Abstract
A long-standing question in the biology of the intracellular bacterium, Chlamydia, has been the structure of the promoter recognized by its RNA polymerase. The ‘RNA polymerase sigma subunit paradox’ refers to the difficulty reconciling the conservation between the RNA polymerases of Chlamydia and Escherichia coli, especially at the level of the promoter-recognition sigma subunit, with the general lack of homology between chlamydial promoters and the E.coli σ70 consensus promoter. While the –10 promoter element appears to be conserved between Chlamydia and E.coli, the structure of the chlamydial –35 promoter element has not been defined. We have investigated the structure of the –35 element of the Chlamydia trachomatis dnaK promoter by measuring the effects of single base pair substitutions on in vitro promoter activity. Most substitutions produced large decreases in promoter activity, which allowed us to define the optimal –35 sequence in the context of the dnaK promoter. We found that the optimal chlamydial –35 promoter sequence is identical to the E.coli σ70 consensus –35 promoter element (TTGACA). These results indicate that the optimal promoter specificities of the major form of chlamydial RNA polymerase and E.coli σ70 RNA polymerase are in fact highly conserved. A further implication of our results is that many chlamydial promoters have a suboptimal promoter structure. We hypothesize that these chlamydial promoters are intrinsically weak promoters that can be regulated during the chlamydial developmental cycle by additional transcription factors.
INTRODUCTION
Chlamydia is a Gram-negative pathogenic bacterium and an obligate intracellular parasite of eukaryotic cells. It has an unusual developmental cycle characterized by two morphologic forms (1–3). The elementary body is an infectious, but metabolically inactive, form. Upon infection of a host cell, it converts into the reticulate body, which is the intracellular, metabolically active form that divides by binary fission as part of the replicative phase of the developmental cycle.
The chlamydial developmental cycle is characterized by the coordinate transcriptional regulation of gene expression (1–3). A major unresolved issue in the field of chlamydial transcription is the structure of the chlamydial promoter. Chlamydial promoters show variability in the –35 element, which has made it difficult to deduce a consensus chlamydial promoter sequence (reviewed in 4,5), and few chlamydial promoters resemble the Escherichia coli σ70 consensus promoter structure. In contrast, the RNA polymerases of Chlamydia and E.coli are highly conserved, especially in the sigma subunit, which recognizes promoter sequences. Stephens has described the lack of conservation of chlamydial promoters in the face of RNA polymerase conservation as the ‘RNA polymerase sigma subunit paradox’ (6). There has also been speculation that chlamydial RNA polymerase may have an altered promoter specificity (7,8).
In the absence of an experimental chlamydial genetics system, promoter structure in Chlamydia has been studied with in vitro transcription methods (7–11) and a heterologous in vivo system in E.coli (12). We previously used an in vitro saturation mutagenesis approach to define the optimal –10 promoter sequence of the Chlamydia trachomatis rRNA promoter (10). This optimal sequence resembles the E.coli σ70 consensus –10 promoter element. However, we were unable to define the optimal –35 promoter sequence of the rRNA promoter because single base substitutions in this region had little effect on promoter activity. Examination of the predicted sequence of other chlamydial promoters also shows a well conserved –10 promoter element but poor sequence conservation in the –35 region (4,10).
To address the ‘RNA polymerase sigma subunit paradox’, and to determine the promoter specificity of chlamydial RNA polymerase, we have defined the optimal –35 sequence of the dnaK promoter. The C.trachomatis dnaK promoter is a heat shock promoter that has recently been shown to be negatively regulated by a heat shock repressor, HrcA (13). We chose to study the dnaK promoter because it is one of the few predicted chlamydial promoters that resembles the E.coli σ70 consensus promoter structure, and it has a 5/6 match in the –35 region. In this report, we demonstrate that substitutions in the –35 region of the dnaK promoter produced large decreases in promoter activity. This mutational approach has allowed us to define for the first time the optimal –35 promoter sequence for a specific class of chlamydial promoters. This optimal chlamydial promoter structure strongly resembles the E.coli σ70 consensus promoter, and suggests that the optimal promoter specificity of chlamydial and E.coli RNA polymerases is highly conserved. These results raise the question of why many chlamydial promoters, including the normally highly transcribed rRNA promoter, do not resemble the optimal promoter structure. We discuss the possibility that many chlamydial promoters are intrinsically weak because of their suboptimal promoter structure, thereby allowing for regulation during the chlamydial developmental cycle by additional transcription factors.
MATERIALS AND METHODS
Reagents
Products were obtained from the following sources and were used according to the manufacturer’s specifications. Restriction enzymes, calf alkaline phosphatase, T4 DNA ligase, rRNasin and Thermus aquaticus DNA polymerase from Promega Biotech (Madison, WI); T4 polynucleotide kinase from New England Biolabs (Beverly, MA); T7 Sequenase DNA polymerase and dideoxynucleotide kit from United States Biochemical Corp. (Cleveland, OH); nucleoside triphosphates, 3′-O-methylguanosine 5′-triphosphate from Amersham Pharmacia Biotech (Arlington Heights, IL); 32P-containing nucleoside triphosphates from ICN Pharmaceuticals, Inc. (Costa Mesa, CA); ampicillin from Fisher Scientific (Pittsburgh, PA); and purified E.coli RNA polymerase from Epicentre (Madison, WI).
DNA manipulation
Nucleic acid preparation and analysis were performed according to standard recombinant DNA protocols, as described previously (10).
Construction of the wild-type dnaK transcription template
The dnaK promoter region (–39 to +6) from C.trachomatis, mouse pneumonitis strain, was amplified by PCR. The promoter insert was cloned upstream of a promoterless G-less cassette transcription template in plasmid pMT504 (10). pMT504 also contains an internal control transcription template consisting of the C.trachomatis rRNA promoter (–53 to +5) upstream of a shorter G-less cassette. Transcription of the plasmid produced a 159 nt test transcript and a 130 nt control transcript.
Construction of dnaK transcription templates containing mutations
Individual mutant promoters were produced by PCR, with the desired mutation introduced on an oligonucleotide primer. Each template contained the dnaK promoter region from –39 to +6. A 5 bp mutation of the –35 region was produced by altering the sequence from –34 to –30 (TTGAC to GGTCA). Eighteen mutant dnaK promoters (–39 to +6), with single base pair substitutions in the –35 region, were produced, so that the effect of all possible single substitutions from –34 to –29 could be tested. The mutant dnaK promoters were cloned upstream of a promoterless G-less cassette transcription template in plasmid pMT504 as previously described (10).
Purification of C.trachomatis RNA polymerase
RNA polymerase was partially purified from C.trachomatis serovar LGV L2 at 20 h post-infection by heparin–agarose chromatography as previously described (9).
In vitro transcription
The following reaction mixture was assembled: 50 mM potassium acetate, 8.1 mM magnesium acetate, 50 mM Tris acetate pH 8.0, 27 mM ammonium acetate, 2 mM DTT, 400 µM ATP, 400 µM UTP, 1.2 µM CTP, 0.06 µM [α-32P]CTP (3000 Ci/mmol), 100 µM 3′-O-methylguanosine 5′-triphosphate Na salt, 18 U rRNasin, 10% glycerol and 1.0 µl heparin–agarose-purified C.trachomatis RNA polymerase or 0.03 U of E.coli σ70 RNA polymerase. Supercoiled DNA template (final concentration 25 nM) was added and the reaction was incubated at 37°C for 15 min. The final reaction volume was 10 µl. The reaction was terminated by the addition of 10 µl of stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol). An aliquot of 7 µl of the sample was electrophoresed on an 8 M urea/6% polyacrylamide gel. Transcripts were visualized by autoradiography and quantified with a BioRad Personal Molecular Imager FX (Hercules, CA).
Calculation of promoter activity
Each transcription reaction produced a test transcript from the dnaK promoter, and a control transcript from the C.trachomatis rRNA promoter. Promoter activity was calculated as the ratio of the test transcript to the control transcript. Relative promoter activity was determined by normalizing the promoter activity of each mutant promoter to that of the wild-type dnaK promoter defined as 100%. Three measurements of relative promoter activity were obtained for each promoter, and a mean and a standard deviation were calculated. Fold changes in promoter activity were obtained by comparing the relative promoter activity of each mutant promoter to that of the wild-type dnaK promoter.
RESULTS
Substitution of the –35 region of the dnaK promoter decreased transcription by C.trachomatis RNA polymerase
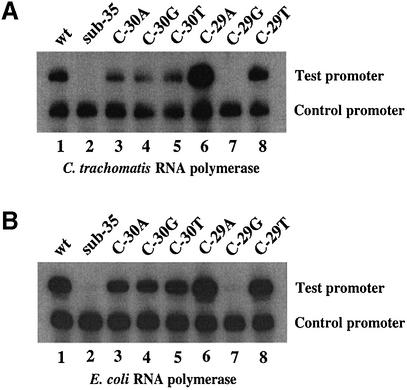
We have previously shown that the C.trachomatis dnaK promoter is transcribed by chlamydial RNA polymerase in vitro (14). To determine if the –35 promoter element is important for transcription, we made a 5 bp substitution of the predicted –35 region, changing the sequence from TTGAC to GGTCA at positions –34 to –30 (Fig. 1). This substitution of the –35 region produced a 22-fold decrease in transcription by C.trachomatis RNA polymerase (Fig. 2A, lanes 1 and 2). The C.trachomatis dnaK promoter is also transcribed by E.coli RNA polymerase (14). The same 5 bp substitution of the –35 region produced a 54-fold decrease in transcription by E.coli RNA polymerase (Fig. 2B, lanes 1 and 2).
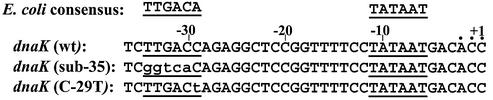
Figure 1.
Sequence of the C.trachomatis dnaK promoter region. A 5 bp substitution in the –35 region (sub-35), and an example of a single base pair substitution (C to T change at –29, C-29T) are shown beneath the wild-type (wt) promoter sequence. The E.coli σ70 consensus sequence is shown for comparison. Predicted –35 and –10 promoter sequences are underlined. Substitutions are shown in lower case type. Dots above the sequence indicate in vivo transcription initiation sites with the numbering shown relative to the position marked +1.
Figure 2.
In vitro transcription of C.trachomatis dnaK promoter templates by C.trachomatis RNA polymerase (A) or E.coli RNA polymerase (B). The dnaK templates contained the wild-type (wt) sequence (lane 1), a 5 bp substitution of the –35 region (sub-35) (lane 2), a single base pair substitution of the wild-type C at position –30 with A (lane 3), G (lane 4) or T (lane 5), or a single base pair substitution of the wild-type C at position –29 with A (lane 6), G (lane 7) or T (lane 8). The C.trachomatis rRNA promoter was used as the control promoter.
Single base substitutions in the –35 region of the dnaK promoter also had an effect on promoter activity
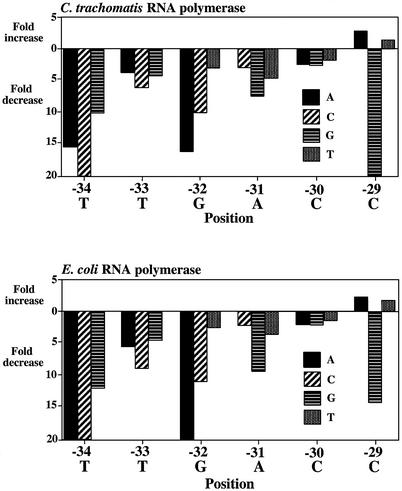
We tested the effect on in vitro transcription of single base substitutions in the predicted –35 region from positions –34 to –29. At each position, we tested each of the three possible substitutions separately. Most of the single base substitutions produced a large effect on transcription by C.trachomatis RNA polymerase. For example, each of the substitutions at positions –34, –33, –32 and –31 decreased promoter activity from 3- to >20-fold (Fig. 3). Point substitutions at –30 had less effect, with substitution of the wild-type C by an A, G or T producing a 2.3-, 2.5- or 1.6-fold decrease in transcription, respectively. Of all the positions in the –35 promoter region, only substitutions at –29 produced an increase in transcription. At this position, substitution of the wild-type C with an A or T produced a 3.1- and 1.2-fold increase, respectively. Examples of transcription of promoters containing single base substitutions are shown in Figure 2A.
Figure 3.
Effects of single base pair substitutions on the C.trachomatis dnaK promoter by C.trachomatis or E.coli RNA polymerases. All three possible substitutions were tested at each position from –34 to –29. The wild-type sequence at each position is shown below each graph. Changes in promoter activity are shown as fold increases or decreases relative to wild-type promoter activity. Decreases >20-fold are not illustrated as extending beyond the bottom axis. Each experiment is the mean of three separate experiments.
Single base substitutions in the –35 region of the dnaK promoter also affected transcription by E.coli RNA polymerase
We also assayed the promoter activity of this series of C.trachomatis dnaK promoter mutations when transcribed by E.coli RNA polymerase. Most of the single base substitutions produced a large decrease in transcription with a pattern similar to that obtained with C.trachomatis RNA polymerase (Figs 2B and 3). The smallest differences were also seen at –30 and –29. At –30, substitution of the wild-type C with an A, G or T, produced a 1.8-, 1.9- or 1.2-fold decrease in transcription, respectively (Fig. 2B). As with C.trachomatis RNA polymerase substitutions of wild-type C at –29 with an A or T produced a slight increase in promoter activity (up 2.4- and 1.7-fold, respectively).
Derivation of an optimal promoter sequence
To determine if there is a sequence preference in the –35 promoter element, we examined each position in the –35 promoter region, comparing the effect of each of the four possible nucleotides on promoter activity. To facilitate this analysis, we determined a relative promoter activity for each mutant promoter by normalizing the promoter activity to that of the wild-type dnaK promoter, which was defined as 100% (Table 1). For example, at position –34, the relative promoter activity when transcribed by C.trachomatis RNA polymerase was 6.5, 3.0, 10.0 or 100% for an A, C, G or T at this position, respectively. Thus, at –34 there was a marked sequence preference for a T and all other substitutions decreased promoter activity significantly. These results are best displayed in Figure 4 where, for each position, the height of the letter representing each nucleotide is proportional to its relative promoter activity. In this manner, the optimal –35 promoter sequence for C.trachomatis RNA polymerase was determined to be TTGACA in the context of the dnaK promoter. The optimal –35 promoter sequence for E.coli RNA polymerase in the context of the C.trachomatis dnaK promoter was TTGA(C/T)A which compares very favorably with the E.coli σ70 consensus –35 promoter element (15).
Table 1. Promoter activities of dnaK promoter templates with single base pair substitutions.
| Position | Wild-type sequence | Relative promoter activity ± SDa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Chlamydia trachomatis RNA polymerase | Escherichia coli RNA polymerase | ||||||||
| A | C | G | T | A | C | G | T | ||
| –34 | T | 6.5 ± 2.3 | 3.0 ± 0.5 | 10.0 ± 2.0 | 100 | 4.0 ± 0.4 | 3.0 ± 0.3 | 8.4 ± 1.4 | 100 |
| –33 | T | 28.8 ± 1.3 | 16.6 ± 3.0 | 24.4 ± 2.3 | 100 | 18.6 ± 1.6 | 11.4 ± 2.3 | 22.6 ± 1.3 | 100 |
| –32 | G | 6.2 ± 1.0 | 9.9 ± 1.6 | 100 | 34.4 ± 4.7 | 4.0 ± 0.2 | 9.1 ± 1.2 | 100 | 41.0 ± 2.3 |
| –31 | A | 100 | 35.2 ± 8.8 | 13.7 ± 3.4 | 22.1 ± 8.7 | 100 | 46.6 ± 3.2 | 10.9 ± 0.7 | 29.2 ± 1.6 |
| –30 | C | 43.0 ± 6.3 | 100 | 40.7 ± 4.3 | 63.8 ± 10.1 | 57.0 ± 4.5 | 100 | 53.2 ± 1.8 | 80.8 ± 7.3 |
| –29 | C | 312.6 ± 12.3 | 100 | 2.1 ± 0.6 | 118.3 ± 26.4 | 242.0 ± 37.7 | 100 | 7.1 ± 1.9 | 168.9 ± 13.0 |
aThe relative promoter activity was determined by normalizing to that of the wild-type dnaK promoter defined as 100%.
Each value is the mean of three separate experiments.
Figure 4.
Base preference for C.trachomatis RNA polymerase (A) or E.coli RNA polymerase (B), based on a mutational analysis of the C.trachomatis dnaK promoter from positions –34 to –29. For each position the height of each letter is proportional to its relative promoter activity. The position relative to the start site of transcription is indicated above the letters.
DISCUSSION
We have defined the optimal –35 promoter sequence of the dnaK promoter by measuring the effect of single base pair substitutions on in vitro transcription by C.trachomatis RNA polymerase. This RNA polymerase contains σ66 (9), the major chlamydial sigma subunit, which is the homolog of E.coli σ70 (16,17). In a previous study of the rRNA promoter we were able to use this approach to define the optimal chlamydial –10 promoter element, but not the –35 element (10). We hypothesize that the mutational approach in the current study was successful because the –35 element of the dnaK promoter resembles the optimal chlamydial promoter structure more closely than the rRNA promoter.
The optimal chlamydial –35 promoter sequence, TTGACA, is identical to the E.coli σ70 consensus –35 promoter element. The strongest sequence preference lies in the first three positions, while the fifth position showed the least sequence specificity (Fig. 4). Our results obtained with E.coli RNA polymerase were very similar, although not identical. Both the optimal chlamydial and E.coli –35 promoter sequences that we derived strongly resemble the E.coli σ70 consensus –35 promoter element (15). Furthermore, the E.coli consensus –35 promoter element also shows greatest sequence conservation in the first three positions. These results, together with our previous definition of the –10 promoter element, demonstrate that there is strong conservation between the –35 and –10 promoter elements of C.trachomatis and other bacteria such as E.coli (15) and Bacillus subtilis (18).
Prior to this study, a major unresolved issue in the field of chlamydial transcription was the ‘RNA polymerase sigma subunit paradox’, as noted by Stephens (6). This paradox referred to the difficulty reconciling the strong sequence conservation between the RNA polymerases of Chlamydia and E.coli, and the lack of consensus homology between chlamydial and E.coli promoters. In fact, it has not been possible to determine a consensus chlamydial –35 promoter sequence (reviewed in 4,5). Our studies show that the optimal promoter sequence recognized by chlamydial and E.coli RNA polymerases is actually very similar, which agrees with the amino acid conservation in the subregions of the RNA polymerase σ subunit that have been shown to recognize promoter elements (16,17). It has been proposed that additional regions of chlamydial σ66 outside these promoter recognition domains may have a role in promoter recognition (12). Our results support the idea that the paradox can be explained at the promoter level, and that there is a lack of sequence conservation among chlamydial promoters because many have a suboptimal promoter sequence, particularly in the –35 element (4,10).
One possible explanation for a suboptimal promoter structure is that these promoters are weak by design so that they can be regulated by transcription factors acting via cis-acting regulatory sequences (4,10,19). Douglas and Hatch have raised the possibility that sequences located in the spacer region between the promoter elements of the C.trachomatis MOMP P2 promoter are required for high level transcription (4,8). We have defined a cis-acting element in the spacer region of the C.trachomatis rRNA promoter that we have called the spacer A/T region because of its location and a sequence preference for A and T residues (10). Spacer A/T regions have also been identified in other chlamydial promoters (19) that we now recognize as having suboptimal –35 promoter elements. We do not know if the Spacer A/T region has an intrinsic ability to increase promoter activity, or whether it serves as a binding site for a positive-acting factor.
An intrinsically weak rRNA promoter with a suboptimal promoter structure is unusual among bacteria. In E.coli, the rRNA promoter closely resembles the consensus promoter sequence and is a strong promoter (20). rRNA is transcribed at high levels so that the ribosomal translational machinery is available for bacterial growth. A weak rRNA promoter that can be upregulated would be more consistent with an organism such as Chlamydia, where there are distinct phases in the developmental cycle, with a metabolically inactive elementary body and a metabolically active reticulate body. Chlamydial rRNA transcription is first detectable at 7 h after infection of a host cell, at about the time the elementary body is converting into a reticulate body (21).
With the definition of the optimal C.trachomatis promoter sequence, we are also able to identify chlamydial promoters that are likely to be strong promoters because they are predicted to have optimal promoter sequences. The promoter for the omcB gene (22), which encodes the 60 kDa cysteine-rich protein, is predicted to be a strong promoter, with good –35 and –10 promoter elements. It also contains a spacer A/T region that has been shown to increase its transcription (19). The dnaK and PCT promoters (23) are also predicted to be strong promoters, but they do not contain an identifiable spacer A/T region. The –35 and –10 elements of the dnaK promoter are well conserved between chlamydial strains, including C.trachomatis (mouse pneumonitis strain, and serovars D and L2) and Chlamydia pneumoniae (C. S. Schaumburg and M. Tan, unpublished observations).
We have defined the optimal –35 promoter sequence recognized by the main form of C.trachomatis RNA polymerase without having had to derive a consensus promoter sequence, which would have required the definition of multiple promoter structures. Using similar methods, we have also derived the optimal –35 promoter sequence that is recognized by E.coli RNA polymerase, and the strong resemblance to the E.coli σ70 consensus promoter provides validation for this approach.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Dr Bert Semler, Allen Chen, Adam Wilson and Hilda Hiu Yin Yu for their valued suggestions and support. This work was supported by a grant from the NIH (AI 44198).
REFERENCES
- 1.Moulder J.W. (1991) Interaction of chlamydiae and host cells in vitro. Microbiol. Rev., 55, 143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyrick P.B. (2000) Intracellular survival by Chlamydia. Cell Microbiol., 2, 275–282. [DOI] [PubMed] [Google Scholar]
- 3.Shaw E.I., Dooley,C.A., Fischer,E.R., Scidmore,M.A., Fields,K.A. and Hackstadt,T. (2000) Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol., 37, 913–925. [DOI] [PubMed] [Google Scholar]
- 4.Hatch T.P. (1999) Developmental Biology. In Stephens,R.S. (ed.), Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. American Society for Microbiology, Washington, DC, pp. 29–67.
- 5.Timms P. and Mathews,S. (2002) Chlamydial Infections: Proceedings of the Tenth International Symposium on Human Chlamydial Infections. In Schachter,J. (ed.), International Chlamydia Symposium, San Francisco, Vol. 10, pp. 585–594.
- 6.Stephens R.S. (1990) Molecular Genetics of Chlamydia. In William,R. and Bowie,E.A. (eds), Chlamydial Infections 1990. Cambridge University Press, Cambridge, UK, pp. 63–72.
- 7.Mathews S.A. and Sriprakash,K.S. (1994) The RNA polymerase of Chlamydia trachomatis has a flexible sequence requirement at the –10 and –35 boxes of its promoters. J. Bacteriol., 176, 3785–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas A.L. and Hatch,T.P. (1996) Mutagenesis of the P2 promoter of the major outer membrane protein gene of Chlamydia trachomatis. J. Bacteriol., 178, 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan M. and Engel,J.N. (1996) Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J. Bacteriol., 178, 6975–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan M., Gaal,T., Gourse,R.L. and Engel,J.N. (1998) Mutational analysis of the Chlamydia trachomatis rRNA P1 promoter defines four regions important for transcription in vitro. J. Bacteriol., 180, 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L., Shi,Y., Douglas,A.L., Hatch,T.P., O’Connell,C.M., Chen,J.M. and Zhang,Y.X. (2000) Identification and characterization of promoters regulating tuf expression in Chlamydia trachomatis serovar F. Arch. Biochem. Biophys., 379, 46–56. [DOI] [PubMed] [Google Scholar]
- 12.Mathews S.A. and Stephens,R.S. (1999) DNA structure and novel amino and carboxyl termini of the Chlamydia sigma 70 analogue modulate promoter recognition. Microbiology, 145, 1671–1681. [DOI] [PubMed] [Google Scholar]
- 13.Wilson A. and Tan,M. (2002) Functional analysis of the heat shock regulator HrcA of Chlamydia trachomatis. J. Bacteriol., 184, 6566–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M., Wong,B. and Engel,J.N. (1996) Transcriptional organization and regulation of the dnaK and groE operons of Chlamydia trachomatis. J. Bacteriol., 178, 6983–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley D.K. and McClure,W.R. (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res., 11, 2237–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel J. and Ganem,D. (1990) A PCR-based approach to cloning sigma factors from eubacteria and its application to the isolation of a sigma70 homolog from Chlamydia trachomatis. J. Bacteriol., 172, 2447–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler J.E., Burgess,R.R., Thompson,N.E. and Stephens,R.S. (1990) Chlamydia trachomatis RNA polymerase major σ subunit. J. Biol. Chem., 265, 13206–13214. [PubMed] [Google Scholar]
- 18.Helmann J.D. (1995) Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res., 23, 2351–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaumburg C.S. and Tan,M. (2000) A positive cis-acting DNA element is required for high level transcription in Chlamydia. J. Bacteriol., 182, 5167–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourse R., Gaal,T., Aiyar,S., Barker,M., Estrem,S., Hirvonen,C. and Ross,W. (1998) Strength and regulation without transcription factors: lessons from bacterial rRNA promoters. Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, Vol. 63, pp. 131–139. [DOI] [PubMed]
- 21.Engel J. and Ganem,D. (1990) Identification and comparison of putative chlamydial promoter elements. In Van der Ploeg,L. (ed.), Immune Recognition and Evasion: Molecular Aspects of Host Parasite Interaction. Academic Press Inc, San Diego, California, pp. 245–260.
- 22.Lambden P.R., Everson,J.S., Ward,M.E. and Clarke,I.N. (1990) Sulfur-rich proteins of Chlamydia trachomatis: developmentally regulated transcription of polycistronic mRNA from tandem promoters. Gene, 87, 105–112. [DOI] [PubMed] [Google Scholar]
- 23.Ricci S., Cevenini,R., Cosco,E., Comanducci,M., Ratti,G. and Scarlato,V. (1993) Transcriptional analysis of the Chlamydia trachomatis plasmid pCT identifies temporally regulated transcripts, anti-sense RNA and sigma 70-selected promoters. Mol. Gen. Genet., 237, 318–326. [DOI] [PubMed] [Google Scholar]