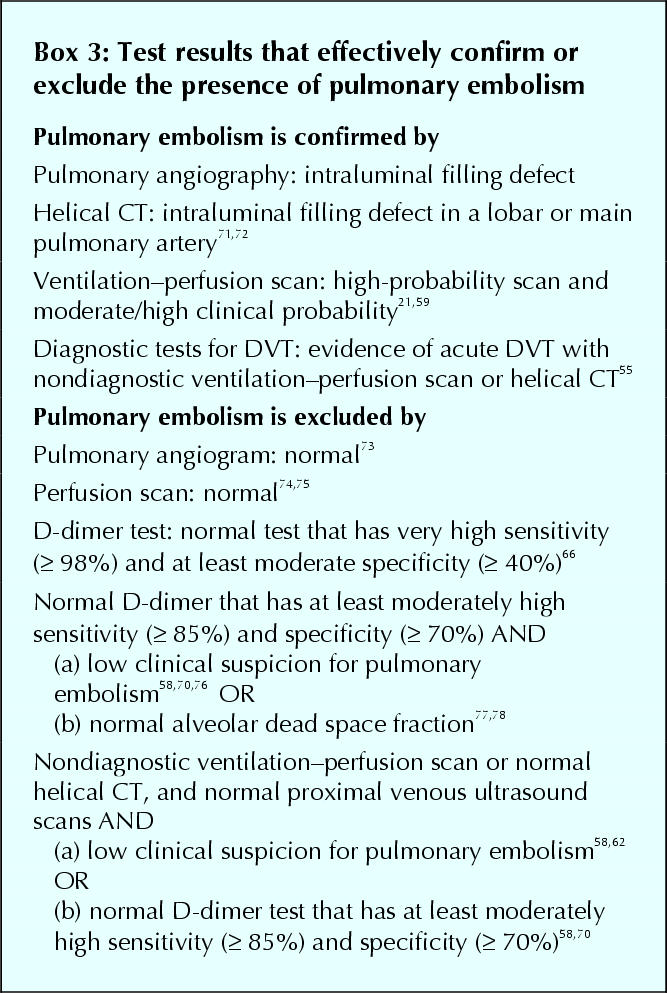
These D-dimer assays have a sensitivity for venous thromboembolism of about 85%–98%.69 The negative likelihood ratio and predictive value with these tests are not high enough to rule out pulmonary embolism in consecutive patients. Consequently, a normal result needs to be combined with another assessment that identifies patients as having a lower pretest probability for pulmonary embolism (e.g., low clinical probability,58,70 nondiagnostic lung scan,58,70 high alveolar dead space fraction77,78). Although neither test on its own can rule out pulmonary embolism, this is achieved by using the 2 tests in combination (Box 3). Such D-dimer assays are more specific than very sensitive D-dimer assays and, therefore, generate fewer false-positive results (e.g., 32%).70 A whole-blood D-dimer assay (SimpliRED, Agen Biomedical, Brisbane, Australia), which can be performed at the bedside in minutes, is one such test that has been extensively evaluated (approximate sensitivity 85%, approximate specificity 70%).58,70,78,79
Box 3.
Ventilation–perfusion lung scanning
Ventilation–perfusion lung scanning has been the usual initial investigation in patients with suspected pulmonary embolism. A normal perfusion scan excludes pulmonary embolism,74,75,80,81 but is found in a minority (about 25%) of patients.21,55,57,59,61,76 Perfusion defects are nonspecific, however, with only about one-third of patients with defects having pulmonary embolism.55,59,60,61,76,81 The probability that perfusion defects are due to pulmonary embolism increases with increasing size and number, the presence of a wedged shape and the presence of a normal ventilation scan (“mismatched” defect).59,60,61 Mismatched perfusion defects that are segmental or larger are termed “high-probability” defects.60 A single mismatched defect is associated with a prevalence of pulmonary embolism of about 80%, whereas this prevalence is ≥ 90% with 3 or more defects.82 High-probability scans occur in about 50% of patients with pulmonary embolism59,60 and about 10% of patients who are tested for pulmonary embolism.57,59,60,83 Therefore, about 65% of patients with suspected pulmonary embolism have intermediate-probability or lower-probability lung scans (see later) and require further testing.57,59,60,83
Computed tomography
Traditional computed tomography (CT) is not suitable for evaluating suspected pulmonary embolism, because it is not feasible to opacify the pulmonary arteries with radiographic contrast for the time required to complete imaging (about 3 minutes) and, even if this could be achieved, motion artifact would interfere with image quality. These problems are overcome by helical CT (also known as spiral or continuous volume CT) as image acquisition can be completed within a single breath hold (e.g., about 20 seconds).84,85 Although helical CT is widely used in clinical practice, 2 recent systematic reviews of studies that evaluated the accuracy of helical CT for the diagnosis of pulmonary embolism concluded that the technique has been inadequately evaluated for this purpose.71,86 Since these reviews, 2 studies have helped to clarify the accuracy, strengths and limitations of helical CT for the diagnosis of pulmonary embolism.72,85,87 In the first, among 299 patients who did not have pulmonary embolism excluded by a negative highly sensitive D-dimer result (pulmonary embolism prevalence of 39%), helical CT had a sensitivity of 70%, a specificity of 91%, a positive likelihood ratio of 8.0, a negative likelihood ratio of 0.3, an overall positive predictive value of 84% and a negative predictive value of 82%.72 The positive predictive value of CT varied by anatomical level: 100% in main pulmonary arteries, 85% in lobar and only 62% in segmental (16% abnormal CT results) pulmonary arteries. Subsegmental pulmonary arteries were not systematically evaluated in this study.72 In the second study, which prospectively compared helical CT to diagnostic lung scanning (normal or high-probability scans) or pulmonary angiography in 230 patients, helical CT had sensitivities of 86% for segmental or larger pulmonary embolisms and 21% for subsegmental pulmonary embolisms (21% of total pulmonary embolisms).85,87 Overall sensitivity for pulmonary embolism was 69% and specificity was 86%.85,87
The combined results from a number of studies suggest that the sensitivity of helical CT for isolated subsegmental pulmonary embolism is about 30%86,87 and that such emboli account for about 20% of symptomatic pulmonary embolism.29,30,31,86,88,89 Because patients with isolated subsegmental pulmonary embolism are also likely to have a substantial risk of recurrence, these emboli cannot be dismissed as clinically unimportant.
Taken together, these findings suggest the following results with helical CT. First, intraluminal filling defects in lobar or main pulmonary arteries have a positive predictive value for pulmonary embolism of at least 85% and can be interpreted in the same way as a high-probability ventilation–perfusion scan. Second, intraluminal defects that are confined to segmental, and particularly subsegmental, pulmonary arteries are nondiagnostic and require further testing. Third, a normal helical CT substantially reduces the probability of pulmonary embolism but does not exclude this diagnosis (i.e., is similar to a “low-probability” ventilation–perfusion scan). A frequency of pulmonary embolism of about 5%, during follow-up or at pulmonary angiography, in patients with nondiagnostic lung scans, normal helical CT scans and normal venous ultrasonography emphasizes that a normal CT scan alone does not exclude pulmonary embolism.72,90,91,92
Magnetic resonance imaging (MRI) has been less well evaluated than helical CT for the diagnosis of pulmonary embolism; however, it appears to have similar accuracy.31,93,94,95 Both helical CT and MRI have the advantage that they may reveal an alternative pulmonary diagnosis, and both examinations may be extended to look for concomitant deep vein thrombosis. MRI also avoids exposure to radiation and radiographic contrast. It is anticipated that the diagnosis of pulmonary embolism by CT and MRI will continue to improve, and modern scanners may already be more accurate than those used in published studies using older technology.85
Tests for deep vein thrombosis
Detection of asymptomatic deep vein thrombosis is an indirect way to diagnose pulmonary embolism.20,55 In the presence of acute pulmonary embolism, deep vein thrombosis is detectable by bilateral ascending venography in about 75%21,22,23 of patients and by compression ultrasonography of the proximal veins in about 50%21,25 of patients (i.e., sensitivity for pulmonary embolism of 75% and 50% respectively). However, among patients with symptomatic pulmonary embolism, there are strong correlations among (1) pulmonary embolism size, (2) the presence of diagnostic findings on ventilation–perfusion scanning or helical CT and (3) the presence of proximal deep vein thrombosis.25 Consequently, the proportion of patients with pulmonary embolism and nondiagnostic findings on ventilation–perfusion scanning (or helical CT) who will have detectable deep vein thrombosis will be lower than the values noted previously (about 30% for compression ultrasonography of the proximal veins).25,55
In practice, ultrasonography of the proximal veins is abnormal in about 5% of patients who have nondiagnostic lung scans.55,57,58,62,76,83 This ultrasound examination can be limited to an assessment of venous compressibility at the inguinal ligament and the mid-popliteal fossa without loss of sensitivity for proximal deep vein thrombosis.96 Because the positive predictive value of an abnormal ultrasound scan is only about 75% in this setting, confirmatory venography should be considered in patients who are more likely to have a false-positive result (e.g., less convincing ultrasound findings, previous venous thromboembolism with the potential for residual abnormalities, negative D-dimer).20,55 Normal bilateral proximal venous ultrasound scans or venograms do not rule out embolism in patients with nondiagnostic lung scans (or helical CT); however, they reduce this probability (negative likelihood ratios of about 0.7 for ultrasonography and about 0.5 for venography). Because absence of deep vein thrombosis is associated with a lower risk of recurrence among patients with pulmonary embolism,20,23,97 the negative likelihood ratios for symptomatic venous thromboembolism during follow-up with negative tests for deep vein thrombosis are expected to be lower than these estimates.
Pulmonary angiography
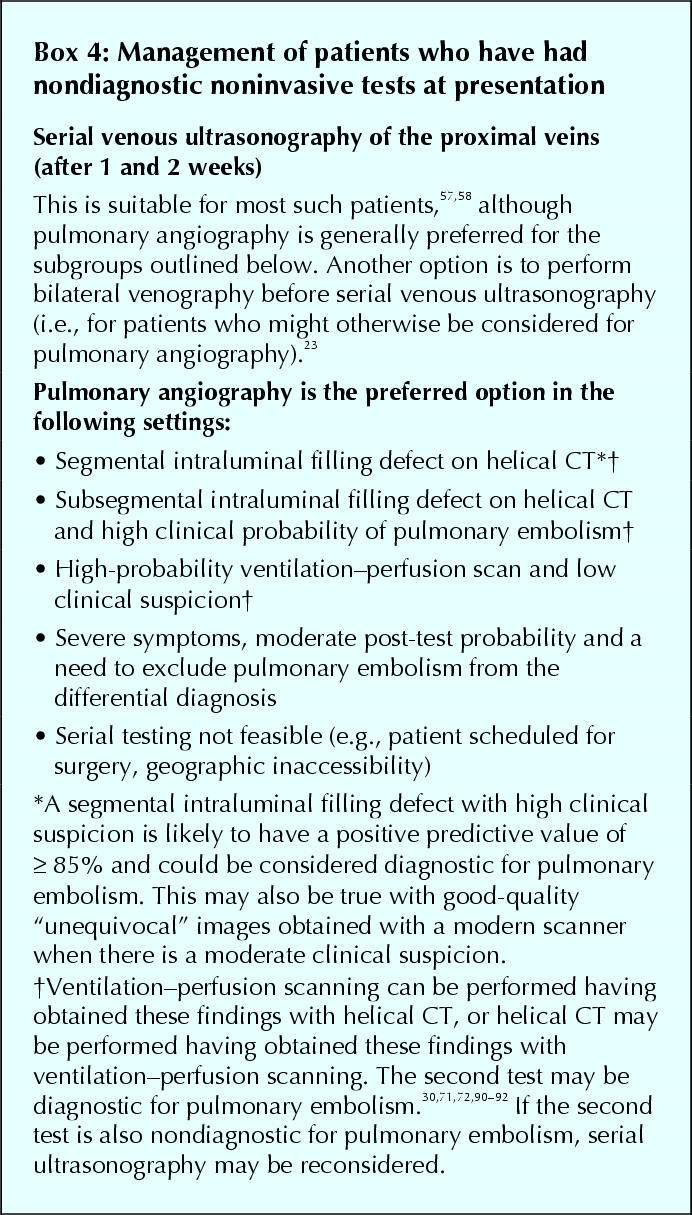
Pulmonary angiography is the criterion standard for the diagnosis of pulmonary embolism, but it is associated with serious side effects (e.g., mortality of about 0.5%),73 is technically demanding to perform, may be difficult to interpret and is costly. It is contraindicated in patients with renal impairment and may not be feasible in the sickest patients.59,60 For these reasons, pulmonary angiography is usually reserved for patients who have had nondiagnostic noninvasive tests for pulmonary embolism when it is considered unsafe to withhold anticoagulation, while performing serial testing to detect evolving proximal deep vein thrombosis (see later), or when it is necessary to establish a diagnosis to manage patients with severe symptoms (Box 4). Of patients with normal pulmonary angiograms, about 1% have an episode of symptomatic venous thromboembolism during the following 6 months;73,98 this is the standard against which the safety of withholding anticoagulant therapy following negative tests for pulmonary embolism is assessed.
Box 4.
Echocardiography
Transthoracic or transesophageal echocardiography may directly visualize embolized thrombi (right heart chambers or central pulmonary arteries) or show right heart hemodynamic changes that indirectly suggest pulmonary embolism.99 Indirect parameters such as unexplained right ventricular dilatation/dysfunction and marked tricuspid regurgitation, which can be detected similarly by transthroracic and transesophageal echocardiography, have a sensitivity of about 50% and a specificity of about 90% for pulmonary embolism.64,100,101,102,103 Transthoracic echocardiography visualizes intracardiac thrombi (usually right atrium) in about 5% of patients with acute pulmonary embolism and generally does not detect emboli in the pulmonary arteries.38,64,100,101,102 Transesophageal echocardiography can visualize thrombi in the central pulmonary arteries (main, right, proximal portion of left) with high specificity (> 90%),101,104 but its sensitivity has not been evaluated in unselected patients with pulmonary embolism (perhaps about 30%).
Because of the limited specificity with the transthoracic approach, the invasiveness of the transesophageal approach and the low sensitivity with both approaches, echocardiography is not suitable as a routine diagnostic test for pulmonary embolism. However, echocardiography shows indirect evidence of pulmonary embolism in about 80% of patients with massive embolism (i.e., ≥ 60% perfusion defects),100 and central emboli can be seen by transesophageal echocardiography in about 70% of the patients who have pulmonary embolism and right ventricular dysfunction.101,104,105 Consequently, echocardiography is valuable in differentiating between massive pulmonary embolism and other causes of hemodynamic compromise. In conjunction with clinical assessment and the results of other noninvasive tests (e.g., venous ultrasonography), echocardiography may enable pulmonary embolism to be diagnosed, or anticoagulants to be withheld, in severely ill patients, at least until it becomes feasible to perform additional testing.100 In addition to its diagnostic role, the echocardiographic finding of right ventricular dysfunction or patent foramen ovale in conjunction with pulmonary embolism indicates a relatively poor short-term prognosis and may encourage the use of more aggressive therapy for pulmonary embolism.2,38,99,106
Combinations of diagnostic tests for pulmonary embolism
When individual tests are nondiagnostic, it may be possible to combine their results to confirm or exclude pulmonary embolism (Box 3). Some of the better studied combinations are described below.
Clinical assessment and ventilation–perfusion lung scanning
The clinical assessment of pulmonary embolism is complementary to ventilation–perfusion lung scanning. A high-probability lung scan with a moderate or high clinical probability of pulmonary embolism is diagnostic (prevalence of pulmonary embolism of ≥ 90%).59,60 All other combinations of clinical probability and abnormal lung scan findings are associated with a prevalence of pulmonary embolism of 10%–50% and, therefore, require further investigation.59,60 Among the patients with these other combinations, the prevalence of pulmonary embolism varies as follows: about 50% with a low clinical suspicion and a high-probability scan, about 10% with a low clinical suspicion and subsegmental, matched perfusion defects (“low-probability” scans) and about 25%, on average, with other combinations.59,60
Clinical assessment and negative D-dimer testing
The combination of a low clinical probability and a negative moderately sensitive D-dimer assay (sensitivity ≥ 85%) has a negative predictive value for pulmonary embolism of about 99%.58,70,76 Two management studies have confirmed the safety of excluding pulmonary embolism with this combination of findings.58,76
Nondiagnostic lung scanning and negative D-dimer testing
The combination of a nondiagnostic lung scan (i.e., an abnormal lung scan result lower than high probability) and a normal moderately sensitive D-dimer assay has been estimated to have a negative predictive value of about 97%.70,76 This combination of findings is currently considered nondiagnostic, particularly if clinical probability is high.
Nondiagnostic lung scanning and normal ultrasound testing for proximal deep vein thrombosis
In general, the combination of a nondiagnostic lung scan and normal bilateral tests for deep vein thrombosis at presentation is nondiagnostic. However, because the negative predictive value of low clinical suspicion, a nondiagnostic scan and normal proximal ultrasound examinations is expected to be about 95%,57,58,62,66 and because there is evidence that patients with such results have a low (≤ 2%) risk of presenting with symptomatic venous thromboembolism during follow-up,58,62 this combination of findings may be considered to exclude the presence of pulmonary embolism (some choose to perform serial venous ultrasonography [see later]).
Nondiagnostic lung scanning, negative D-dimer testing and normal ultrasound testing for proximal deep vein thrombosis
When this combination of findings includes a moderately sensitive D-dimer test, this is estimated to have a negative predictive value of about 98%.58,70,76 Although this approach has not been well tested prospectively, excluding the presence of pulmonary embolism with this combination of findings is reasonable. However, serial venous ultrasonography should be considered when there is a high clinical probability of pulmonary embolism.
Helical CT scanning in combination with other tests
Based on the estimated prevalence of pulmonary embolism with different CT findings (see earlier) and extrapolating from studies that evaluated patients with nondiagnostic lung scans, various combinations of test results are expected to exclude the presence of pulmonary embolism when combined with a normal helical CT (Box 3).
Management of patients with nondiagnostic results of combined noninvasive tests
Depending on the tests that have been performed and local referral patterns, results of noninvasive testing are nondiagnostic in 30%–60% of the patients with suspected pulmonary embolism.55,56,57,58,59,60,62,66,70,72,76 Overall, these patients have a prevalence of pulmonary embolism of about 20%,55,57,58,59,60,66,70,72,76 which is too high to ignore and too low to treat. Pulmonary angiography can be performed in these patients66,76 but adherence to this recommendation is poor.107 An alternative is to forgo definitive testing for pulmonary embolism but to use knowledge of the predictable natural history of venous thromboembolism to manage patients in a way that is safe (Box 1). For most patients with initial nondiagnostic testing for pulmonary embolism that includes normal ultrasound scans of the proximal veins, this can be achieved by withholding treatment unless proximal deep vein thrombosis is detected on repeat ultrasound examinations during follow-up (Box 4).
Withholding anticoagulants and pulmonary angiography on the basis of serial normal ultrasounds of the proximal veins
The 20% of patients with initial nondiagnostic tests for pulmonary embolism (including normal bilateral proximal venous ultrasound scans) who have had the condition also have either small residual deep vein thrombosis (usually confined to the calf) or no residual deep vein thrombosis. These patients are at risk of recurrent pulmonary embolism if the small residual thrombi extend or if a new deep vein thrombosis forms, with the highest risk period being within 2 weeks of presentation.32,33,66,98,108 However, before such patients have a recurrent episode of pulmonary embolism, they must first redevelop proximal deep vein thrombosis. Performing serial venous ultrasounds over a 2-week period in all patients with nondiagnostic tests for pulmonary embolism enables those who are progressing toward recurrent pulmonary embolism to be detected, and treated, before recurrent embolism.20,56,57,58 Ultrasound scans of the proximal veins become abnormal during serial testing in about 2% of patients.56,57,58 Those who do not develop an abnormal ultrasound have a low subsequent risk of symptomatic venous thromboembolism that is similar to the rate observed following normal pulmonary angiography58 (about 1% over 6 months).56,57,58
Clinical follow-up after completing diagnostic testing
After the presence of pulmonary embolism has been excluded or after serial ultrasonography has been completed in those who could not have pulmonary embolism excluded on the day of presentation, there remains a small risk of symptomatic venous thromboembolism within the next 3 months (about 1%).57,58,59,66,72,76,98 Consequently, patients who are not diagnosed with pulmonary embolism should routinely be advised to return if they develop new symptoms suggestive of deep vein thrombosis or pulmonary embolism. Because most episodes of symptomatic venous thromboembolism that occur among these patients during follow-up are deep vein thrombosis or nonfatal pulmonary embolisms (e.g., 1 fatal pulmonary embolism among 16 events in 4 recent studies57,58,66,76), re-evaluation of patients with persistent or recurrent symptoms serves as an additional safety measure.
Diagnosis of pulmonary embolism in pregnancy
Pregnant patients with suspected pulmonary embolism can be managed similarly to nonpregnant patients, with the following modifications. First, ultrasonography of the proximal veins can be performed as an initial test; patients with unequivocal evidence of deep vein thrombosis can be presumed to have pulmonary embolism. Second, the amount of radioisotope used for the perfusion scan can be reduced and the duration of scanning extended. Third, if pulmonary angiography is performed, the brachial approach with abdominal screening is preferable to reduce fetal radiation exposure. Fourth, in the absence of safety data relating to helical CT in pregnancy, this is discouraged (if it is necessary, abdominal screening should be used).
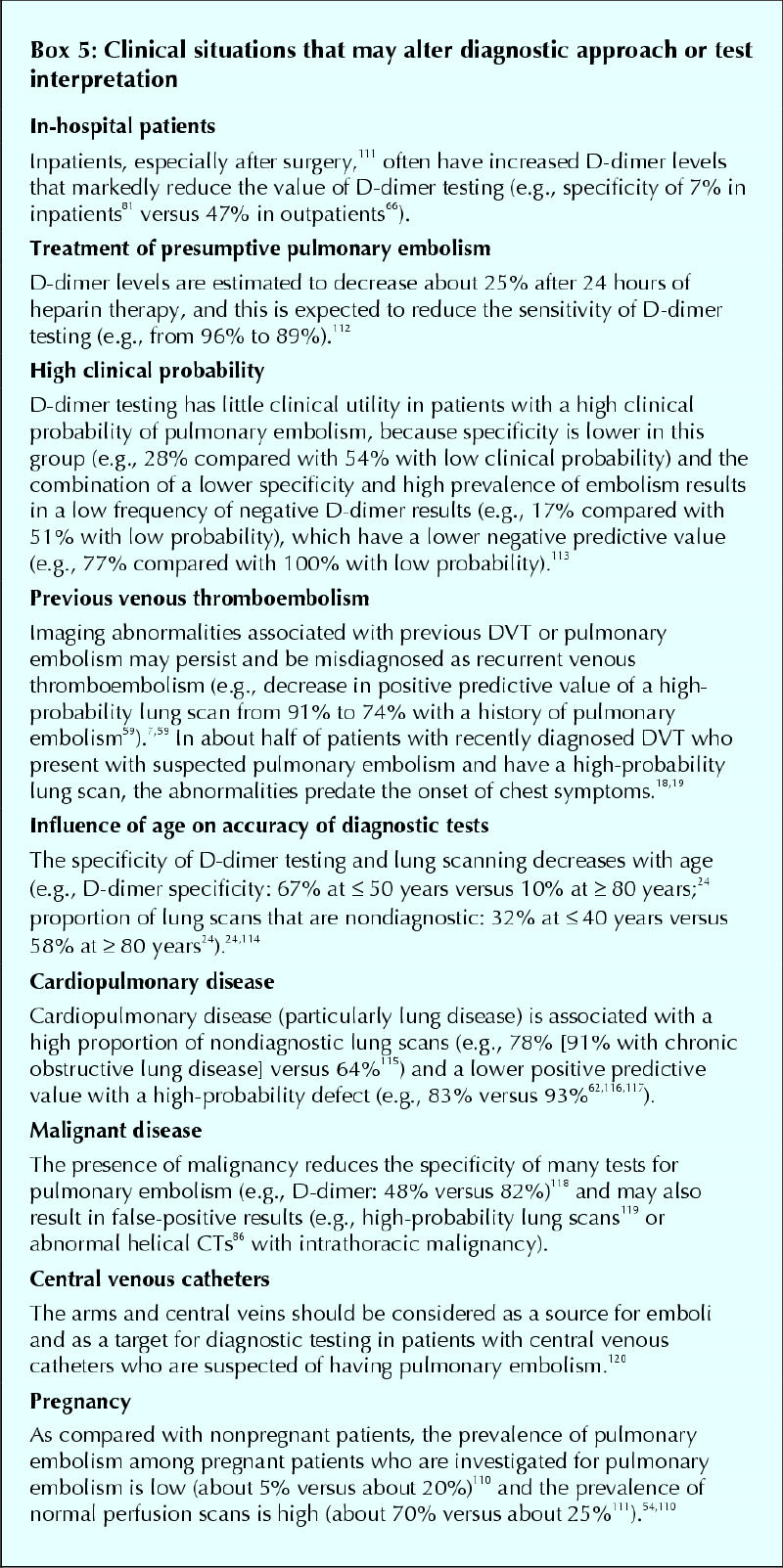
It is likely that the risk of inaccurate diagnosis of pulmonary embolism during pregnancy far exceeds the risks of radiation exposure with diagnostic testing.109,110 Recent studies indicate that the prevalence of pulmonary embolism tends to be low, and the frequency of normal lung scans high, in pregnant patients who are investigated for pulmonary embolism (Box 5111,112,113,114,115,116,117,118,119,120).54,110
Box 5.
Algorithms for the diagnosis of pulmonary embolism
There are many valuable tests (including clinical assessment) that may be used, singly or in combination, to confirm or exclude the presence of pulmonary embolism with a high degree of confidence (Box 3). Availability of testing and differences among patient presentations (Box 5) will influence the diagnostic approach used.
A number of prospectively validated algorithms have been published that emphasize the use of different initial noninvasive tests in conjunction with ventilation–perfusion lung scanning. These include structured clinical assessment and serial venous ultrasonography;57 sensitive D-dimer assay, empirical clinical assessment and venous ultrasonography at presentation only;66 and clinical assessment, moderately sensitive D-dimer assay and serial venous ultrasonography.58 Based on these studies and others that have been discussed, such an algorithm is presented in Fig. 1. Algorithms that incorporate helical CT require further validation.
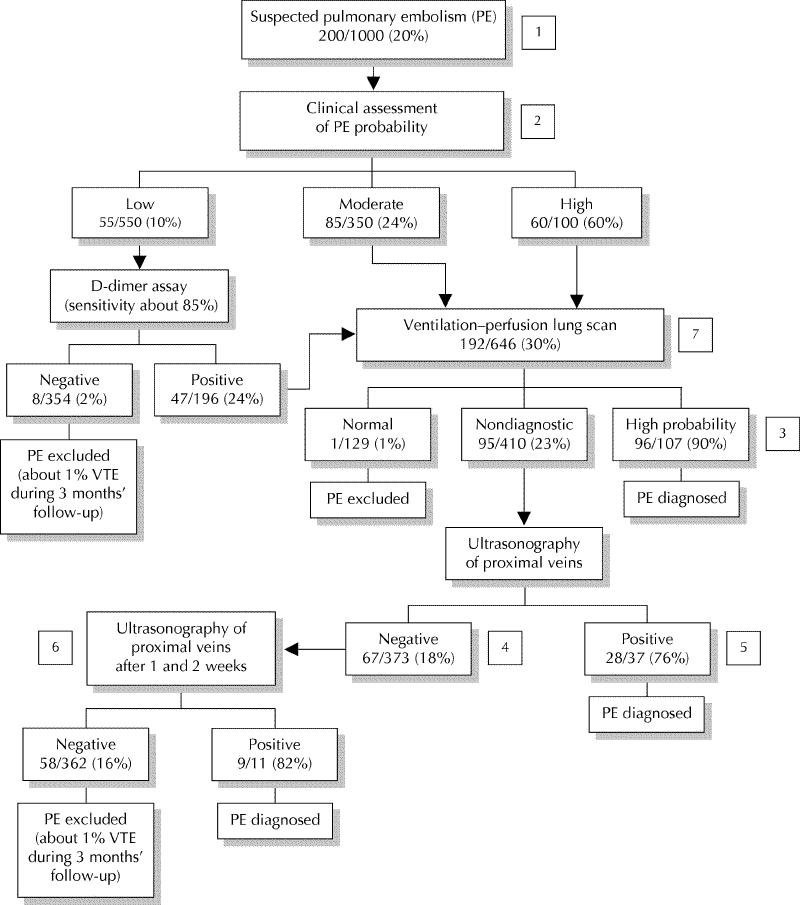
Fig. 1: A diagnostic algorithm for pulmonary embolism (estimated frequencies of test results and associated prevalences of pulmonary embolism for a hypothetical cohort of 1000 outpatients) [1]. If a very sensitive D-dimer assay is used, it can be the first test performed: a negative result excludes pulmonary embolism regardless of clinical assessment category and a positive test can be followed by a ventilation–perfusion scan [2]. A ventilation–perfusion scan can be performed as the initial test without using clinical assessment of the probability of pulmonary embolism as part of the diagnostic process [3]. Pulmonary angiography or helical CT may be considered if the clinical assessment of pulmonary embolism probability is low, particularly if a D-dimer test has not been done [4]. Additional testing (e.g., helical CT, bilateral venography) may be considered if overall assessment suggests a high probability of pulmonary embolism (e.g., 50%–80%), symptoms are severe or cardiopulmonary reserve is poor [5]. Venography should be considered if there is an increased risk of a false-positive ultrasound result (e.g., previous venous thromboembolism, equivocal ultrasound findings, preceding findings suggest low probability of pulmonary embolism [e.g., ≤ 10%]) [6]. It is reasonable not to repeat ultrasound testing, or to do only 1 more ultrasound after 1 week, if preceding findings suggest a low probability of pulmonary embolism (e.g., ≤ 10%) [7]. If helical CT is used in place of ventilation–perfusion lung scanning: (i) intraluminal filling defects in segmental or larger pulmonary arteries are generally diagnostic for pulmonary embolism; (ii) all other findings (i.e., a normal CT scan or intraluminal filling defects confined to the subsegmental pulmonary arteries) are nondiagnostic and can be managed as shown for a nondiagnostic lung scan.
Supplementary Material
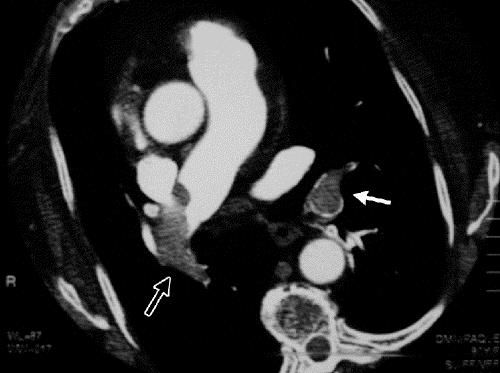
Figure. Helical CT of the pulmonary arteries with intraluminal filling defects in the lobar artery of the left lower lobe (solid arrow) and the main artery of the right lung (open arrow) in a patient with a chest deformity.
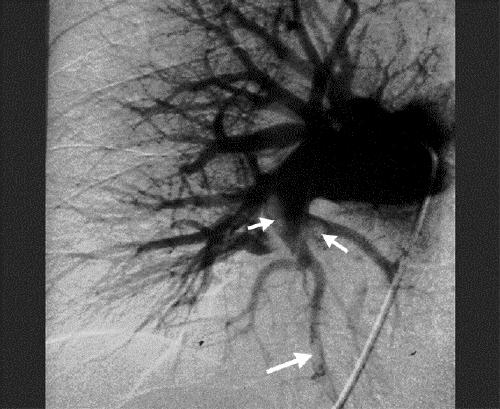
Figure. Conventional pulmonary angiogram of the right lung with intraluminal filling defects in the lobar artery and segmental and subsegmental arteries of the lower lobe.
Acknowledgments
I thank Shannon Bates for reviewing and improving an earlier version of this article.
Footnotes
This article has been peer reviewed.
Dr. Kearon is a Research Scholar of the Heart and Stroke Foundation of Canada.
Competing interests: None declared.
Correspondence to: Dr. Clive Kearon, 70 Wing, Rm. 39, Henderson General Hospital, 711 Concession St., Hamilton ON L8V 1C3
References
- 1.Pineda LA, Hathwar VS, Grant BJ. Clinical suspicion of fatal pulmonary embolism. Chest 2001;120:791-5. [DOI] [PubMed]
- 2.Kearon C. Natural history of venous thromboembolism. Semin Vasc Med 2001; 1:27-37. [DOI] [PubMed]
- 3.Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet 1969;2:230-2. [DOI] [PubMed]
- 4.Nicolaides AN, Kakkar VV, Field ES, Renney JTG. The origin of deep vein thrombosis: a venographic study. Br J Radiol 1971;44:653-63. [DOI] [PubMed]
- 5.Cogo A, Lensing AWA, Prandoni P, Hirsh J. Distribution of thrombosis in patients with symptomatic deep-vein thrombosis: implications for simplifying the diagnostic process with compression ultrasound. Arch Intern Med 1993; 153: 2777-80. [PubMed]
- 6.Fraser DG, Moody AR, Morgan PS, Martel AL, Davidson I. Diagnosis of lower-limb deep venous thrombosis: a prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med 2002;136:89-98. [DOI] [PubMed]
- 7.Kearon C, Julian JA, Newman TE, Ginsberg JS, for the McMaster Diagnostic Imaging Practice Guidelines Initiative. Non-invasive diagnosis of deep vein thrombosis. Ann Intern Med 1998;128:663-77. [DOI] [PubMed]
- 8.Moser KM, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med 1981;94:439-44. [DOI] [PubMed]
- 9.Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet 1985;2(8454):515-8. [DOI] [PubMed]
- 10.Hull R, Hirsh J, Sackett DL, Powers P, Turpie AGG, Walker I. Combined use of leg scanning and impedance plethysmography in suspected venous thrombosis. An alternative to venography. N Engl J Med 1977;296:1497-500. [DOI] [PubMed]
- 11.Hull R, Hirsh J, Sackett DL, Taylor DW, Carter C, Turpie AGG, et al. Replacement of venography in suspected venous thrombosis by impedance plethysmography and 125I-fibrinogen leg scanning. Ann Intern Med 1981; 94:12-5. [DOI] [PubMed]
- 12.Heijboer H, Buller HR, Lensing AWA, Turpie AGG, Colly LP, ten Cate WJ. A comparison of real-time compression ultrasonography with impedance plethysmography for the diagnosis of deep-vein thrombosis in symptomatic outpatients. N Engl J Med 1993;329:1365-9. [DOI] [PubMed]
- 13.Doyle DJ, Turpie AGG, Hirsh J, Best C, Kinch D, Levine MN, et al. Adjusted subcutaneous heparin or continuous intravenous heparin in patients with acute deep vein thrombosis. Ann Intern Med 1987;107:441-5. [DOI] [PubMed]
- 14.Huisman MV, Buller HR, ten Cate J, van Royen EA, Vreeken J, Kersten MJ, et al. Unexpected high prevalence of silent pulmonary embolism in patients with deep venous thrombosis. Chest 1989;95:498-502. [DOI] [PubMed]
- 15.Moser KM, Fedullo PF, LittleJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA 1994; 27: 223-5. [PubMed]
- 16.Dorfman GS, Cronan JJ, Tupper TB, Messersmith RN, Denny DF, Lee CH. Occult pulmonary embolism: a common occurrence in deep venous thrombosis. AJR Am J Roentgenol 1987;148:263-6. [DOI] [PubMed]
- 17.Nielsen HK, Husted SE, Krusell LR, Fasting H, Hansen PC, Hansen HH. Silent pulmonary embolism in patients with deep venous thrombosis. Incidence and fate in a randomized, controlled trial of anticoagulation versus no anticoagulation. J Intern Med 1994;235:457-61. [DOI] [PubMed]
- 18.Monreal M, Ruiz J, Fraile M, Bonet M, Davant E, Muchart J, et al. Prospective study on the usefulness of lung scan in patients with deep vein thrombosis of the lower limbs. Thromb Haemost 2001;85:771-4. [PubMed]
- 19.Girard P, Decousus M, LaPorte S, Buchmuller A, Herve P, Lamer C, et al. Diagnosis of pulmonary embolism in patients with proximal deep vein thrombosis: specificity of symptoms and perfusion defects at baseline and during anticoagulant therapy. Am J Respir Crit Care Med 2001;164:1033-7. [DOI] [PubMed]
- 20.Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med 1998;129:1044-9. [DOI] [PubMed]
- 21.Hull RD, Hirsh J, Carter CJ, Jay RM, Dodd PE, Ockelford PA, et al. Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med 1983;98:891-9. [DOI] [PubMed]
- 22.Girard P, Musset D, Parent F, Maitre S, Phlippoteau C, Simonneau G. High prevalence of detectable deep venous thrombosis in patients with acute pulmonary embolism. Chest 1999;116:903-8. [DOI] [PubMed]
- 23.Kruit WHJ, de Boer AC, Sing AK, van Roon F. The significance of venography in the management of patients with clinically suspected pulmonary embolism. J Intern Med 1991;230:333-9. [DOI] [PubMed]
- 24.Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357-61. [DOI] [PubMed]
- 25.Galle C, Papazyan JP, Miron MJ, Slosman D, Boumameaux H, Perrier A. Prediction of pulmonary embolism extent by clinical findings, D-dimer level and deep vein thrombosis shown by ultrasound. Thromb Haemost 2002;86:1156-60. [PubMed]
- 26.Hull RD, Raskob GE, Coates G, Panju AA, Gill GJ. A new noninvasive management strategy for patients with suspected pulmonary embolism. Arch Intern Med 1989;149:2549-55. [PubMed]
- 27.Sijens PE, van Ingen HE, van Beek EJ, Berghout A, Oudkerk M. Rapid ELISA assay for plasma D-dimer in the diagnosis of segmental and subsegmental pulmonary embolism. A comparison with pulmonary angiography. Thromb Haemost 2000;84:156-9. [PubMed]
- 28.de Monye W, Sanson BJ, MacGillavry MR, Pattynama PM, Buller HR, Berg-Huysmans AA, et al. Embolus location affects the sensitivity of a rapid quantitative D-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 2002;165:345-8. [DOI] [PubMed]
- 29.Stein PD, Henry JW. Prevalence of acute pulmonary embolism in central and subsegmental pulmonary arteries and relation to probability interpretation of ventilation/perfusion lung scans. Chest 1997;111:1246-8. [DOI] [PubMed]
- 30.de Monye W, van Strijen MJ, Huisman MV, Kieft GJ, Pattynama PM. Suspected pulmonary embolism: prevalence and anatomic distribution in 487 consecutive patients. Advances in New Technologies Evaluating the Localisation of Pulmonary Embolism (ANTELOPE) Group. Radiology 2000;215:184-8. [DOI] [PubMed]
- 31.Oudkerk M, van Beek EJ, Wielopolski P, van Ooijen PM, Brouwers-Kuyper EM, Bongaerts AH, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet 2002;359:1643-7. [DOI] [PubMed]
- 32.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet 1960;1:1309-12. [DOI] [PubMed]
- 33.Hull R, Delmore T, Genton E, Hirsh J, Gent M, Sackett D, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979;301:855-8. [DOI] [PubMed]
- 34.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998;279:458-62. [DOI] [PubMed]
- 35.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med 1999;159:445-53. [DOI] [PubMed]
- 36.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 1995;108:978-81. [DOI] [PubMed]
- 37.Bell WR, Simon TL. Current status of pulmonary embolic disease: pathophysiology, diagnosis, prevention, and treatment. Am Heart J 1982;103:239-61. [DOI] [PubMed]
- 38.Goldhaber SZ, Visni L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-9. [DOI] [PubMed]
- 39.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000;101:2817-22. [DOI] [PubMed]
- 40.Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, et al. Aleptase versus heparin in acute pulmonary embolism: randomized trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341: 507-11. [DOI] [PubMed]
- 41.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997;134:479-87. [DOI] [PubMed]
- 42.Blackmon JR, Sautter RD, Wagner HN. Urokinase pulmonary embolism trial: phase I results. JAMA 1970;214:2163-72. [PubMed]
- 43.Levine M, Hirsh J, Weitz J, Cruickshank M, Neemeh J, Turpie AGG, et al. A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest 1990;98:1473-9. [DOI] [PubMed]
- 44.PIOPED Investigators. Tissue plasminogen activator for the treatment of acute pulmonary embolism. Chest 1990;97:528-33. [DOI] [PubMed]
- 45.Dalen JE, Alpert JS, Hirsh J. Thrombolytic therapy for pulmonary embolism. Is it effective? Is it safe? When is it indicated? Arch Intern Med 1997; 157: 2550-6. [PubMed]
- 46.Paraskos JA, Adelstein SJ, Smith RE, Rickman FD, Grossman W, Dexter L, et al. Late prognosis of acute pulmonary embolism. N Engl J Med 1973; 289: 55-8. [DOI] [PubMed]
- 47.Hall RJC, Sutton GC, Kerr IH. Long-term prognosis of treated acute massive pulmonary embolism. Br Heart J 1977;39:1128-34. [DOI] [PMC free article] [PubMed]
- 48.Riedel M, Stanek V, Widimsky J. Longterm follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151-8. [DOI] [PubMed]
- 49.Heijboer H, Jongbloets LMM, Buller HR, Lensing AWA, ten Cate JW. Clinical utility of real-time compression ultrasonography for diagnostic management of patients with recurrent venous thrombosis. Acta Radiol 1992; 33: 297-300. [PubMed]
- 50.Prandoni P, Cogo A, Bernardi E, Villalta S, Polistena P, Simioni P, et al. A simple ultrasound approach for detection of recurrent proximal vein thrombosis. Circulation 1993;88:1730-5. [DOI] [PubMed]
- 51.Kearon C. Epidemiology of venous thromboembolism. Semin Vasc Med 2001; 1: 7-25. [DOI] [PubMed]
- 52.Kearon C, Salzman E, Hirsh J. Epidemiology, pathogenesis, and natural history of venous thrombosis. In: Colman R, Hirsh J, Marder JV, Clowes AW, George JN, editors. Hemostasis and thrombosis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001.
- 53.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416-20. [PubMed]
- 54.Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med 2001;161:92-7. [DOI] [PubMed]
- 55.Turkstra F, Kiujer PMM, van Beek E Jr, Brandjes DPM, ten Cate JW, Buller HR. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med 1997;126:775-81. [DOI] [PubMed]
- 56.Hull RD, Raskob GE, Ginsberg JS, Panju AA, Brill-Edwards P, Coates G, et al. A noninvasive strategy for the treatment of patients with suspected pulmonary embolism. Arch Intern Med 1994;154:289-97. [PubMed]
- 57.Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AGG, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 1998;129:997-1005. [DOI] [PubMed]
- 58.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001;135:98-107. [DOI] [PubMed]
- 59.The PIOPED investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990;263(20):2753-9. [DOI] [PubMed]
- 60.Hull RD, Hirsh J, Carter CJ, Raskob GE, Gill GJ, Jay RM, et al. Diagnostic value of ventilation-perfusion lung scanning in patients with suspected pulmonary embolism. Chest 1985;88:819-28. [DOI] [PubMed]
- 61.Miniati M, Pistolesi M, Marini C, Di Ricco G, Formichi B, Prediletto R, et al. Value of perfusion lung scan in the diagnosis of pulmonary embolism: results of the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISA-PED). Am J Respir Crit Care Med 1996;154:1387-93. [DOI] [PubMed]
- 62.Perrier A, Miron MJ, Desmarais S, De Moerloose P, Slosman D, Didier D, et al. Using clinical evaluation and lung scan to rule out suspected pulmonary embolism: Is it a valid option in patients with normal results of lower-limb venous compression ultrasonography? Arch Intern Med 2000;160:512-6. [DOI] [PubMed]
- 63.Miniati M, Prediletto R, Formichi B, Marini C, Di Ricco G, Tonelli L et al. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 1999;159:864-71. [DOI] [PubMed]
- 64.Miniati M, Monti S, Pratali L, Di Ricco G, Marini C, Formichi B, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110: 528-35. [DOI] [PubMed]
- 65.Perrier A, Desmarais S, Goehring C, De Moerloose P, Morabia A, Unger PF, et al. D-dimer testing for suspected pulmonary embolism in outpatients. Am J Respir Crit Care Med 1997;156:492-6. [DOI] [PubMed]
- 66.Perrier A, Desmarais S, Miron MJ, De Moerloose P, Lepage R, Slosman D, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet 1999;353:190-5. [DOI] [PubMed]
- 67.Sanson BJ, Lijmer JG, Mac Gillavry MR, Turkstra F, Prins M, Buller H. Comparison of a clinical probability estimate and two clinical models in patients with suspected pulmonary embolism. Thromb Haemost 2002;83:199-203. [PubMed]
- 68.Bates SM, Ginsberg JS. Comparison of a clinical probability estimate and two clinical models in patients with suspected pulmonary embolism: commentary. Thromb Haemost 2002;83:182-4. [PubMed]
- 69.Lee AYY, Ginsberg JS. Laboratory diagnosis of venous thromboembolism. Baillieres Clin Haematol 1998;11:587-604. [DOI] [PubMed]
- 70.Ginsberg JS, Wells PS, Kearon C, Anderson D, Crowther M, Weitz JI, et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism. Ann Intern Med 1998;129:1006-11. [DOI] [PubMed]
- 71.Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Ann Intern Med 2000;132:227-32. [DOI] [PubMed]
- 72.Perrier A, Howarth N, Didier D, Loubeyre P, Unger PF, De Moerloose P, et al. Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism. Ann Intern Med 2001;135:88-97. [DOI] [PubMed]
- 73.Stein PD, Athanasoulis C, Alavi A, Greenspan RH, Hales CA, Saltzman HA, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992;85:462-8. [DOI] [PubMed]
- 74.van Beek E Jr, Kuyer PMM, Schenk BE, Brandjes DPM, ten Cate JW, Büller HR. A normal perfusion lung scan in patients with clinically suspected pulmonary embolism: frequency and clinical validity. Chest 1995;108:170-3. [DOI] [PubMed]
- 75.Hull RD, Raskob GE, Coates G, Panju AA. Clinical validity of a normal perfusion lung scan in patients with suspected pulmonary embolism. Chest 1990; 97:23-6. [DOI] [PubMed]
- 76.de Groot MR, van Marwijk KM, Pouwels JG, Engelage AH, Kuipers BF, Buller HR. The use of a rapid D-dimer blood test in the diagnostic work-up for pulmonary embolism: a management study. Thromb Haemost 1999;82: 1588-92. [PubMed]
- 77.Rodger MA, Jones G, Rasuli P, Raymond F, Djunaedi H, Bredeson CN, et al. Steady-state end-tidal alveolar dead space fraction and D-dimer: bedside tests to exclude pulmonary embolism. Chest 2001;120:115-9. [DOI] [PubMed]
- 78.Kline JA, Israel EG, Michelson EA, O'Neil BJ, Plewa MC, Portelli DC. Diagnostic accuracy of a bedside D-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism: a multicenter study. JAMA 2001;285:761-8. [DOI] [PubMed]
- 79.Wells PS, Brill-Edwards P, Stevens P, Panju A, Patel A, Douketis J, et al. A novel and rapid whole-blood assay for D-dimer in patients with clinically suspected deep vein thrombosis. Circulation 1995;91:2184-7. [DOI] [PubMed]
- 80.Kipper MS, Moser KM, Kortman KE, Ashburn WL. Longterm follow-up of patients with suspected pulmonary embolism and a normal lung scan. Perfusion scans in embolic suspects. Chest 1982;82:411-5. [DOI] [PubMed]
- 81.Miron MJ, Perrier A, Bounameaux H, De Moerloose P, Slosman DO, Didier D, et al. Contribution of noninvasive evaluation to the diagnosis of pulmonary embolism in hospitalized patients. Eur Respir J 1999;13:1365-70. [DOI] [PubMed]
- 82.Stein PD, Henry JW, Gottschalk A. Mismatched vascular defects. An easy alternative to mismatched segmental equivalent defects for the interpretation of ventilation/perfusion lung scans in pulmonary embolism. Chest 1993;104:468-72. [DOI] [PubMed]
- 83.Perrier A, Bounameaux H, Morabia A, De Moerloose P, Slosman D, Didier D, et al. Diagnosis of pulmonary embolism by a decision analysis-based strategy including clinical probability, D-dimer levels, and ultrasonography: a management study. Arch Intern Med 1996;156:531-6. [PubMed]
- 84.Robinson PJA. Ventilation-perfusion lung scanning and spiral computed tomography of the lungs: competing or complementary modalities. Eur J Nucl Med 1996;23:1547-53. [DOI] [PubMed]
- 85.de Monye W, Pattynama PMT. Contrast-enhanced spiral computed tomography of the pulmonary arteries; an overview. Semin Thromb Hemost 2001; 27:33-9. [DOI] [PubMed]
- 86.Mullins MD, Becker DM, Hagspiel KD, Philbrick JT. The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism. Arch Intern Med 2000;160:293-8. [DOI] [PubMed]
- 87.van Strijen M, de Monye W, Kieft GJ, Bloem JL. Diagnosis of pulmonary embolism with spiral CT: a prospective cohort study in 617 consecutive patients [abstract]. Radiology 1999;213(Suppl):127.
- 88.Oser RF, Zuckerman DA, Gutierrez FR. Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology 1996;199:31-5. [DOI] [PubMed]
- 89.Quinn MF, Lundell CJ, Klotz TA. Reliability of selective pulmonary arteriography in the diagnosis of pulmonary embolism. Am J Roentgenol 1987;149: 469-71. [DOI] [PubMed]
- 90.Ferretti GR, Bosson JL, Buffaz PD, Ayanian D, Pison C, Blanc F, et al. Acute pulmonary embolism: role of helical CT in 164 patients with intermediate probability at ventilation-perfusion scintigraphy and normal results at duplex US of the legs. Radiology 1997;205:453-8. [DOI] [PubMed]
- 91.Ost D, Rozenshtein A, Saffran L, Snider A. The negative predictive value of spiral computed tomography for the diagnosis of pulmonary embolism in patients with nondiagnostic ventilation-perfusion scans. Am J Med 2001;110:16-21. [DOI] [PubMed]
- 92.Lorut C, Ghossains M, Horellou MH, Achkar A, Fretault J, Laaban JP. A noninvasive diagnostic strategy including spiral computed tomography in patients with suspected pulmonary embolism. Am J Respir Crit Care Med 2000; 162: 1413-8. [DOI] [PubMed]
- 93.Meaney JFM, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336:1422-7. [DOI] [PubMed]
- 94.Gupta A, Frazer CK, Ferguson JM, Kumar AB, Davis SJ, Fallon MJ, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology 1999;210:353-9. [DOI] [PubMed]
- 95.Sostman HD, Layish DT, Tapson VF. Prospective comparison of helical CT and MR imaging in patients with clinically suspected pulmonary embolism. J Magn Reson Imaging 1996;6:275-81. [DOI] [PubMed]
- 96.MacGillavry MR, Sanson BJ, Buller HR, Brandjes DP. Compression ultrasonography of the leg veins in patients with clinically suspected pulmonary embolism: Is a more extensive assessment of compressibility useful? Thromb Haemost 2000;84:973-6. [PubMed]
- 97.Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost 2000;84:548-52. [PubMed]
- 98.Henry JW, Relyea B, Stein PD. Continuing risk of thromboemboli among patients with normal pulmonary angiograms. Chest 1995;107:1375-8. [DOI] [PubMed]
- 99.Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med 2002;136:691-700. [DOI] [PubMed]
- 100.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, et al. Utility of an integrated clinical, echocardiographic, and venous ultrasonographic approach for triage of patients with suspected pulmonary embolism. Am J Cardiol 1998;82:1230-5. [DOI] [PubMed]
- 101.Leibowitz D. Role of echocardiography in the diagnosis and treatment of acute pulmonary thromboembolism. J Am Soc Echocardiogr 2001;14:921-6. [DOI] [PubMed]
- 102.Steiner P, Lund GK, Debatin JF, Steiner D, Nienaber C, Nicolas V, et al. Acute pulmonary embolism: value of transthoracic and transesophageal echocardiography in comparison with helical CT. AJR Am J Roentgenol 1996;167:931-6. [DOI] [PubMed]
- 103.McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol 1996;78:469-73. [DOI] [PubMed]
- 104.Pruszczyk P, Torbicki A, Pacho R, Chlebus M, Kuch-Wocial A, Pruszynski B, et al. Noninvasive diagnosis of suspected severe pulmonary embolism: transesophageal echocardiography vs spiral CT. Chest 1997;112:722-8. [DOI] [PubMed]
- 105.Vieillard-Baron A, Qanadli SD, Antakly Y, Fourme T, Loubieres Y, Jardin F, et al. Transesophageal echocardiography for the diagnosis of pulmonary embolism with acute cor pulmonale: a comparison with radiological procedures. Intensive Care Med 1998;24:429-33. [DOI] [PubMed]
- 106.Konstantinides S, Geibel A, Kasper W, Olschewski M, Blumel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation 1998;97:1946-51. [DOI] [PubMed]
- 107.Schluger N, Henschke C, King T, Russo R, Binkert B, Rackson M, et al. Diagnosis of pulmonary embolism at a large teaching hospital. J Thorac Imaging 1994;9:180-4. [DOI] [PubMed]
- 108.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, et al. The clinical course of pulmonary embolism. N Engl J Med 1992;326:1240-5. [DOI] [PubMed]
- 109.Ginsberg JS, Hirsh J, Rainbow AJ, Coates G. Risks to the fetus of radiologic procedures used in the diagnosis of maternal venous thromboembolic disease. Thromb Haemost 1989;61:189-96. [PubMed]
- 110.Chan WS, Ray JG, Murray S, Coady GE, Coates G, Ginsberg JS. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162(10):1170-5. [DOI] [PubMed]
- 111.Douketis JD, McGinnis J, Ginsberg JS. The clinical utility of a rapid bedside D-dimer assay for screening of deep vein thrombosis following orthopaedic surgery. Thromb Haemost 1997;78:1300-1. [PubMed]
- 112.Couturaud F, Kearon C, Bates SM, Ginsberg JS. Decrease in sensitivity of D-dimer for acute venous thromboembolism after starting anticoagulant therapy. Blood Coagul Fibrinolysis. 2002;13:241-6. [DOI] [PubMed]
- 113.Bates SM, Grand'Maison A, Johnston M, Naguit I, Kovacs MKJ, Ginsberg JS. A latex D-dimer reliably excludes venous thromboembolism. Arch Intern Med 2001;161:447-53. [DOI] [PubMed]
- 114.Stein PD, Gottschalk A, Saltzman HA, Terrin ML. Diagnosis of acute pulmonary embolism in the elderly. J Am Coll Cardiol 1991;18:1452-7. [DOI] [PubMed]
- 115.Lesser BA, Stein PD, Chen J, Hales CA, Greenspan RH. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest 1992;102:17-22. [DOI] [PubMed]
- 116.Stein PD, Coleman RE, Gottschalk A, Saltzman HA, Terrin ML, Weg JG. Diagnostic utility of ventilation/perfusion lung scans in acute pulmonary embolism is not diminished by pre-existing cardiac or pulmonary disease. Chest 1991; 100:604-6. [DOI] [PubMed]
- 117.Stein PD, Gottschalk A, Henry JW, Shivkumar K. Stratification of patients according to prior cardiopulmonary disease and probability assessment based on the number of mismatched segmental equivalent perfusion defects. Approaches to strengthen the diagnostic value of ventilation/perfusion lung scans in acute pulmonary embolism. Chest 1993;104:1461-7. [DOI] [PubMed]
- 118.Lee A, Julian J, Levine M, Weitz J, Kearon C, Wells P, et al. Clinical utility of a rapid whole-blood D-dimer assay in patients with cancer who present with suspected acute deep venous thrombosis. Ann Intern Med 2000;131:417-23. [DOI] [PubMed]
- 119.Stein PD, Gottschalk A. Critical review of ventilation/perfusion lung scans in acute pulmonary embolism. Prog Cardiovasc Dis 1994;37:13-24. [DOI] [PubMed]
- 120.Prandoni P, Polistena P, Bernardi E, Cogo A, Casara D, Verlato F, et al. Upper-extremity deep vein thrombosis - risk factors, diagnosis, and complications. Arch Intern Med 1997;157:57-62. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


