Abstract
Initiation of protein synthesis on the hepatitis C virus (HCV) mRNA involves a structured element corresponding to the 5′ untranslated region and constituting an internal ribosome entry site (IRES). The domain IIId of the HCV IRES, an imperfect RNA hairpin extending from nucleotides 253 to 279 of the viral mRNA, has been shown to be essential for translation and for the binding of the 40S ribosomal subunit. We investigated the properties of a series of antisense 2′-O-methyloligoribonucleotides targeted to various portions of the domain IIId. Several oligomers, 14–17 nt in length, selectively inhibited in vitro translation of a bicistronic RNA construct in rabbit reticulocyte lysate with IC50s <10 nM. The effect was restricted to the second cistron (the Renilla luciferase) located downstream of the HCV IRES; no effect was observed on the expression of the first cistron (the firefly luciferase) which was translated in a cap-dependent manner. Moreover, antisense 2′-O-methyloligoribonucleotides specifically competed with the 40S ribosomal subunit for binding to the IRES RNA in a filter- retention assay. The antisense efficiency of the oligonucleotides was nicely correlated to their affinity for the IIId subdomain and to their ability to displace 40S ribosomal subunit, making this process a likely explanation for in vitro inhibition of HCV-IRES-dependent translation.
INTRODUCTION
Hepatitis C virus (HCV), a member of the Flaviviridae family, is an enveloped positive-strand RNA virus with a genomic sequence of ∼9600 bases. This genomic RNA encodes a single polyprotein that is processed by both host peptidases and virally encoded proteases to afford at least 10 distinct structural and non-structural proteins (1–4). The genome also contains two untranslated regions, one at each end of the sequence. The 5′ untranslated region (UTR) functions as an internal ribosome entry site (IRES) that allows for cap-independent initiation of translation (5–7).
The minimal IRES includes nearly the entire 5′-UTR of the message (5). The proposed secondary structure of the HCV IRES which comprises four different domains is phylogenetically highly conserved (8) and is critical for translation initiation. This region recruits the 43S particles; the 40S subunit binds to the lower part of sub-domain III and to domain IV (9–11). In particular, the IIId sub-domain whose structure adopts the characteristic loop E fold (12,13) is protected from chemical modification and RNase cleavage upon 40S binding (12,14). The role played by this domain IIId in translation initiation makes it a potential drug target in the HCV RNA genome (13).
Oligonucleotides are ligands of interest for selective regulation of gene expression. We developed the SELEX procedure against various RNA motifs in order to identify aptamers able to recognize folded RNA structures (15). We previously characterized DNA (16) and RNA oligomers (17) selective for the TAR RNA element of HIV-1. A phosphoramidate analog derived from a selected RNA aptamer is a competitor of the viral protein Tat and an inhibitor of TAR-dependent transcription in an in vitro assay (18,19). We recently characterized a new RNA recognition motif through in vitro selection carried out against individual hairpins of the HCV mRNA. Apical loop–internal loop interaction drives the binding of RNA aptamers to domain IV of the IRES and to the stem–loop structure SL1 of the 3′-UTR of the HCV mRNA (20).
In an attempt to generate selective high affinity ligands of sub-domain IIId of the HCV IRES we performed in vitro selection against this imperfect hairpin. However, the selected RNA oligomers were generally antisense sequences. We optimized these anti-IIId antisense oligomers and derived strong inhibitors of in vitro translation. The best 2′-O-methyloligoribonucleotides are characterized by an IC50 <10 nM in a cell-free assay and are able to displace the 40S ribosomal subunit from the IRES.
MATERIALS AND METHODS
Oligonucleotides
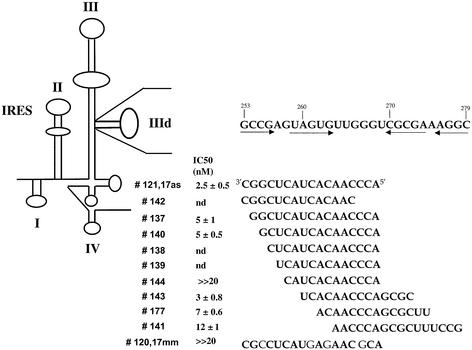
The 3′ biotinylated IIId RNA hairpin of the HCV IRES (5′-GCGCCGAGUAGUGUUGGGUCGCGAAAGGCGC), antisense RNA and DNA sequences were chemically synthesized on an Expedite 8909 synthesiser. The antisense 2′-O-methyloligonucleotides complementary to the IIId domain (Fig. 2) were synthesized on a solid phase from base-protected 1-(2-O-methyl-3-O (2-cyanoethoxy (diisopropylamino)-phosphino)-5-(4,4′dimethoxytrityl)-β-D-nucleoside by using 1H-tetrazole as activator.
Figure 2.
2′-O-methyl antisense oligonucleotides complementary to the IIId domain of the HCV IRES. The secondary structure of the IRES is schematized to the left. The sequence of the IIId domain is indicated to the right above that of antisense oligomers. The arrows refer to the IIId stem of the imperfect hairpin. The number of these antisense sequences is given as well as their IC50 deduced from in vitro translation assays in RRL (see Materials and Methods). nd, not determined.
Two different primers, T7IRES (5′-TAATACGACTCACTATAGCCAGCCCCCTGATGG) containing the T7 promoter (underlined) and IRES3′ (5′-GGATTCGTGCTCATG GTGCAC) from Genset (Paris, France) were used for PCR amplification and production of the IRES. All oligonucleotides were purified on denaturing polyacrylamide gels and were generally >95% pure.
Electrophoretic mobility shift assay
In order to evaluate the dissociation constant (Kd) of the complexes formed by the domain IIId and various antisense oligonucleotides, electrophoretic mobility shift assays (EMSAs) were performed at 23°C. In general, 0.1 or 1 nM of 32P 5′ end-labeled IIId domain was incubated at 23°C with different concentrations of antisense oligomers for 20 min at 23°C in 10 µl of 20 mM HEPES pH 7.3 at 20°C, containing 20 mM sodium acetate, 140 mM potassium acetate and 3 mM magnesium acetate (R buffer). Samples were run on a 15% (w/v) 19:1 acrylamide/bisacrylamide non-denaturing gel in 50 mM Tris–acetate (pH 7.3 at 20°C), 3 mM magnesium acetate, at 90 V (4 V/cm) for 15 h and quantified by Instant Imager analysis (Hewlett Packard).
Kd values were calculated from data-point fitting using Kaleidagraph™ 3.0 (Abelbeck Software), according to the equation:
[complex] = {Kd + [antisense0] + [RNA] ± [(Kd + [antisense0] + [RNA])2 – [antisense0] [RNA]]1/2}/2
[RNA] being the concentration of free 32P 5′end-labeled target RNA hairpin, i.e. domain IIId and [antisense]0 corresponding to the total concentration of unlabeled antisense oligonucleotide.
Plasmids
The bicistronic plasmid termed pIRF contains the coding sequence for the firefly luciferase under the control of the cytomegalovirus promoter followed by HCV genomic sequence (HCV nucleotides 1–371) and the coding sequences for the Renilla luciferase in the pcDNA3.1 Zeo vector (Invitrogen). This vector was a gift from Dr Annie Cahour (Hopital Pitié-Salpétrière, Paris).
The plasmid pGEM2HRV2 provided by Hélène Jacquemin-Sablon (Institut Bergonié, Bordeaux) consists of the human rhinovirus 2 genomic sequence (HRV nucleotides 10–611) which includes the HRV IRES followed by a coding region for a slightly truncated form of the influenza virus NS1 protein and finally the complete NS1 3′-UTR (21). Translation experiments were monitored by the incorporation of [35S]methionine in translation assay.
In vitro transcription
Uncapped RNAs for in vitro translation assays were produced from the plasmids pIRF and pGEM2HRV2, linearized by digestion for 2 h at 37°C with XhoI and EcoRI, respectively. The linearized sequences were purified by the nucleospin kit (Macherey-Nagel). After precipitation with 10 vol of butanol, the dried pellet was resuspended in 10 µl of diethylpyrocarbonate-treated water (1 µg/µl). RNAs were synthesized for 4 h using Ampliscribe™ T7 High Yield Transcription Kit (TEBU).
IRES RNA was synthesized by in vitro transcription of DNA fragments obtained by PCR amplification from the pCV-H77 molecular clone (22). The PCR was performed with oligonucleotides T7 IRES and IRES3′ as primers using 2.5 U of AmpliTaq gold DNA polymerase (Perkin Elmer) for 30 cycles. The PCR product was transcribed for 4 h at 37°C using the MEGAscript kit (Ambion). The RNAs were precipitated and quantified by UV-absorbance at 260 nm. The RNA products were checked by electrophoresis on polyacrylamide gel containing 7 M urea in TBE buffer (90 mM Tris–borate pH 8, 1 mM EDTA) and appeared as a single band exhibiting the expected mobility compared to size markers. For cell transfection, capped RNAs were produced form the Xho-linearized pIRF plasmid using RiboMAX™ large scale RNA production kit (Promega) complemented with 3 mM m7G(5′) ppp (5′) G (Invitrogen).
In vitro translation assay
In vitro translation was performed in 30 µl of a mixture containing 15 µl of rabbit reticulocyte lysate (RRL; Promega) supplemented with R buffer, 2 µl of amino acids at 1 mM each and 50 ng of pIRF mRNA. This mRNA amount was in the translation linear response range for this batch of lysate. After 60 min incubation at 30°C in the presence or absence of the desired antisense oligonucleotide, the translation level of the Renilla and firefly genes was evaluated by Dual-Luciferase Reporter Assay System (Promega) using a luminometer (Lumat Berthold). The effect of antisense oligomers was evaluated by measuring the ratio between Renilla and firefly luciferases in the presence and in the absence of antisense sequence, respectively.
The pGEM2HRV2 RNA (200 ng) or the pIRF RNA (300 ng) was translated in a mixture containing 21 µl of RRL, 1 µl of amino acids without methionine, 4 µl of [35S]methionine (10 µCi/µl). After 60 min incubation at 30°C in R buffer supplemented or not with antisense oligonucleotides, the reactions were processed for SDS polyacrylamide gel electrophoresis, and the dried gels submitted to autoradiography using Hyperfilm (Kodak).
Cell culture and RNA transfection
Huh7 (human hepatoma) cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine and 1% non-essential amino acids, at 37°C in a 5% CO2 atmosphere. All culture reagents were purchased from Invitrogen. The day before RNA transfection, 8.104 cells were seeded into a 24-well plate. For transfection, 3 nM of RNA derived from pIRF vector were mixed with or without oligonucleotide at different concentrations (0.1–25 nM). This mixture was then added to 200 µl of DMEM (without fetal calf serum) containing 5 µl of DMRIE-C reagent (Invitrogen). The lipid–RNA complex was added to Huh7 cells, previously rinsed two times with DMEM (without fetal calf serum), and incubated for 18 h at 37°C. Firefly and Renilla luciferase activities were then determined using the Dual-Luciferase™ reporter assay system (Promega).
For two step transfection the oligonucleotide was mixed in 200 µl of DMEM containing 5 µl of DMRIE-C. This mixture was added to Huh cells and incubated at 37°C for 2, 6 or 17 h prior to transfection of capped RNA (3 nM) in 200 µl of DMEM containing 4 µl of DMRIE-C. The cells were incubated for 24 h at 37°C. Luciferase activity were then evaluated as described above.
Purification of the 40S ribosomal subunit
40S ribosomal subunits were isolated essentially as described (23) from RRL freshly prepared according to Jagus (24). RRL was thawed on ice and supplemented with protease inhibitors (complete mini-EDTA free from Boehringer). After centrifugation at 30 000 r.p.m. for 4 h at 4°C in a Sorvall T856 rotor, the pellet was resuspended in 2 ml/tube of 5 mM Tris–HCl buffer pH 7.5 containing 0.25 M sucrose, 0.1 mM EDTA and 1 mM DTT. KCl was added to the polysome suspension up to a final concentration of 0.5 M followed by gentle rocking for 30 min at 4°C. Samples were then ultracentrifuged at 40 000 r.p.m. for 4 h at 4°C in a Sorvall T856 rotor. The pellet was resuspended in 20 mM Tris–HCl buffer pH 7.5 containing 50 mM KCl, 4 mM MgCl2 and 2 mM DTT. Puromycin was added up to a concentration of 1 mM and the ribosome suspension was incubated for 10 min on ice then for 10 min at 37°C, followed by addition of KCl up to a final concentration of 0.5 M. The ribosome suspension was then layered onto a 10–30% sucrose gradient in 20 mM Tris–HCl pH 7.5 containing 0.5 M KCl, 3 mM MgCl2, 2 mM DTT and spun at 22 000 r.p.m. in a Beckman SW28 rotor at 4°C for 15 h. The 40S subunits were recovered by fractionating the gradient and monitoring the absorbance at 280 and 260 nm. Fractions containing the 40S subunit were pooled, concentrated and dialyzed against 0.25 M sucrose in 20 mM Tris–HCl buffer pH 7.5 containing 10 mM KCl, 1 mM MgCl2, 1 mM DTT and 0.1 mM EDTA, using Centricon 50 spin concentrators (Amicon) prior to storage at –20°C. Concentration of 40S subunit solutions was determined by absorption spectrophotometry (1 OD260nm = 50 pmol). The binding properties of 40S subunits were evaluated by filter binding assay using the HCV IRES RNA.
Filter binding assays
To generate IRES RNA–40S subunit complexes, 32P end-labeled IRES RNA was first heated to 65°C for 2 min then cooled to room temperature. RNA IRES at 10 nM (final concentration) was then added to a tube containing folding/binding buffer (20 mM Tris–HCl pH 7.4, 100 mM potassium acetate, 200 mM potassium chloride, 2.5 mM magnesium acetate, 1 mM DTT). 40S subunit at 10 nM and the desired oligonucleotide from 1 to 50 nM were added to the reactions (final volume 100 µl) and incubated at 30°C for 10 min before loading on 0.45 µm nitrocellulose filters (Gelman). The filters were presoaked in the binding buffer, assembled in a dot blot apparatus, and the samples were added directly on the filter under vacuum. The filters were washed with the binding buffer, then removed, dried and counted. All measurements were carried out with IRES RNA as an internal standard. Reported values are the average of at least three independent experiments.
RESULTS
In vitro selection against domain IIId generates antisense sequences
In an attempt to identify aptamers targeted to the structured domain IIId of the HCV IRES we carried out in vitro selection using the RNA library and the conditions described previously (20). Briefly, 1013 different RNA candidates with a randomized window of 30 nt were mixed at room temperature with biotinylated IIId RNA in 20 mM HEPES pH 7.3 containing 20 mM sodium acetate, 140 mM potassium acetate and 3 mM magnesium acetate. Following eight rounds of enrichment as described (20) the selected sequences were cloned and sequenced. Most of them showed streches up to 12 nt complementary to the apical loop and to the 5′ strand of the IIId domain (not shown). In addition using MFOLD, these regions were predicted to be displayed in an unfolded context. In other words, in contrast to previous selections performed against various RNA structures (17,20,25) the selection process identified sequences antisense to the IIId domain.
Starting from these results, we therefore decided to investigate the properties of antisense oligonucleotides targeted to the region of the IIId domain which generated most of the selected sequences, i.e., the 5′ strand and the apical loop. As a lead we evaluated an heptadecaoligoribonucleotide (17as) complementary to positions 253–269 of the HCV mRNA in an in vitro translation assay with the pIRF bicistronic RNA. The antisense oligomer 17as induced 50% reduction of the expression of the second cistron (the Renilla luciferase gene) under the control of the HCV IRES at a concentration of ∼10 nM. The inhibition was dose-dependent and no effect was observed on the translation of the first cistron (the firefly luciferase gene) (Fig. 1). The control oligomer 17mm corresponding to 17as in which four mismatches were introduced (Fig. 2) was not inhibitory under the same conditions.
Figure 1.
Effect of RNA oligomers 17as and 17mm on the in vitro translation of luciferase transcripts. The pIRF mRNA was translated in RRL in the presence of oligonucleotides 17as (antisense) or 17mm (mismatched) as indicated in Materials and Methods. The Renilla (R-luc) or firefly luciferase (F-luc) activity was calculated with respect to a sample without oligonucleotide. Triangle, F-luc/17as; square, R-Luc/17mm; diamond, F-luc/17mm; circle, R-luc/17as. Each data point is the average of three independent experiments.
Screening of 2′-O-methyl,oligoribonucleotide antisense molecules for translation inhibitory effect
The deoxy version of the antisense oligoribonucleotide 17as was a poorer inhibitor (IC50 ≈ 100 nM) of in vitro translation than the RNA 17as (not shown). This is likely related to the lower affinity of the DNA complementary sequence for the target RNA site as compared to that of the RNA homolog and to the fact that in RRL the RNase H contribution to antisense effects of oligodeoxynucleotide sequences is low if not reduced to zero (26).
In order to generate nuclease-resistant antisense sequences we therefore considered 2′-O-methyl derivatives which retain the A-type helix geometry characteristic of RNA and display even an increased affinity for RNA than unmodified RNA itself (27). Starting from the 17mer oligoribonucleotide complementary to nucleotides 253–269 of the HCV IRES, we derived a series of 2′-O-methyl antisense sequences against the IIId domain (Fig. 2). The parent antisense oligomer (#121) corresponding to 17as was shortened either from the 5′ or from the 3′ end to generate sequences #142, #137, #140, #138, #139 and #144, whose length ranged from 16 to 12 nt. These seven antisense oligomers are complementary to part of the 5′ strand and of the loop of the IIId imperfect hairpin, i.e., look like sequences obtained the most frequently in the SELEX experiment (see above). We also synthesized oligomers complementary to the loop and to the 3′ strand of the stem (#143, #177 and #141). In addition, we prepared a control sequence (#120) derived from the RNA oligomer 17mm and therefore corresponding to the antisense #121 with four point mutations generating CC and GG mismatches.
We investigated the effect of these antisense oligomers using the bicistronic luciferase construct pIRF in which the Renilla gene was under the control of the HCV IRES. As few as 10 nM of oligonucleotides #121, #137, #140, #143 and #177 strongly inhibited (>70%) IRES-dependent in vitro translation in RLL (not shown). This inhibition was dose dependent; significant inhibition was still observed at 1 nM of the strongest antisense oligomers. In contrast a modest inhibition (<40%) was observed at 10 nM with oligomers #138, #139, #142 and #144, whereas #141 was able to inhibit IRES translation at a moderate level (≈60%) at the same concentration. As expected, the mismatched oligomer #120 had a much reduced inhibitory effect than the antisense sequence #121. At such low concentrations (≤10 nM) the expression of the firefly cistron was not affected (<5%) even in the presence of the strongest Renilla luciferase inhibitors (not shown).
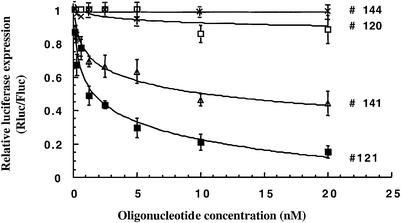
Following this screening, we carried out cell-free translation assays with most of the antisense oligomers in a more quantitative manner to determine the doses at which the Renilla luciferase synthesis was 50% inhibited (IC50). The results indicated that #121 and #143 were strong inhibitors of IRES-dependent translation characterized by an IC50 of 2.5 ± 0.5 nM and 3 ± 0.8 nM respectively (Figs 2 and 3). The oligomer #120 used as a control induced a very weak inhibition (∼15% at 20 nM compared with 85% for the corresponding antisense sequence #121). No significant inhibition (<10%) of the firefly luciferase was observed in the presence of #121 up to 20 nM (data not shown). Antisense oligomers #177 and #137 were also potent inhibitors (IC50 ≈ 5–7 nM) of Renilla luciferase expression but slightly less efficient than #121 (Fig. 2). The oligomer #141 was a moderate but selective translation inhibitor (IC50 ≈ 12 ± 1 nM) whereas no effect was observed with the 12mer #144 (Fig. 3).
Figure 3.
In vitro translation screening of anti-IIId oligonucleotides. Dose-dependent inhibition of pIRF RNA in vitro translation by 2′-O-methyl antisense oligomers #121, #141, #120 and #144. The luciferase expression (Rluc/Fluc) is given relative to a translation reaction in the absence of antisense oligonucleotide. Each data point is the average of three independent experiments (± standard deviation).
To further assess the specificity of the effect displayed by #121, we performed an in vitro translation assay with a construct pGEM2HRV2 containing the human rhinovirus 2 IRES upstream of the coding region of a truncated form of the influenza virus NS1 protein. This IRES does not contain a target site complementary to the sequence #121. The translation of this construct resulted in the synthesis of a 28 kDa protein. As shown in Figure 4, oligonucleotide #121 at either 1 or 10 nM did not reduce the amount of the 28 K protein (lanes 4 and 5) in contrast to what was observed for the 36 K Renilla luciferase protein (lane 10). Note that in lane 10 no effect was detected on the production of the firefly luciferase (61 K). The mismatched oligomer #120 had no effect on either construct at 1 or 20 nM (lanes 2, 3, 7 and 8). Taken together, these results demonstrate that anti-IIId antisense 2′-O-methyloligoribonucleotides are highly specific of the HCV IRES and that the translation inhibition is related to the selective binding of antisense sequences to their target: mutating the antisense or changing the IRES sequence abolishes the inhibitory effect.
Figure 4.
Specific inhibition of HCV IRES-dependent translation by antisense oligonucleotide #121. Autoradiograph of gel showing NS1 (28 K), Renilla (36 K) and firefly (61 K) in vitro translation products from pGEM2HRV2 (lanes 1–5) or pIRF mRNA (lanes 6–10). Lanes 1 and 6, reaction without oligonucleotide. Lanes 2, 3, 7 and 8, reactions with 1 nM or 20 nM of oligonucleotide #120, respectively. Lanes 3, 4, 9 and 10, 1 nM or 20 nM of oligonucleotide #121, respectively. See Materials and Methods for experimental details.
Inhibition of translation in cultured cells
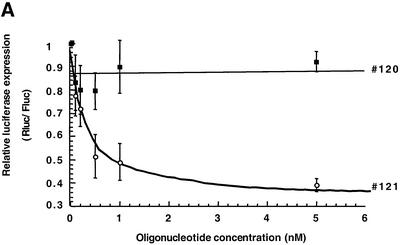
Antisense oligonucleotides targeted to the IIId domain proved to be efficient translation inhibitors in a cell free assay (RRL; see above). It was therefore of interest to check whether an effect could be detected in a more complex model. We co-transfected a mixture of our luciferase bicistronic transcript (pIRF) and of oligomer #121 in Huh7 cells. A 50% reduction of Renilla luciferase expression was observed following co-transfection with about 1 nM oligonucleotide (Fig. 5A). This inhibition was selective, as the mismatched oligonucleotide #120 induced only a 15% decrease of luciferase activity at concentrations up to 20 nM. We still observed an inhibition when the transfection of the bicistronic mRNA was delayed with respect to that of the oligonucleotide (Fig. 5B). The effect could be detected at 2, 6 or 17 h post-oligonucleotide transfection, however, the inhibition was of lower amplitude than when the target RNA and the antisense #121 were co-transfected. In contrast, the reverse situation (transfection of the mRNA first followed by that of the oligonucleotide 6 h later) did not result in translational reduction.
Figure 5.
Effect of antisense oligonucleotides on the translation of luciferase mRNA in transfected Huh7 cells. (A) Oligonucleotides #120 and #121 were co-transfected with RNA obtained from the pIRF construct under the conditions described in Materials and Methods. The luciferase expression (Rluc/Fluc) is given relative to a translation reaction in the absence of antisense oligonucleotide. The mean values from three independent experiments are indicated (± standard deviation). (B) Oligonucleotide #121 was transfected 2# (circle), 6# (square) or 17 h (triangle) prior to RNA transfection (see Materials and Methods for details).
Antisense oligonucleotide binding to the IRES IIId domain
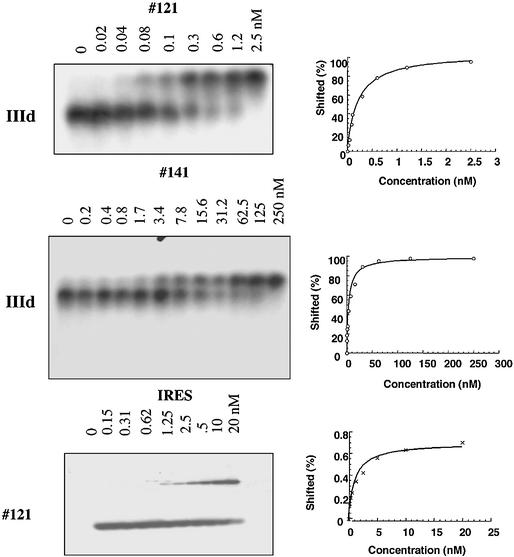
We quantitated the binding of oligonucleotides to the IIId domain of the HCV IRES, using EMSA. Addition of increasing amounts of antisense oligomers to labeled IIId RNA as described in Materials and Methods resulted in a low mobility species corresponding to the oligonucleotide-IIId RNA complex (Fig. 6). The analysis of the titration curve indicated that #121 binds to IIId with a Kd of 0.24 ± 0.08 nM and #141, which displayed a moderate level of IRES translation inhibition, binds to IIId with a Kd of 7.5 ± 0.7 nM. The mismatched oligomer #120 and the 12mer #144 (data not shown) did not give rise to any shifted band, indicating that they did not form stable complexes with the IIId RNA at concentrations up to 250 nM.
Figure 6.
Electrophoretic mobility shift assays of oligomer/RNA complexes. Electrophoretic mobility shift assays were performed at 23°C as described in Materials and Methods. IIId RNA was 32P 5′-end-labeled and the concentrations of unlabeled partners (oligonucleotides #121 or #141) are indicated at the top of each autoradiography. In the lower panel IRES RNA was added to the 32P-5′end-labeled oligonucleotide #121, at the concentration indicated at the top of each lane. The dissociation constants (Kds) were deduced from the densitometric analysis of the autoradiograph shown to the right (see Materials and Methods).
We then investigated the ability of oligonucleotide #121 to bind to the intact IRES. Increasing amounts of full length IRES RNA were added to 32P-labeled #121 and the complex was analyzed by EMSA (Fig. 6). The oligomer was still able to bind to its target although with a reduced affinity (Kd = 2.2 nM ± 0.6) compared to the IIId domain. No retarded band was detected when the antisense oligomer was mixed with the negative IRES RNA (not shown), thus demonstrating the specificity of the association.
Competition between antisense oligonucleotides and 40S ribosome subunit for IRES binding
The domain IIId is known to be involved in the binding of the 40S ribosomal subunit to the HCV IRES (10,12,14,28,29). It was therefore of interest to determine whether anti-IIId oligonucleotides competed out the ribosomal subunit. To this end full length (nucleotides 1–355) radiolabeled IRES RNA was incubated with purified 40S subunit from RRL and the resulting complexes were analyzed by filter retention. Curve fitting analysis of titration experiments, performed as described in Materials and Methods, showed that the HCV IRES RNA binds to the 40S subunit with a Kd of ∼5 nM in Tris buffer at 30°C (data not shown), in excellent agreement with previous results (10).
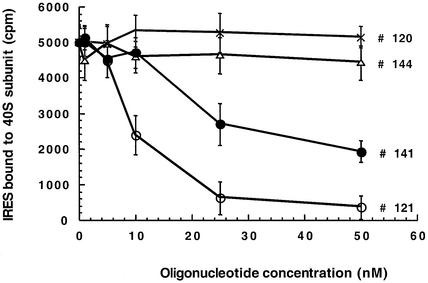
We then performed competition experiments between #121 and rabbit 40S subunit using the same assay. Results given in Figure 7 show that #121 displaces very efficiently the 40S subunit: retention of IRES was reduced by 50% in the presence of ∼8 nM of #121. Similar results were obtained when the 40S and the oligomer were added simultaneously or when the oligonucleotide #121 was added to the pre-formed 40S–IRES complex. In contrast, the mismatched oligomer #120 did not affect 40S subunit binding to the IRES RNA suggesting that the competition was directly related to oligonucleotide-IIId RNA complex formation. The oligomer #141 was a weaker competitor of the ribosomal subunit than the sequence #121, 50% displacement being achieved at oligonucleotide concentrations >20 nM whereas the antisense 12mer #144 had no effect on 40S–IRES RNA complex formation. Therefore there is a direct relationship between the oligonucleotide affinity for the IIId RNA and the ability of these oligomers to compete with 40S ribosomal subunit for IRES binding.
Figure 7.
Competition between antisense oligonucleotides and 40S ribosomal subunit for IRES binding. Binding curves of IRES RNA to 40S particles in presence of various oligonucleotides were deduced from filter retention assays (see Materials and Methods). The mean values of three independent experiments are indicated (± standard deviation).
DISCUSSION
We investigated the properties of a series of 2′-O-methyloligoribonucleotides targeted to domain IIId of the HCV IRES. Five out of the 10 antisense oligonucleotides are selective inhibitors of in vitro translation of the Renilla luciferase gene, which is the second cistron of our bicistronic construct, placed under the control of the HCV IRES (Fig. 2). These antisense oligonucleotides are remarkably efficient as they are characterized by an IC50 <10 nM in the rabbit reticulocyte cell-free medium. These oligomers 14–17 nt long are complementary to the 3′ strand (#121, 137, 140) or to the top part of the IIId hairpin (#143, 177). Due to the presence of non-canonical base pairs in the IIId upper stem the oligomers straddling the IIId loop do not form hairpins themselves; consequently they could hybridize to their RNA target without prior unfolding, thus retaining their full efficiency.
Over the last six years several studies used antisense RNAs (30), ribozymes (31,32) or antisense oligonucleotides targeted to the HCV IRES to inhibit in vitro translation of mRNAs driven by this viral internal entry site. Both unmodified (33) and chemically modified oligomers were used (34–40). These included phosphorothioate, 2′-modified derivatives, morpholino analogs and alpha anomers. The most active sequences in in vitro translation assays in RRL were characterized by an IC50 of ∼100 nM, i.e., more than one order of magnitude higher than what we obtained in our study for the most efficient 2′-O-methyloligomers (Fig. 2). The translation initiation codon and the region in its vicinity (about nucleotides 330–370) were generally targeted in these previous studies (34–36,40). A systematic screen using oligodeoxynucleotide-induced cleavage by RNase H to map the accessible sites in the HCV IRES also identified the initiator AUG region as a good target for antisense oligodeoxynucleotides using this criterion (38). Hanecak et al. (36) targeted the IIId domain by phosphorothioate oligodeoxynucleotides which were poor inhibitors of in vitro translation, likely due to the modest affinity of these derivatives for RNA targets. A morpholino 20mer targeted also to this domain did not show better inhibitory activity (37).
In vitro selection that we carried out against the IIId imperfect hairpin of the HCV IRES failed to identify RNA structures able to interact strongly with the target in contrast to previous studies performed with HCV or HIV hairpins (17,20). In the case of HIV, RNA hairpin aptamers characterized by a six membered loop, complementary to the TAR one flanked by selected G,A residues conferred to this loop–loop (‘kissing’) complex a high stability [Kd ≈ 20 nM; (25)]. For HCV, the rational design of an anti-IIId domain RNA ligand on the basis of this anti-TAR aptamer led to an oligomer of moderate affinity: an RNA hairpin with an 8 bp stem and a 6 nt loop complementary to the apical loop of the IIId domain, flanked by a G and a A residue, mimicking the anti-TAR aptamer, bound to the target RNA with a Kd >300 nM under the selection conditions (not shown). This might mean that the IIId stem–loop is not prone to loop–loop interactions. Given the similarity between the TAR loop (5′-CUGGGA) and the IIId loop (5′-UUGGGU) this indicates that kissing complex formation is extremely sensitive to the loop sequence. The ability to form loop–loop complexes might also depend on the top parts of the stems which constitute the critical helix–helix junctions. Our selection generated essentially antisense RNA oligomers which displayed an affinity at least 6-fold higher (Kd <50 nM) than the rationally designed anti-IIId ‘aptamer’ described above. This might be related to the relatively low stability of the upper stem of domain IIId which includes several non-Watson–Crick base pairs, thus promoting the invasion by complementary (antisense) sequences. The best antisense molecules were not present among the selected sequences of our SELEX experiment. This might be related firstly to the low number (20) of sequences analyzed after cloning. The outcome of the selection, i.e., the absence of folded aptamers did not encourage us to sequence and evaluate more clones. We cannot exclude that the selection was not completed and that we should have performed a few more rounds under more stringent conditions. However, the results of the selection were clear enough to indicate that antisense sequences were excellent ligands of the IIId domain. From this standpoint our SELEX experiment was fruitful.
Beyond the sensitivity of a given site to antisense oligonucleotides, the variability of the target sequence should also be considered as far as therapeutic perspectives are concerned. In this respect the comparison of different HCV genomes shows nucleotide substitutions in the region surrounding the initiator AUG codon (41) whereas the stem–loop IIId is absolutely conserved among all HCV genotypes, therefore constituting a more attractive target for antisense sequences. This strong conservation of the sequence of the IIId domain is undoubtedly related to its function, notably in the interaction of the IRES with the 40S ribosomal subunit (10,12,14,28). Point mutations in this IIId area are detrimental to 40S subunit binding and to IRES function (10). In particular, the G residues in the apical loop (G266, 267, 268) are essential for ribosomal subunit binding, as well as the Cs at positions 254 and 255. G266, 267 and 268 are strongly protected from reaction with kethoxal or dimethylsulfate in the IRES-40S subunit complex (12). Interestingly, our antisense sequences cover the key residues G266 to G268 in the loop, thus likely interfering with the functional contacts between the IIId domain and the ribosomal subunit. Indeed, we observed an excellent correlation between the antisense effect (IC50), the affinity of the oligonucleotide for the IIId RNA or the IRES (Kd) and the competition efficiency with the 40S ribosomal subunit. The detailed analysis of the behavior of oligonucleotides #121, #141, #144 as representatives of strong, moderate and inefficient antisense oligonucleotides show that the strong translation inhibition observed with #121 (IC50 = 2.5 nM) is related to a potent competition with 40S subunit. Conversely the oligonucleotides which do not displace the 40S–IRES complex (#144; #120) do not prevent in vitro translation. Therefore the reduction of Renilla luciferase expression by anti-IIId 2′-O-methyl, oligoribonucleotides might originate in the inhibition of the formation of the initiation complex.
Antisense oligonucleotides have been successfully used in transformed hepatocytes to inhibit HCV gene expression. Phosphorothioate and 2′ methoxyethoxy, phosphodiester antisense oligonucleotides have been shown to inhibit HCV RNA production and core protein synthesis upon delivery with lipofectin (36). The same phosphorothioate 20mer targeted to the initiator AUG region (nucleotides 330–349) was also shown to reduce HCV gene expression in the liver of BALB/c mice infected with an HCV-vaccinia virus recombinant (42). An antisense oligonucleotide targeted to the same region of the HCV IRES is being evaluated in a phase I/II clinical trial (43). Our 2′-O-methylribonucleotide 17mer sequence targeted to the IIId domain retained its antisense potency in cultured Huh7 cells, at least when the RNA–oligomer complex is perfomed. The effect observed when the oligonucleotide was present in the cell prior to transfection of the target RNA indicates that the hybridization still takes place inside the cell. The failure to inhibit translation when RNA is transfected first might be related to the difficulty to compete out pre-formed initiation complexes even though filter binding assays showed that the antisense oligomer displaced the 40S ribosomal subunit. The luciferase activity detected under these conditions is contributed partly by the enzyme synthesized prior to oligonucleotide transfection. It is also worth mentioning that our oligomers were assayed at much lower concentrations than that used in previous studies. The properties exhibited by anti-IIId oligomers in vitro together with previous results using other oligomers and different targets on the HCV mRNA encourage us to investigate antisense oligomers with improved affinity. Work along this line is in progress in our laboratory.
Acknowledgments
ACKNOWLEDGEMENTS
We are indebted to Dr J. Michel for the synthesis of 2′-O-methyloligoribonucleotides and to S. Reigadas for the production of IRES RNA. We are grateful to Dr A. Cahour, Paris and H. Jacquemin-Sablon, Bordeaux, for the gift of bicistronic constructs and to Dr C. Boiziau for helpful discussion. This work has been supported by a contract from the European Union (QOL-2000-3.1.2) and by a grant from the Réseau Fondamental Hépatite C. L.A.-C. was supported by a grant from the Association pour la Recherche contre le Cancer.
REFERENCES
- 1.Bartenschlager R., Ahlborn-Laake,L., Mous,J. and Jacobsen,H. (1994) Kinetic and structural analyses of hepatitis C virus polyprotein processing. J. Virol., 68, 5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grakoui A., Wychowski,C., Lin,C., Feinstone,S.M. and Rice,C.M. (1993) Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol., 67, 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C., Lindenbach,B.D., Pragai,B.M., McCourt,D.W. and Rice,C.M. (1994) Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J. Virol., 68, 5063–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santolini E., Migliaccio,G. and La Monica,N. (1994) Biosynthesis and biochemical properties of the hepatitis C virus core protein. J. Virol., 68, 3631–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rijnbrand R., Bredenbeek,P., van der Straaten,T., Whetter,L., Inchauspé,G., Lemon,S. and Spaan,W. (1995) Almost the entire 5′ non-translated region of hepatitis C virus is required for cap-independent translation. FEBS Lett., 365, 115–119. [DOI] [PubMed] [Google Scholar]
- 6.Tsukiyama-Kohara K., Iizuka,N., Kohara,M. and Nomoto,A. (1992) Internal ribosome entry site within hepatitis C virus RNA. J. Virol., 66, 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Le,S.Y., Ali,N. and Siddiqui,A. (1995) An RNA pseudoknot is an essential structural element of the internal ribosome entry site located within the hepatitis C virus 5′ noncoding region. RNA, 1, 526–537. [PMC free article] [PubMed] [Google Scholar]
- 8.Honda M., Beard,M.R., Ping,L.H. and Lemon,S.M. (1999) A phylogenetically conserved stem–loop structure at the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J. Virol., 73, 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pestova T.V., Shatsky,I.N., Fletcher,S.P., Jackson,R.J. and Hellen,C.U. (1998) A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev., 12, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieft J.S., Zhou,K., Jubin,R., Murray,M.G., Lau,J.Y. and Doudna,J.A. (1999) The hepatitis C virus internal ribosome entry site adopts an ion-dependent tertiary fold. J. Mol. Biol., 292, 513–529. [DOI] [PubMed] [Google Scholar]
- 11.Kieft J.S., Zhou,K., Jubin,R. and Doudna,J.A. (2001) Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA, 7, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukavsky P.J., Otto,G.A., Lancaster,A.M., Sarnow,P. and Puglisi,J.D. (2000) Structures of two RNA domains essential for hepatitis C virus internal ribosome entry site function. Nature Struct. Biol., 7, 1105–1110. [DOI] [PubMed] [Google Scholar]
- 13.Klinck R., Westhof,E., Walker,S., Afshar,M., Collier,A. and Aboul-Ela,F. (2000) A potential RNA drug target in the hepatitis C virus internal ribosomal entry site. RNA, 6, 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolupaeva V.G., Pestova,T.V. and Hellen,C.U. (2000) An enzymatic footprinting analysis of the interaction of 40S ribosomal subunits with the internal ribosomal entry site of hepatitis C virus. J. Virol., 74, 6242–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toulmé J.J. (2001) New candidates for true antisense. Nat. Biotechnol., 19, 17–18. [DOI] [PubMed] [Google Scholar]
- 16.Boiziau C., Dausse,E., Yurchenko,L. and Toulmé J.J. (1999) DNA aptamers selected against the HIV-1 trans-activation-responsive RNA element form RNA–DNA kissing complexes. J. Biol. Chem., 274, 12730–12737. [DOI] [PubMed] [Google Scholar]
- 17.Ducongé F. and Toulmé,J.J. (1999) In vitro selection identifies key determinants for loop-loop interactions: RNA aptamers selective for the TAR RNA element of HIV-1. RNA, 5, 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darfeuille F., Cazenave,C., Gryaznov,S., Ducongé,F., Di Primo,C. and Toulmé,J.J. (2001) RNA and N3′→P5′ kissing aptamers targeted to the trans-activation responsive (TAR) RNA of the human immunodeficiency virus-1. Nucleosides Nucleotides Nucleic Acids, 20, 441–449. [DOI] [PubMed] [Google Scholar]
- 19.Darfeuille F., Arzumanov,A., Gryaznov,S., Gait,M.J., Di Primo,C. and Toulmé,J.J. (2002) Loop–loop interaction of HIV-1 TAR RNA with N3′→P5′ deoxyphosphoramidate aptamers inhibits in vitro Tat-mediated transcription. Proc. Natl Acad. Sci. USA, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldaz-Carroll L., Tallet,B., Dausse,E., Yurchenko,L. and Toulmé,J.J. (2002) Apical loop-internal loop interactions: a new RNA–RNA recognition motif identified through in vitro selection against RNA hairpins of the hepatitis C virus mRNA. Biochemistry, 41, 5883–5893. [DOI] [PubMed] [Google Scholar]
- 21.Borman A. and Jackson,R.J. (1992) Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology, 188, 685–696. [DOI] [PubMed] [Google Scholar]
- 22.Yanagi M., Purcell,R.H., Emerson,S.U. and Bukh,J. (1997) Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl Acad. Sci. USA, 94, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pestova T.V., Hellen,C.U. and Shatsky,I.N. (1996) Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol., 16, 6859–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagus R. (1987) Translation in cell-free systems. Methods Enzymol., 152, 267–276. [DOI] [PubMed] [Google Scholar]
- 25.Ducongé F., Di Primo,C. and Toulmé,J.J. (2000) Is a closing “GA pair” a rule for stable loop–loop RNA complexes? J. Biol. Chem., 275, 21287–21294. [DOI] [PubMed] [Google Scholar]
- 26.Cazenave C., Frank,P. and Büsen,W. (1993) Characterization of ribonuclease H activities present in two cell-free protein synthesizing systems, the wheat germ extract and the rabbit reticulocyte lysate. Biochimie, 75, 113–122. [DOI] [PubMed] [Google Scholar]
- 27.Lamond A.I. (1993) 2′-O-Alkyloligoribonucleotides: Probes for studying the biochemistry and cell biology of RNA processing. Biochemical Society Transactions, 21, 1–8. [DOI] [PubMed] [Google Scholar]
- 28.Spahn C.M., Kieft,J.S., Grassucci,R.A., Penczek,P.A., Zhou,K., Doudna,J.A. and Frank,J. (2001) Hepatitis C virus IRES RNA-induced changes in the conformation of the 40S ribosomal subunit. Science, 291, 1959–1962. [DOI] [PubMed] [Google Scholar]
- 29.Lytle J.R., Wu,L. and Robertson,H.D. (2001) The ribosome binding site of hepatitis C virus mRNA. J. Virol., 75, 7629–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakita T., Moradpour,D., Tokushihge,K. and Wands,J.R. (1999) Antiviral effects of antisense RNA on hepatitis C virus RNA translation and expression. J. Med. Virol., 57, 217–222. [PubMed] [Google Scholar]
- 31.Sakamoto N., Wu,C.H. and Wu,G.Y. (1996) Intracellular cleavage of hepatitis C virus RNA and inhibition of viral protein translation by hammerhead ribozymes. J. Clin. Invest., 98, 2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber A., He,C.Y., Polyak,S.J., Gretch,D.R., Barr,D. and Kay,M.A. (1996) Elimination of hepatitis C virus RNA in infected human hepatocytes by adenovirusmediated expression of ribozymes. J. Virol., 70, 8782–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakita T. and Wands,J.R. (1994) Specific inhibition of hepatitis C virus expression by antisense oligodeoxynucleotides. In vitro model for selection of target sequence. J. Biol. Chem., 269, 14205–14210. [PubMed] [Google Scholar]
- 34.Alt M., Eisenhardt,S., Serwe,M., Renz,R., Engels,J.W. and Caselmann,W.H. (1999) Comparative inhibitory potential of differently modified antisense oligodeoxynucleotides on hepatitis C virus translation. Eur. J. Clin. Invest., 29, 868–876. [DOI] [PubMed] [Google Scholar]
- 35.Brown-Driver V., Eto,T., Lesnik,E., Anderson,K.P. and Hanecak,R.C. (1999) Inhibition of translation of hepatitis C virus RNA by 2′-modified antisense oligonucleotides. Antisense Nucleic Acid Drug Dev., 9, 145–154. [DOI] [PubMed] [Google Scholar]
- 36.Hanecak R., Browndriver,V., Fox,M.C., Azad,R.F., Furusako,S., Nozaki,C., Ford,C., Sasmor,H. and Anderson,K.P. (1996) Antisense oligonucleotide inhibition of hepatitis C virus gene expression in transformed hepatocytes. J. Virol., 70, 5203–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jubin R., Vantuno,N.E., Kieft,J.S., Murray,M.G., Doudna,J.A.,. Lau,J.Y.N. and Baroudy,B.M. (2000) Hepatitis C Virus Internal Ribosome Entry Site (IRES) Stem Loop IIId Contains a Phylogenetically Conserved GGG Triplet Essential for Translation and IRES Folding. J. Virol., 74, 10430–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima W.F., Brown-Driver,V., Fox,M., Hanecak,R. and Bruice,T.W. (1997) Combinatorial screening and rational optimization for hybridization to folded hepatitis C virus RNA of oligonucleotides with biological antisense activity. J. Biol. Chem., 272, 626–638. [PubMed] [Google Scholar]
- 39.Seki M. and Honda,Y. (1995) Phosphorothioate antisense oligodeoxynucleotides capable of inhibiting hepatitis c virus gene expression: in vitro translation assay. J. Biochem. (Tokyo), 118, 1199–1204. [DOI] [PubMed] [Google Scholar]
- 40.Vidalin O., Major,M.E., Rayner,B., Imbach,J.L., Trepo,C. and Inchauspé,G. (1996) In vitro inhibition of hepatitis C virus gene expression by chemically modified antisense oligodeoxynucleotides. Antimicrob. Agents Chemother., 40, 2337–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier A.J., Tang,S. and Elliott,R.M. (1998) Translation efficiencies of the 5′ untranslated region from representatives of the six major genotypes of hepatitis C virus using a novel bicistronic reporter assay system. J. Gen. Virol., 79, 2359–2366. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., Hanecak,R., Brown-Driver,V., Azad,R., Conklin,B., Fox,M.C. and Anderson,K.P. (1999) Antisense oligonucleotide inhibition of hepatitis C virus (HCV) gene expression in livers of mice infected with an HCV-vaccinia virus recombinant. Antimicrob. Agents Chemother., 43, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witherell G.W. (2001) ISIS-14803 (Isis Pharmaceuticals). Curr. Opin. Investig. Drugs, 2, 1523–1529. [PubMed] [Google Scholar]