Abstract
Considerable interest has been focused on telomerase because of its potential use in assays for cancer diagnosis, and for anti-telomerase drugs as a strategy for cancer chemotherapy. A number of assays based on the polymerase chain reaction (PCR) have been developed for evaluation of telomerase activity. To overcome the disadvantages of the conventional telomerase assay [telomeric repeat amplification protocol (TRAP)] related to PCR artifacts and troublesome post-PCR procedures, we have developed a telomeric repeat elongation (TRE) assay which directly measures telomerase activity as the telomeric elongation rate by biosensor technology using surface plasmon resonance (SPR). 5′-Biotinylated oligomers containing telomeric repeats were immobilized on streptavidin-pretreated dextran sensor surfaces in situ using the BIACORE apparatus. Subsequently, the oligomers associated with the telomerase extracts were elongated in the BIACORE apparatus. The rate of TRE was calculated by measuring the SPR signals. We examined elongation rates by the TRE assay in 18 cancer and three normal human fibroblast cell lines, and 12 human primary carcinomas and matching normal tissues. The elongation rates increased in a concentration- and time-dependent manner. Those of cancer cells were two to 10 times higher than fibroblast cell lines and normal tissues. Telomerase activities and its inhibitory effects of anti-telomerase agents as measured by both the TRE and TRAP assays showed a good correlation. Our assay allows precise quantitative comparison of a wide range of human cells from somatic cells to carcinoma cells. TRE assay is suitable for practical use in the assessment of telomerase activity in preclinical and clinical trials of telomerase-based therapies, because of its reproducibility, rapidity and simplicity.
INTRODUCTION
The telomere is a unique chromosomal structure consisting of tandem GT-rich repeats (TTAGGG)n, which protects the termini of linear chromosomes from fusion and degradation (1). In normal somatic cells, chromosomes lose ∼50–200 nt of telomeric sequence per cell division because of the so-called DNA end-replication problem (2). This progressive telomere shortening with each cell division leads to cellular senescence (1). In contrast to somatic cells, immortal and germ cells have a mechanism to maintain their telomeric length. Telomerase is a ribonucleoprotein enzyme complex, including a unique reverse transcriptase that elongates telomeric DNA (1). Its activity is thought to be required for the development of cellular immortality and oncogenesis (1). Thus, considerable interest is focused on telomerase because of its potential uses in assays for cancer diagnosis, research into cell biology and for anti-telomerase drugs as a strategy for cancer chemotherapy (1).
A number of telomerase assays have been developed over the last decade (3–7). They are categorized into two major subgroups: polymerase chain reaction (PCR)-based and non-PCR-based methods. Telomerase activity was initially measured in vitro by a primer extension assay, in which telomerase synthesizes telomeric repeats onto oligonucleotide (TTAGGG)n primers (3,4). However, this telomerase assay is not sensitive enough to detect telomerase activity in tissue samples. To increase the sensitivity of telomerase detection, Kim et al. (5) developed a telomeric repeat amplification protocol (TRAP) assay, based on PCR. The TRAP assay still had several limitations for quantifying telomerase activity, as described by the authors (5). The major limitations of the TRAP assay are related to PCR-derived artifacts. There have been several attempts to improve the methods for quantification of telomerase activity, such as including internal controls, a standard for telomerase-positive cell lines or a quantification control, as supplied in the commercially available TRAP assays. Despite these efforts, the disadvantages of the TRAP assay have remained for the necessary post-PCR procedures that involve the separation of the PCR products by gel electrophoresis and their evaluation by phosphorimager or densitometry.
Biosensor technology using surface plasmon resonance (SPR) is broadly applicable for measurement of biological activity (8). SPR techniques can measure small local changes in refractive index, linked directly to alterations in concentration on a surface (8). Buckle et al. (9) showed that SPR allows quantification of the relatively small changes in mass associated with polymerization. Recent reports have also demonstrated that real-time elongation of oligonucleotides by DNA polymerase or reverse transcriptase could be measured by SPR (10–11).
Here, we describe our development of the telomeric repeat elongation (TRE) assay, a rapid biosensor chip assay using SPR for the quantitative assessment of telomerase activity. The TRE assay eliminates PCR-related artifacts and gives many advantages (no labeling is required; excellent reproducibility and rapidity; elimination of PCR-related artifacts; easy handling using automated one-step analysis).
MATERIALS AND METHODS
Strategy of the TRE assay
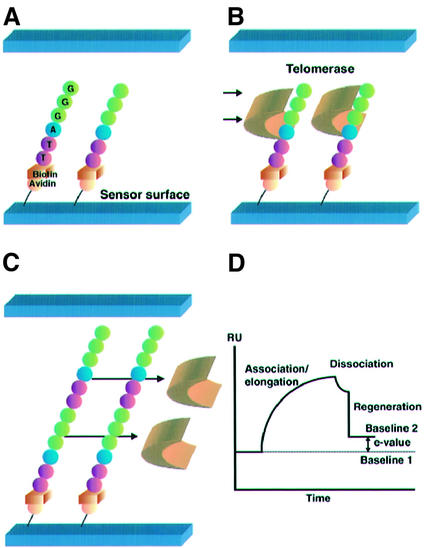
The constitution of, and reactions on, the sensor chip surface that are used in the TRE assay are illustrated in Figure 1. Initially, 5′-biotinylated oligomers (TS-5′B) containing telomeric repeats were immobilized on streptavidin-pretreated dextran sensor surfaces in situ using the BIACORE apparatus (BIACORE 3000; BIACORE AB, Uppsala, Sweden). After removing excessive oligomers by washing with a 100-µl pulse of 1% SDS in 10 mM HEPES, telomerase extracts were injected across the sensor surface, and we carried out an association/elongation phase of oligomers and telomerase at flow rates of 5 µl/min. Subsequently, regeneration was performed by washing with a 100-µl pulse of 1% SDS in 10 mM HEPES at flow rates of 100 µl/min to remove all bound proteins. SPR measurements were then conducted using a BIACORE 3000 machine (BIACORE AB). SPR signals were expressed in resonance units (RU; 1 RU = 1 pg/mm2) and real-time results were represented as a sensorgram. We defined the elongation value (E-value) as the difference of signals, in RU, between the baseline value before telomerase extract injection (baseline 1) and after the regeneration phase (baseline 2). The rate of TRE was calculated using the E-value.
Figure 1.
Strategy for the TRE assay. (A) The TS-5′B oligomers containing the telomeric repeat sequence (TTAGGG) were immobilized on the streptavidin-pretreated dextran sensor chip surface. This gave the initial baseline on the sensorgram [baseline 1 in (D)]. (B) Telomerase extracts were injected through the flow cell and bound to the TS-5′B oligomers. Elongation of the immobilized oligomers occurred immediately. This gave the association/elongation phase on the sensorgram. (C) After finishing the injection of the telomerase extracts, dissociation of binding proteins was observed. Regeneration was performed to remove all bound proteins. (D) A sensorgram of the TRE assay. Real-time monitoring was carried out throughout the association/elongation, dissociation and regeneration phases. The difference between baselines 1 and 2 was defined as the E-value, which was equivalent to the weight of the elongated nucleotides.
Samples and preparation of telomerase extracts
Eighteen cancer cell lines and three normal fibroblast cell lines were maintained under recommended conditions. These cell lines included three breast cancers (MCF-7, T47-D, ZR75-1; ATCC, Rockville, MD), five esophageal cancers (TE-2, -3, -4, -5, -6; Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan), five gastric cancers (MKN7, MKN28, MKN45, GCIY, GT3TKB; RCB, Tsukuba, Japan), five colonic cancers (SW480, SW837, SW948, SW1417, LS513; ATCC) and three normal fibroblast cell lines (CSC 2F0-C25 and ACBRI #469; CSC, Kirkland, WA; and CCD 1076Sk; ATCC). Tissues of human carcinomas and matching normal mucosa were obtained from 12 patients (five esophageal, five gastric and two colonic carcinomas). Telomerase extracts were prepared by the conventional method (12). All extracts were diluted to 0.5 mg/ml by 1× CHAPS buffer.
Immobilization of oligomers containing telomeric repeats
The TS-5′B oligomers were as follows: biotin-5′-AATCCGTCGAGCAGAGTTAGGGTTAGGGTTAGGGTTAGGGTTAG-3′. Identical oligomers that were biotinylated at the 3′ end (TS-3′B) were also prepared as negative control. The oligomers (0.125 µg/ml) in 75 µl were injected across a streptavidin-pretreated dextran sensor surface (SA chip; BIACORE AB), in situ in the BIACORE apparatus (BIACORE 3000) at 5 µl/min in 10 mM HEPES, pH 7.4/150 mM NaCl/10 mM MgCl2 at 37°C. After 1500 RU of oligomers had been immobilized, the chip surface was washed three times to remove excess oligomers using a 100-µl pulse of 1% SDS in 10 mM HEPES.
Telomerase extract binding to immobilized DNA
Telomerase extracts were diluted in a TRE buffer (10 mM HEPES, pH 7.4/150 mM NaCl/10 mM MgCl2/2.5 mM dNTP/10 mM EGTA) to various concentrations. They were applied at 5 µl/min across the chip surface for various injection times, at 37°C. The chip surface was regenerated by washing with a 100-µl pulse of 1% SDS, in 10 mM HEPES, which removed all protein, at a flow rate of 100 µl/min. The regeneration conditions were determined by preliminary studies using NaCl, Gly-HCl and NaOH at several concentrations. After regeneration, the flow rate was returned to 5 µl/min. We defined the E-value as the difference of RU between the baseline levels before extract injection and after the regeneration phase. All values were determined from three to six independent assays, and the coefficients of variation of all data were confirmed to be below 5%.
Microrecovery of elongated oligomers and amplification of 6-bp ladders
First, the mouse monoclonal anti-biotin antibody (DAKO, Glostrup, Denmark) was immobilized on the CM5 sensor chip surface (BIACORE AB) using an amine coupling kit (BIACORE AB). The TS-5′B oligomers were injected across the sensor chip and bound the anti-biotin antibody, with up to 3000 RU. Subsequently, the TRE assay was performed as described above. The ‘microrecover’ and ‘bypasswash’ programs of the BIACORE 3000 were used for the automatic recovery of elongated oligomers. Eluted samples of the sensor surface were treated with phenol/chloroform and then amplified by PCR using the TRAP assay primers. The 5′ end of the forward primer was labeled with FAM. The PCR products were analyzed using an ABI PRISM 310 Genetic Analyzer with GeneScan Software (ABI, Foster City, CA).
TRAP assay
The TRAP assay was performed using a TRAPeze telomerase detection kit (Intergen, Purchase, NY). In brief, 2 µg of protein extract was applied to the TRAP reaction mixture. For each assay, a negative control and 0.1 amol of the quantification standard oligonucleotide R8 were used. Telomerase activity was represented as total product generated (TPG) as described previously (13). One unit of TPG was defined as 0.001 amol of telomerase substrate primers extended by telomerase present in the extract, with at least three telomeric repeats (13).
Treatment of telomerase inhibitor for cancer cell lines
Two breast cancer cell lines (MCF-7 and T47-D) were subjected to an evaluation of the inhibitory effect of anti-telomerase agents. Two types of telomerase inhibitors were examined. Azidothymidine (AZT) is a reverse transcriptase inhibitor that has been studied in great detail, as a possible model compound for a telomerase inhibitor (14). Tamoxifen (TAM) is a hormonal agent (anti-estrogen) touted as the endocrine treatment of breast cancer. Estrogen activates telomerase via direct and indirect effects on the hTERT promotor (15). TAM down-regulates hTERT mRNA (15). Cell culture and drug exposure have been described (14,15). Telomerase activity was evaluated by TRE and TRAP assays.
RESULTS
Preliminary studies
Preliminary experiments to optimize reaction conditions used a cancer cell line (SW480; ATCC) and a human dermal fibroblast cell line (CSC 2F0-C25). The optimal buffering and temperature conditions were: 10 mM HEPES, pH 7.4/150 mM NaCl/10 mM MgCl2/2.5 mM dNTP each/10 mM EGTA at 37°C, with a flow rate of 5 µl/min.
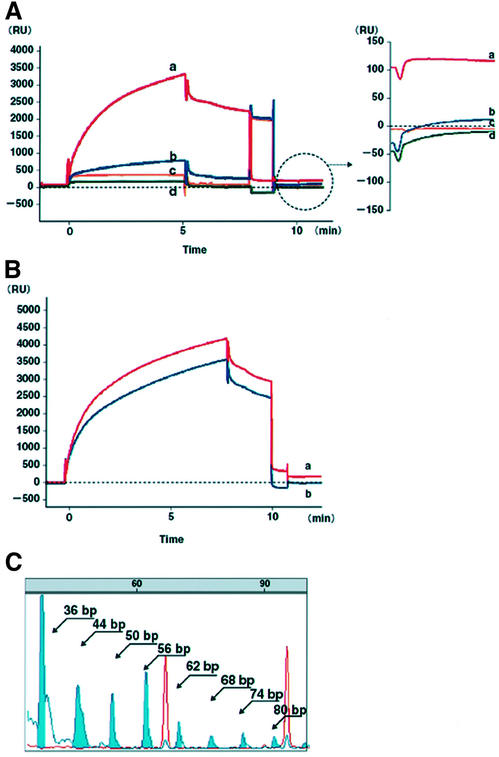
When extracts of the telomerase-positive cell line SW480 were injected, the sensorgram immediately showed a steep slope (Fig. 2A), which gradually became a gentle slope but did not reach a plateau. Equilibrium was not observed during sample injection on any sensorgrams, because this phase involved both the protein binding to the TS-5′B oligomers and elongation of the oligomers. The heat-inactivated telomerase control did not bind to the TS-5′B oligomers, and showed no significant increase of the E-value above the background level (<5 RU) (Fig. 2A). Bound protein was seen in the samples diluted in TRE buffer that did not contain dNTP, and only a slight elongation of the oligomers was observed (Fig. 2A). No elongation of the TS-5′B oligomers occurred in the absence of telomerase extracts (Fig. 2A). Oligomers biotinylated at the 3′ end (TS-3′B) were also used as a negative control: telomerase extracts bound to the TS-3′B oligomers, but no 5′ to 3′ elongation occurred since the 3′ end was immobilized on the sensor chip (Fig. 2B). To eliminate the activity of any polymerase, we also immobilized non-telomeric oligomers [biotin-5′-AATCCGTCGAGCAGAGTGTTGGTCAGGCTGATCTCAAA: estrogen response element (ERE)] which were potential estrogen response elements of hTERT promotor sequences. No significant increase of E-value above the background level was obtained (<5 RU, Fig. 2A).
Figure 2.
Sensorgrams of the TRE assay, and an electropherogram of 6-bp ladders amplified from recovered TS-5′B oligomers from the sensor chip surface. (A) The TRE assays on SW480 (red line, E-value 118 RU). Bound protein was seen in the sample diluted in the TRE buffer without dNTP, in which a slight elongation of the oligomers was observed (blue line, E-value 12 RU). The heat-inactivated telomerase extract control did not bind to the TS-5′B oligomers, and exhibited no significant increase of E-value above the baseline level (yellow line, –1.2 RU). No difference was observed between the initial baseline and that after the regeneration phase in the TRE assay without telomerase extracts (green line, E-value –4 RU). (B) The TRE assay using oligomers biotinylated at the 5′-biotinylated (TS-5′B; red line) and 3′-biotinylated (TS-3′B; blue line) in SW480. Extracts bound to TS-3′B but did not elongate them because of the immobilized 3′ end on the sensor chip. (C) Six-base pair ladders were detected as blue peaks from 44 to 80 bp. Red peaks represent size markers (TAMURA 500, ABI).
To confirm oligomers elongation on the sensor chip surface, we recovered the TS-5′B oligomers and used these as a template for PCR with primers from the TRAP assay. Six-base pair ladders were detected from 44 to 80 bp (Fig. 2C).
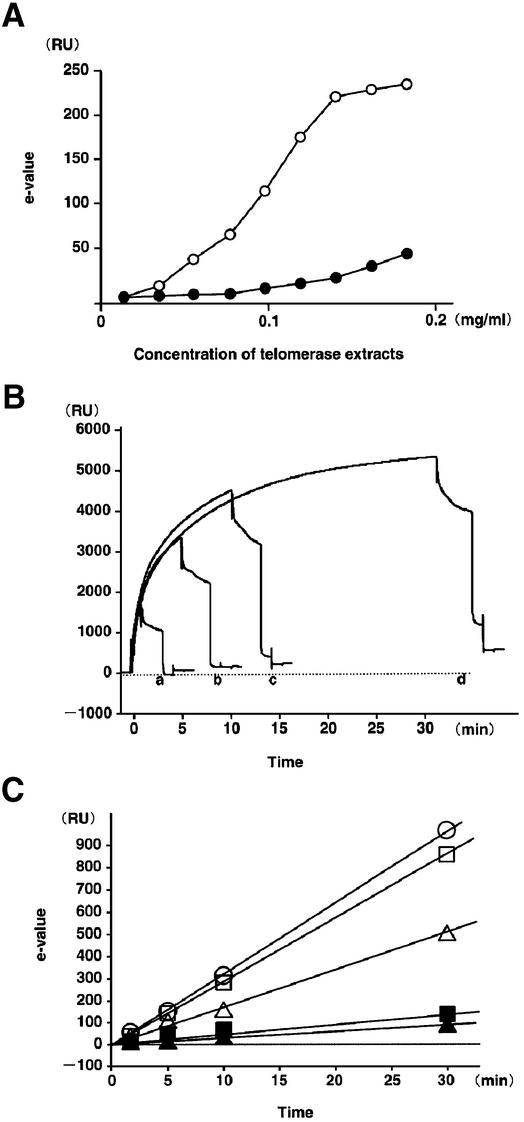
E-values of SW480 for a 10-min injection increased in a concentration-dependent manner from 0.025 to 0.150 mg/ml of cell extract (total amounts of telomerase extracts, 1.25– 7.5 µg), but those of a normal dermal fibroblast cell line (CSC 2F0-C25) were below the background level (<5 RU) until 0.05 mg/ml of cell extract was used (Fig. 3A). We examined several cancer and fibroblast cell lines by TRE assay for 1-, 5-, 10- and 30-min injections, using 0.1 mg/ml of cell extract, and found that their E-values increased linearly (Fig. 3B and C). The coefficient of variation at each time point was below 5%. The increased value in RU following immobilization was used to determine moles of DNA at the chip surface, using an empirical relationship in which 1500 RU was equivalent to 1.8 ng of DNA (1.276 × 10–13 mol of TS-5′B oligomer) (9). An extension of one base on all TS-5′B oligomers would thus result in an increase of ∼33.8 RU. This value is well above the background level and should be observable. The elongation rate was constant in each cell line during any injection time (Fig. 3C and Table 1).
Figure 3.
Relationships between E-values and concentration of telomerase extracts or injection-time. (A) Relationship between E-values and concentration of telomerase extracts. A colon cancer cell line, SW480 (open circles) and a normal fibroblast cell (CSC 2F0-C25, closed circles). (B) Sensorgrams of SW480 for 1- (a), 5- (b), 10- (c) and 30- min (d) injections at 0.1 mg/ml. (C) E-values with several different injection times, used in human cancer cell lines (open circles, T47-D; open squares, MCF7; open triangles, SW480) and normal fibroblast cell lines (closed squares, CSC 2F0-C25; closed triangles, ACBRI #469).
Table 1. Telomerase elongation rate in various human biological samples.
| Cell line | Elongation rate (mer/min) | Tissue samplea | Elongation rate (mer/min) |
|---|---|---|---|
| Breast cancer | Esophageal cancer | ||
| T47-D | 0.872 | EC-T1 | 0.368 |
| MCF7 | 0.721 | EC-N1 | 0.056 |
| ZR75-1 | 0.684 | EC-T2 | 0.624 |
| Esophageal cancer | EC-N2 | 0.089 | |
| TE5 | 0.691 | EC-T3 | 0.452 |
| TE2 | 0.395 | EC-N3 | 0.012 |
| TE3 | 0.388 | EC-T4 | 0.553 |
| TE4 | 0.302 | EC-N4 | 0.026 |
| TE6 | 0.255 | EC-T5 | 0.321 |
| Gastric cancer | EC-N5 | 0.023 | |
| MKN7 | 0.739 | Gastric cancer | |
| MKN45 | 0.393 | GC-T1 | 0.669 |
| GCIY | 0.370 | GC-N1 | 0.091 |
| GT3TKB | 0.331 | GC-T2 | 0.882 |
| MKN28 | 0.206 | GC-N2 | 0.102 |
| Colon cancer | GC-T3 | 0.396 | |
| SW1417 | 0.632 | GC-N3 | 0.056 |
| SW480 | 0.446 | GC-T4 | 0.338 |
| SW837 | 0.441 | GC-N4 | 0.052 |
| SW948 | 0.303 | GC-T5 | 0.463 |
| LS513 | 0.231 | GC-N5 | 0.032 |
| Normal fibroblast | Colon cancer | ||
| CSC 2F0-C25 | 0.113 | CC-T1 | 0.662 |
| ACBRI #469 | 0.106 | CC-N1 | 0.052 |
| CCD 1076Sk | 0.086 | CC-T2 | 0.712 |
| CC-N2 | 0.067 | ||
aT, tumor tissue; N, non-tumor tissue.
We could not systemically determine the lower limit of detection of tumor cells by the dilution method, since the normal fibroblast cells used in the present study exhibited weak telomerase activity. But when we dilute 100 T47 breast cancer cells in 1 × 105 normal fibroblast cells (CCD 1076Sk) and 0.1 mg/ml of extract were injected for 10 min (total amounts of telomerase extracts, 5 µg), we found a 10 RU increase of E-value in comparison with extracts without tumor cells. No significant increase of E-value was confirmed when 10 tumor cells were diluted in 1 × 105 CCD 1076Sk cells. The limit of detection of tumor cells was 1 tumor cell/1000 background cells.
TRE assay using biological samples and comparison with TRAP assay
We examined elongation rates for 18 cancer cell lines and three normal human fibroblast cell lines. Tissues from human carcinomas and matching normal mucosa were obtained from 12 patients (five esophageal, five gastric and two colonic carcinomas), and examined for telomerase activity using the TRE assay. The elongation rates of cancer cells were found to be two to 10 times higher than those of normal mucosa and normal fibroblast cell lines (Table 1). The fibroblast cell lines gave elongation rates ranging from 0.086 mer/min to 0.133 mer/min, giving total elongations of 2.4 mer to 3.9 mer for a 30-min injection.
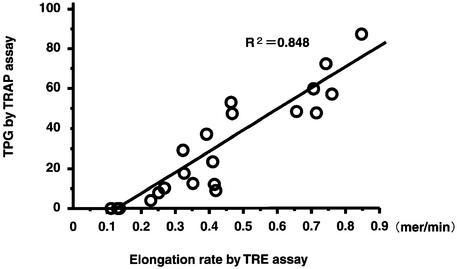
We then examined the same samples of cancer and normal fibroblast cell lines using a standard TRAP assay with the TRAPeze telomerase detection kit (Intergen). Telomerase activities as measured by both the TRE and TRAP assays showed a good correlation between the two assays in cancer cell lines (Fig. 4). Telomerase activities of fibroblast cell lines could be evaluated quantitatively by the TRE assay, but failed to amplify a 6-bp ladder by the TRAP assay. Because the minimum length of TRE for successful visualization of TRAP-laddering is 6 nt, the elongation rates of fibroblast cell lines (2.4–3.9 mer in 30 min), which were detected by the TRE assay, might be too low to be amplified in the TRAP assay.
Figure 4.
Correlation between the results of telomerase activities, as measured by the TRE and TRAP assays.
Inhibitory effects of anti-telomerase agents in breast cancer cell lines
AZT inhibited telomerase activity by 50–70% in two breast cancer cell lines (MCF-7 and T47-D) according to TRE and TRAP assays. 17β-Estradiol (E2) elevated the telomerase activity (120–150%) and TAM down-regulated it at the non-treatment level. Results of both assays were well correlated (Fig. 5).
Figure 5.
Inhibitory effects of telomerase activity for AZT (A) and TAM (B) in two breast cancer cell lines (MCF-7 and T47-D).
DISCUSSION
In this paper, we have described how we implemented a Biosensor chip assay for direct measurement of telomerase activity, using elongation rate and avoiding PCR-related artifacts and troublesome post-PCR procedures associated with various biological samples. The TRE assay gives many advantages over previous methods (i.e. no labeling is required; reproducibility and rapidity; PCR-related artifacts are eliminated; easy handling using an automated one-step analysis). The most significant advantage of the TRE assay is that the telomerase activity of somatic cells or tissues can be evaluated quantitatively.
The TRAP assay is currently the most sensitive assay available for detecting telomerase activity in low cell numbers, which would be possible, for example, using fewer than 10 cells with high telomerase expression (16). However, in the case of low telomerase expression (for example in somatic cells), the concentration of protein extracts used in the TRAP assay must be increased. High protein concentration in the reaction mixture inhibits Taq DNA polymerase in the conventional TRAP assay, which means that the assay cannot be used effectively to detect low levels of telomerase expression. Our present results show that the telomerase activity of somatic cells may be more than 10 times less than that of carcinomas. We have found that the TRE assay is suitable for the precise quantitative comparison of telomerase activity, in a wide range of human cells from somatic cells to carcinoma cells.
Most human somatic cells or tissues express low or undetectable levels of telomerase activity. Hence, continual cell renewal seen in liver cirrhosis leads to telomere shortening, resulting in cellular senescence and end-stage organ failure (1,17). Thus, in theory, external intervention to increase telomerase activity may prolong the life span of target cells and avoid organ failure (1,17). On the other hand, up-regulation of telomerase activity would increase the risk of cancer development. Precise assessment of telomerase activity, from levels found in somatic cells to those found in cancer cells, is needed to allow safe clinical trials of telomere/telomerase therapeutic agents.
The application of the TRE assay would naturally extend to the screening for modulators of telomerase activity as therapeutic agents, but the BIACORE apparatus and sensor chips are expensive. Telomerase down-regulation and telomere shortening would compel cancer cells to undergo apoptosis, while up-regulation and elongation might be able to prolong the life span of target cells and thus preserve organs (1,17). The TRE assay is intended for practical use in the assessment of telomerase activity in pre-clinical and clinical trials of such telomerase-based therapies, because of its rapidity and simplicity.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by Grants-in-Aid 193671332, 13770705 and 13770093 from the Japanese Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Greider C.W. (1996) Telomere length regulation. Annu. Rev. Biochem., 65, 337–365. [DOI] [PubMed] [Google Scholar]
- 2.Hayflic L. and Moorhead,P.S. (1961) The serial cultivation of human diploid cell strains. Exp. Cell Res., 25, 585–621. [DOI] [PubMed] [Google Scholar]
- 3.Shay J.W. (1994) Analysis of telomerase and telomeres. In Adolph,K.W. (ed.), Methods in Molecular Genetics, Part C, Genes and Chromosome Analysis. Academic Press, New York, Vol. 5, pp. 263–280.
- 4.Raymond E., Sun,D., Izbicka,E., Mangold,G., Silvas,E., Windle,B., Sharma,S., Soda,H., Laurence,R., Davidson,K. and von Hoff,D.D. (1999) A human breast cancer model for the study of telomerase inhibitors based on a new biotinylated-primer extension assay. Br. J. Cancer, 80, 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim N.W., Piatyszek,M.A., Prowse,K.R., Harley,C.B., West,M.D., Ho,P.L., Coviello,G.M., Wright,W.E., Weinrich,S.L. and Shay,J.W. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science, 266, 2011–2015. [DOI] [PubMed] [Google Scholar]
- 6.Sun D., Hurley,L.H. and von Hoff,D.D. (1998) Telomerase assay using biotinylated-primer extension and magnetic separation of the products. Biotechniques, 25, 1046–1051. [DOI] [PubMed] [Google Scholar]
- 7.Lackey D.B. (1998) A homogeneous chemiluminescent assay for telomerase. Anal. Biochem., 263, 57–61. [DOI] [PubMed] [Google Scholar]
- 8.Myszka D.G. (1999) Survey of the 1998 optical biosensor literature. J. Mol. Recognit., 12, 390–408. [DOI] [PubMed] [Google Scholar]
- 9.Buckle M., Williams,R.M., Negroni,M. and Buc,H. (1996) Real time measurements of elongation by a reverse transcriptase using surface plasmon resonance. Proc. Natl Acad. Sci. USA, 93, 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond P.W. and Cech,T.R. (1998) In vitro selection and characterization of Bcl-X(L)-binding proteins from a mix of tissue-specific mRNA display libraries. Biochemistry, 37, 5162–5172. [DOI] [PubMed] [Google Scholar]
- 11.Pemberton I.K. and Buckle,M. (1999) Real time in vitro analysis of transcription by RNA polymerase on immobilized DNA fibres. J. Mol. Recognit., 12, 322–327. [DOI] [PubMed] [Google Scholar]
- 12.Counter C.M., Avilion,A.A., LeFeuvre,C.E., Stewart,N.G., Greider,C.W., Harley,C.B. and Bacchetti,S. (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J., 5, 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelhardt M., Mackenzie,K., Drullinsky,P., Silver,R.T. and Moore,M.A. (2000) Telomerase activity and telomere length in acute and chronic leukemia, pre- and post-ex vivo culture. Cancer Res., 60, 610–617. [PubMed] [Google Scholar]
- 14.Murakami J., Nagai,N., Shigemasa,K. and Ohama,K. (1999) Inhibition of telomerase activity and cell proliferation by a reverse transcriptase inhibitor in gynaecological cancer cell lines. Eur. J. Cancer, 35, 1027–1034. [DOI] [PubMed] [Google Scholar]
- 15.Kyo S., Takakura,M., Kanaya,T., Zhuo,W., Fujimoto,K., Nishio,Y., Orimo,A. and Inoue,M. (1999) Estrogen activates telomerase. Cancer Res., 59, 5917–5921. [PubMed] [Google Scholar]
- 16.Kim N.W. and Wu,F. (1997) Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 25, 2595–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph K.L., Chang,S., Millard,M., Schreiber-Agus,N. and DePinho,R.A. (2000) Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science, 287, 1253–1258. [DOI] [PubMed] [Google Scholar]