Abstract
Rrp43p is a Saccharomyces cerevisiae exosome subunit involved in pre-rRNA processing which is found both in the nucleus and in the cytoplasm. So far, no function has been assigned to the cytoplasmic fraction of Rrp43p. We have addressed Rrp43p function by analyzing mRNA stability in three rrp43 temperature-sensitive (ts) strains, which carry different ts alleles (rrp43-1, rrp43-2 and rrp43-3), and by analyzing Rrp43p interactions with the remaining exosome subunits. In the ts strains, endogenous mRNAs (ACT1 and PAB1), as well as a heterologous reporter mRNA (CATpG) showed longer half-lives, relative to a control strain carrying wild-type RRP43. The mutants also accumulated a degradation intermediate of the reporter mRNA that is typical of defective mRNA decay. These results allow us to propose that Rrp43p is required for mRNA degradation. Rrp43p interacts with the exosome complex via Rrp46p, as determined by two-hybrid analyses. Interestingly, the rrp43 ts mutant proteins do not interact with Rrp46p, indicating that the ts phenotype may be caused by disruption of the Rrp43p– Rrp46p interaction. The ts strains also showed a pre-rRNA processing defect, which is consistent with previous studies on Rrp43p function.
INTRODUCTION
In eukaryotes, extensive post-transcriptional processing is generally necessary for the synthesis of mature RNAs. Transfer RNAs (tRNAs) undergo 5′ and 3′ processing and intron removal. mRNA processing includes 5′-capping, excision of introns, 3′-cleavage and polyadenylation and, in some cases, editing of specific nucleotides. In addition, degradation of mRNA is of special interest because it is important for the control of gene expression. Studies utilizing the yeast Saccharomyces cerevisiae have identified two mRNA degradation pathways. mRNAs either undergo shortening of the polyadenylate tail, followed by decapping and 5′→3′ exonucleolytic digestion, or they are first deadenylated and subsequently degraded by 3′→5′ digestion (1,2). Three of the four rRNAs are synthesized from a single pre-rRNA. This pre-rRNA contains the coding sequences of the 18S, 5.8S and 25–28S rRNAs flanked by two external transcribed sequences (5′ ETS and 3′ ETS) and two internal transcribed sequences (ITS1 and ITS2, respectively) (3). During rRNA processing, which is best characterized in S.cerevisiae (see Fig. 1), these spacer sequences are removed by a sequential series of endo- and exonucleolytic cleavages (3–5).
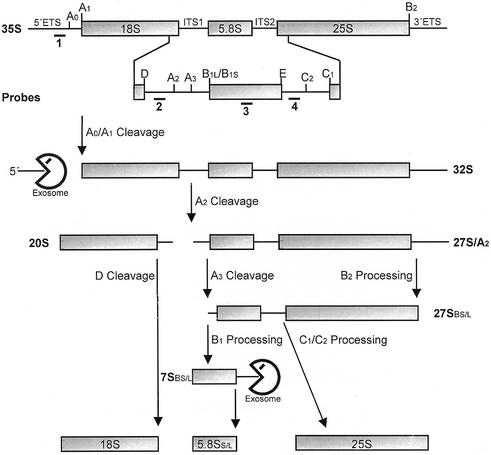
Figure 1.
Structure of the 35S pre-rRNA and major intermediates of the rRNA processing pathway in S.cerevisiae. The 35S pre-rRNA contains the sequences of the mature 18S, 5.8S and 25S rRNAs separated by two internal transcribed spacers (ITS1 and ITS2) and flanked by two external transcribed spacers (5′ ETS and 3′ ETS). Processing of the 35S pre-rRNA starts with endonucleolytic cleavages at sites A0 (in 5′ ETS) and A1, originating a 5′ ETS fragment and a 32S pre-rRNA containing the mature 5′ end of the 18S rRNA. Subsequent processing at site A2 (in ITS1) yields the 20S and the 27SA2 pre-rRNAs. The former is processed at site D to 18S rRNA while the latter is processed into the mature 5.8S and 25S rRNAs by two alternative pathways. The major pathway involves cleavage at sites A3 and B2, generating a shorter 5′ end, and the mature 3′ end of the 25S rRNA. 5′→3′ exonucleolytic cleavage originates the mature 5′ end of 5.8S rRNA, and processing at sites C1 and C2 separates the 7S pre-rRNA from mature 25S rRNA. The 7S pre-rRNA is finally 3′→5′ processed to generate mature 5.8S rRNA. In the minor pathway, the 27SA2 pre-rRNA is processed by a mechanism that produces a 5.8S rRNA with an extra 7–8 nt at the 5′ end. Numbers below the 35S pre-rRNA indicate the position of the probes used in hybridization experiments. The exosome is directly involved in 3′→5′ processing of 7S pre-rRNA and 5′ ETS degradation, but Rrp43p depletion also affects processing of 18S and 25S rRNA precursors.
In S.cerevisiae, several factors involved in mRNA degradation also participate in rRNA, snRNA and snoRNA biosynthesis. The 5′→3′ exonucleases Rat1p and Xrn1p degrade single-stranded RNA and excised pre-rRNA sequences, and are responsible for the formation of the mature 5′-end of the 5.8S and 25S rRNAs (6–9). The exosome is a conserved eukaryotic protein complex containing multiple 3′→5′ exonucleases (10–15). Eight homologs of yeast exosome subunits have recently been found in Trypanosoma brucei and been shown to be involved in rRNA processing (16). In addition, an exosome-like complex was identified in Arabdopsis thaliana, which contains AtRrp41p, a protein that complements Rrp41p function in yeast (17). The exosome was initially identified as a five-subunit complex required for processing of the 5.8S rRNA 3′-end (Fig. 1) (14). More recently, six other exosome subunits were identified that co-purify with ProtA–Rrp4p on an IgG–Sepharose column and form nuclear and cytoplasmic sub-complexes (10). The exosome subunits Rrp4p, Rrp41p/Ski6p and Csl4p/Ski4p have been shown to participate in 3′→5′ degradation of deadenylated mRNA (18,19). The mammalian exosome was reported to participate in degradation of ARE (A-U-rich element)-containing RNAs in a cell-free system (20) and the mammalian homolog of yeast Rrp45p, PM-Scl75, interacts with AREs, mediating the rapid degradation of ARE-containing mRNAs (21). Based on recent data, a model has been proposed in which the exosome degrades mRNA in a decapping-independent mechanism that requires Ski2p, Ski3p, Ski7p and Ski8p in yeast (2). However, there is evidence that the mammalian exosome is functionally linked to mRNA decapping. Following deadenylation, the mammalian exosome degrades the mRNA 3′→5′, in a process coupled to decapping (22). The subunits Rrp4p and Rrp41p/Ski6p are also involved in snRNAs and snoRNAs processing (12,13). Except for Rrp6p, all the other exosome subunits are essential for yeast cell viability (10,14,23). Up to now, exonuclease activity has been demonstrated in vitro for the subunits Rrp4p, Rrp41p/Ski6p, Rrp44p/Dis3p and Rrp6p (11,14); however, all of the subunits have sequence similarity with bacterial RNases (10,24). So far, it is unclear why cells need so many RNases with similar function. Detailed studies are still required to understand the specific function of most exosome subunits.
Rrp43p is one of the exosome subunits identified by co-purification with ProtA–Rrp4p (14) and was shown to interact with Nip7p, a protein required for 25S rRNA synthesis and 60S ribosome biogenesis (25,26). In addition to defects in 3′-end formation of 5.8S (14), depletion of Rrp43p also blocks processing of the 27S pre-rRNA, formation of the 20S pre-rRNA and leads to accumulation of an aberrant 23S pre-rRNA (27). Rrp43p shows sequence similarity to Escherichia coli RNase PH (14); however, an RNase activity for Rrp43p has not yet been demonstrated. Subcellular localization studies have detected Rrp43p in the nucleolus, nucleoplasm and cytoplasm (10,26). It is assumed that the nucleolar and nuclear fractions of Rrp43p function in pre-rRNA processing and degradation of excised spacer sequences. No function has been demonstrated for the cytoplasmic fraction of Rrp43p, but its presence in this cell compartment, together with the fact that other exosome subunits are involved in mRNA decay, suggest that it may also be involved in mRNA degradation. Furthermore, there is no information about the mechanism of Rrp43p interaction with the exosome complex.
Previous studies on Rrp43p function were based on conditional expression using the regulated GAL1 promoter, which is repressed in the presence of glucose and strongly induced by galactose (14,27). This type of conditional strain has to be maintained in galactose-containing media, resulting in production of the GAL1-regulated protein in the cell in excess of normal levels. In this work, we used a mutagenesis strategy to generate rrp43p temperature-sensitive (ts) mutants in order to investigate Rrp43p function on mRNA degradation. We show that, at the restrictive temperature, certain amino acid substitutions cause significant accumulation of mRNA and abolish Rrp43p function in rRNA processing. In addition, the two-hybrid system (28) was used to analyze potential Rrp43p interactions with other exosome subunits. These analyses revealed that Rrp43p interacts with the exosome only via Rrp46p, and that the rrp43 ts mutants no longer interact with Rrp46p.
MATERIALS AND METHODS
DNA analysis methods and plasmid construction
DNA cloning and electrophoretic analysis were performed as described by Sambrook et al. (29). DNA sequencing was performed by the Big Dye method (Perkin Elmer). A cDNA library fused to the GAL4 activation domain was obtained from the American Type Culture Collection (ATCC 87002). Plasmids used in this study are summarized in Table 1 and cloning strategies are briefly described below. The lexA:: RRP43 fusion used in the two-hybrid screen was constructed by inserting a 1.2 kb BamHI–SalI RRP43 fragment into pBTM116 (30), which had been digested with the same restriction enzymes, generating plasmid pBTM-RRP43. GAL4 activation domain fusions with the exosome subunits were constructed by inserting PCR-amplified open reading frames (ORFs) into the BamHI and SalI sites of pGAD-C (31), generating pGAD-RRP4, pGAD-RRP40, pGAD-RRP41, pGAD-RRP42, pGAD-RRP44, pGAD-RRP45, pGAD-RRP46, pGAD-MTR3, pGAD-CSL4 and pGAD-RRP6. Plasmid pCFUS-RRP43 was constructed by inserting a 1.2 kb BamHI–SalI RRP43 fragment into pGFP-C-FUS (32), which was digested with the same enzymes to remove the GFP coding sequence. In this plasmid, RRP43 is under control of the MET25 promoter. Plasmid pACT-RRP46, which bears the gene encoding a hybrid protein of the Gal4p activation domain and Rrp46p, was isolated from strain YCO45 that showed positive two-hybrid interaction with Rrp43p. Plasmids pCFUS-rrp43-1, pCFUS-rrp43-2 and pCFUS-rrp43-3 were isolated from mutant strains rrp43-1, rrp43-2 and rrp43-3, respectively (see below). Plasmid YCp111GAL-RRP43 was constructed by inserting an XbaI–SalI RRP43 fragment (from pGFP-RRP43) (26) into YCp111GAL-NIP7 (N.I.T.Zanchin, unpublished results), digested with XbaI and SalI. It results in a HIS6–RRP43 fusion controlled by the GAL1 promoter. Plasmid pCATpG was constructed by inserting the CAT coding region downstream of the PGK1 promoter in the plasmid YCplac111 (33). Subsequently, a 20 nt long poly(G) oligo was inserted into an XbaI restriction site, downstream of the CAT stop codon. Plasmids containing wild-type and mutant RRP43 alleles fused to the HA epitope were contructed as follows. A DNA fragment encoding the HA epitope was isolated from vector pACT2 (obtained from Clontech) by digestion with BglII and BamHI. This fragment was inserted into the BamHI restriction site of the vectors pCFUS-RRP43, pCFUS-rrp43-1, pCFUS-rrp43-2 and pCFUS-rrp43-3, which is located upstream of the RRP43 coding sequence, resulting in N-terminal HA fusions. The resulting vectors are listed in Table 1.
Table 1. Plasmids used in this study.
| Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| pACT-RRP46 | GAL4::RRP46, LEU2 2 µm | This study |
| pBTM116 | lexA DNA-binding domain, TRP1 2 µm | (30) |
| pBTM-RRP43 | lexA::RRP43, TRP1 2 µm | This study |
| pGAD-424 | GAL4 activation domain, LEU2 2 µm | (30) |
| pGAD-C | GAL4 activation domain, LEU2 2 µm | (31) |
| pACT-RRP46 | GAL4::RRP46, LEU2 2 µm | This study |
| pGAD-RRP4 | GAL4::RRP4, LEU2 2 µm | This study |
| pGAD-RRP40 | GAL4::RRP40, LEU2 2 µm | This study |
| pGAD-RRP41 | GAL4::RRP41, LEU2 2 µm | This study |
| pGAD-RRP42 | GAL4::RRP42, LEU2 2 µm | This study |
| pGAD-RRP44 | GAL4::RRP44, LEU2 2 µm | This study |
| pGAD-RRP45 | GAL4::RRP45, LEU2 2 µm | This study |
| pGAD-RRP46 | GAL4::RRP46, LEU2 2 µm | This study |
| pGAD-MTR3 | GAL4::MTR3, LEU2 2 µm | This study |
| pGAD-CSL4 | GAL4::CSL4, LEU2 2 µm | This study |
| pGAD-RRP6 | GAL4::RRP6, LEU2 2 µm | This study |
| YCplac111 | pUC19-MCS, LEU2, CEN4 | (33) |
| YCp111GAL-RRP43 | GAL1::His-RRP43, LEU2, CEN4 | This study |
| pCFUS-RRP43 | MET25::RRP43, CEN6, URA3 | This study |
| pCFUS-rrp43-1 | MET25::rrp43-1, URA3, CEN6 | This study |
| pCFUS-rrp43-2 | MET25::rrp43-2, URA3, CEN6 | This study |
| pCFUS-rrp43-3 | MET25::rrp43-3, URA3, CEN6 | This study |
| pCATpG | PGK1::CATpG, LEU2, 2 µm | This study |
| pCFUS-HARRP43 | MET25::HA-RRP43, CEN6, URA3 | This study |
| pCFUS-HArrp43-1 | MET25::HA-rrp43-1, URA3, CEN6 | This study |
| pCFUS-HArrp43-2 | MET25::HA-rrp43-2, URA3, CEN6 | This study |
| pCFUS-HArrp43-3 | MET25::HA-rrp43-3, URA3, CEN6 | This study |
Yeast strains, media and genetic techniques
Yeast strains used in this work are listed in Table 2. Yeast strains were maintained as described (34). Glucose or galactose was added as carbon source to yeast extract–peptone medium (YP) or synthetic medium (YNB) to a final concentration of 2%. The RRP43 gene disruption has been described previously (27), as has the construction of a conditional strain for RRP43, DG458, which bears a plasmid-encoded copy of RRP43 under the control of the GAL1 promoter. Strain YCO43 was constructed by replacing the endogenous plasmid of strain DG458 with plasmid YCp111GAL-RRP43 (LEU2). Strains rrp43-1, rrp43-2 and rrp43-3 were isolated in a screen to identify temperature-sensitive mutations in RRP43 (see results). Strains carrying the RRP43 alleles containing an N-terminal HA epitope tag were generated by transforming strain YCO43 with vectors pCFUS-HARRP43, pCFUS-HArrp43-1, pCFUS-HArrp43-2 and pCFUS-HArrp43-3. YCO43 carries a vector with RRP43 under the control of the GAL1 promoter that is repressed in the presence of glucose. The pCFUS-encoded, HA-tagged proteins are under the control of the MET25 promoter. The resulting strains (Table 2) carrying both the wild-type and the mutant proteins fused to the HA epitope were able to grow in glucose-containing medium at 25°C, showing that the HA-tagged proteins can complement the chromosomal deletion of RRP43 (Fig. 2D).
Table 2. Yeast strains.
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| DG458 | MATa ade2-1 leu2-3, 112 his3-11, 15 trp1-1 ura3-1 can1-100 rrp43::KAN p(URA3 GAL::ProtA::RRP43) | (27) |
| YCO43 | DG458/YCp111-GAL::His-RRP43 | This study |
| YCO44 | YCO43/pCFUS-RRP43 | This study |
| rrp43-1 | YCO43/pCFUS-rrp43-1 | This study |
| rrp43-2 | YCO43/pCFUS-rrp43-2 | This study |
| rrp43-3 | YCO43/pCFUS-rrp43-3 | This study |
| L40 | MATa his3d200 trp1-901 leu2-3, 311 ade2 lys2-801am URA3::(lexAop)8-lacZ LYS2::(lexAop)4-HIS3 | (37) |
| L40-41 | L40, pBTM-NIP7, pACT-NOP8 | (26) |
| L40-61 | L40, pBTM-NIP7, pACT-RRP43 | (26) |
| YCO45 | L40, pBTM-RRP43 | This study |
| YCO46 | L40, pBTM-RRP43, pACT | This study |
| YCO47 | YCO43/pCFUS-HARRP43 | This study |
| YCO48 | YCO43/pCFUS-HArrp43-1 | This study |
| YCO49 | YCO43/pCFUS-HArrp43-2 | This study |
| YCO50 | YCO43/pCFUS-HArrp43-3 | This study |
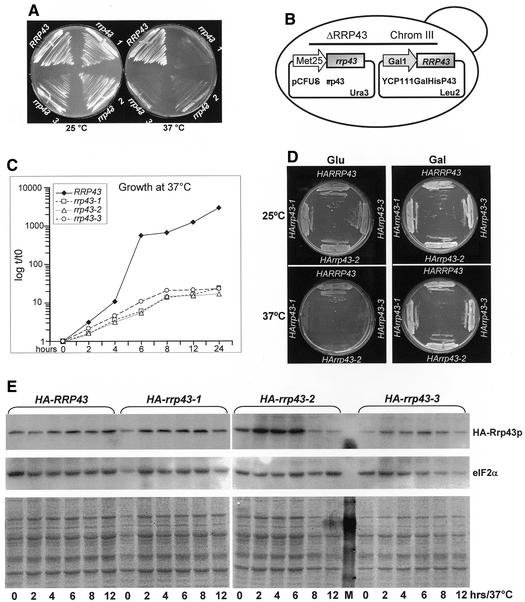
Figure 2.
Isolation of PCR-generated mutants of RRP43 with a temperature-sensitive phenotype. (A) Control strain YCO44 (ΔRRP43/pCFUS-RRP43) and temperature-sensitive rrp43 mutant strains obtained by mutagenic PCR transformation of strain YCO43 (ΔRRP43/YCp111-GAL::His-RRP43) plated onto YNB(–Ura) medium containing glucose and incubated at either 25 or 37°C. (B) Important genetic features of the strains tested. (C) Growth curves of strains containing the wild-type copy of RRP43 or the ts alleles rrp43-1, rrp43-2 and rrp43-3 at the restrictive temperature in liquid medium. (D) Complementation of the chromosomal RRP43 deletion by alleles containing N-terminal HA epitope fusions. Strains YCO47 (HARRP43), YCO48 (HA-rrp43-1), YCO49 (HA-rrp43-2) and YCO50 (HA-rrp43-3) were incubated in YNB minimal medium containing either glucose or galactose at 25 and 37°C. All strains contain the YCp111GAL-RRP43 vector encoding the wild-type copy of RRP43 under the GAL1 promoter, which allows for them to grow at the non-permissive temperature in galactose-containing medium (left panel). (E) Analysis of mutant protein levels. Whole cell extracts were isolated from strains containing HA-tagged proteins incubated at 37°C for the indicated times. M, molecular weight marker. (Upper panel) HA-tagged proteins detected by immunoblotting using an antibody against the HA epitope. (Center panel) The membrane was reprobed with a rabbit polyclonal antiserum raised against the translation factor eIF2α. (Lower panel) Coomassie stained PVDF membrane used in the immunoblot assay.
In vitro mutagenesis and isolation of rrp43 temperature-sensitive (ts) alleles
The method for mutagenesis and in vivo recombination was performed essentially as described previously (35). Briefly, plasmid DNA template (pCFUS-RRP43) was linearized with XhoI and diluted to 10 ng/µl. PCRs (100 µl) contained 1 mM dGTP, dCTP and dTTP and 0.2 mM dATP. MgCl2 was added to an end concentration of 3 mM. Reactions were pre-heated to 94°C and submitted to 30 cycles of 1 min at 94°C, 1 min at 55°C, 1 min at 72°C. The sequences of the oligonucleotides used in the PCR were: 5′-TCACTCTAGACGGATCCTGGAAATGGCTGAAAGTACC-3′ for the forward primer and 5′-GTTTGTCGACTCCTTTCGACAGTCATCAG-3′ for the reverse primer. Yeast strain YCO43 was transformed (36) with total PCR plus 100 ng of pCFUS-RRP43 linearized with EcoRI and plated onto YNB medium, containing glucose and lacking uracil and leucine. In such a way, transformants in which recombination between the PCR product and pCFUS-RRP43 (URA3) had occurred were selected (Fig. 2B). Approximately 1700 transformants were obtained that were able to grow on glucose-containing medium selective for the genetic marker (URA3) of vector pCFUS-RRP43 (Table 1) at 25°C. These transformants were replica plated using the same medium and incubated at 25 or 37°C. Strains rrp43-1, rrp43-2 and rrp43-3 showed stable temperature-sensitive phenotypes and were selected for further analysis (Fig. 2A). In order to test whether the ts phenotype was due to mutations in the copy of RRP43 present in pCFUS, the three mutant strains were incubated on YNB plates containing either glucose or galactose at 37°C. At this stage of analysis, all of the strains still contained plasmid YCp111GAL-RRP43 and, therefore, were able to grow on galactose-containing plates at 37°C, because the wild-type copy of RRP43 is being expressed, whereas on glucose, with GAL::RRP43 repressed, only the control strain YCO44 grew well at 37°C (Fig. 2).
Yeast two-hybrid screen for proteins that interact with Rrp43p
The host strain for the two-hybrid screen, YCO45 (Table 2), is a derivative of strain L40 (37) containing both yeast HIS3 and E.coli lacZ genes as reporters for two-hybrid interaction integrated into the genome. Strain YCO45 bears plasmid pBTM-RRP43, which encodes a hybrid protein containing the lexA DNA-binding domain and the full-length RRP43 ORF. Large-scale transformation of YCO45 was performed with a yeast cDNA library fused to the GAL4 activation domain (pACT-cDNA, ATCC 87002). Transformants were plated directly onto medium lacking histidine for immediate selection of Rrp43p interacting proteins. His+ clones were tested for lacZ expression by transferring cells to nitrocellulose filters and analyzing for β-galactosidase (β-Gal) activity (38). β-Gal activity of strains analyzed in two-hybrid experiments was quantitated using cell extracts isolated from these strains in buffer Z using ONPG as substrate (30). Strains L40-41 and L40-61 were used as positive controls and strain YCO46 was used as a negative control for the two-hybrid interaction (26) (Table 2).
RNA analysis
Exponentially growing cultures of yeast strains were shifted from 25 to 37°C. At various times, samples were collected and quickly frozen in a dry ice–ethanol bath. Total RNA was isolated from yeast cells by a modified hot phenol method (39). For mRNA analysis, yeast strains were incubated for 4 or 8 h at 37°C, when 20 µg/ml thiolutin (40) was added and samples were taken at various time points after addition of thiolutin. RNAs were separated by electrophoresis on 1.3% agarose gels, following denaturation with glyoxal (29). RNA gels were submitted to northern blotting using Hybond nylon membranes (Amersham). Membranes were probed with 32P-labeled oligonucleotides complementary to specific regions of the 35S pre-rRNA using the hybridization conditions described previously (25) and analyzed in a phosphorimager (Molecular Dynamics). The oligonucleotide probes used were: 1, 5′-GGTCTCTCTGCTGCCGGAAATG-3′; 2, 5′-GCTCTCATGCTCTTGCCAAAAC-3′; 3, 5′-CGTATCGCATTTCGCTGCGTTC-3′; 4, 5′-CTCACTACCAAACAGAAT GTTTGAGAAGG-3′. mRNAs were analyzed by probing with random primer labeling (Gibco BRL) of DNA fragments corresponding to PAB1, MFA2, CAT and ACT1 ORFs. Blots were quantified by using a phosphorimager (Molecular Dynamics). The 25S rRNA and the scR1 RNA were used as internal controls for mRNA half-life quantitation. The value obtained at each time point for the test RNA was divided by the value obtained at the same time point for the 25S rRNA or for the scR1 RNA. The graphs were constructed using relative values where time point 0 was considered as 1. The graphs show the average of seven northern blots for the ACT1 mRNA, five blots for the PAB1 mRNA and eight blots for the CATpG mRNA. The mRNA half-life corresponds to the time when the initial amount of mRNA was 50% reduced (Table 3). Three northern blots were also performed to analyze MFA2 mRNA decay (data not shown). mRNA decay of the reporter mRNA (CATpG) was analyzed by transforming the ts mutant strains and the control strain with plasmid pCATpG. Yeast strains containing this plasmid were grown on selective medium for 4 or 8 h at 37°C, when thiolutin was added, and fractions were collected at various times as described above. CATpG intermediates were analyzed as follows: total RNA was extracted, resolved on 4% polyacrylamide–7 M urea gels, transferred to Hybond nylon membrane (Amersham) using an electroblotter (Hoeffer) and hybridized with an oligonucleotide specific to the poly(G) of the reporter mRNA. For normalization of the amount of RNA loaded on gels, a probe specific to the RNA polymerase III transcript scR1 was used (41). Metabolic labeling of rRNA was performed as described previously (34). Exponentially growing cultures of strains YCO44, rrp43-1, rrp43-2 and rrp43-3 were incubated at 37°C for 4 h in YNB-glucose medium lacking methionine. Subsequently, cells were pulse–chase labeled with 100 µCi/ml [methyl-3H]methionine (Amersham Pharmacia) for 2 min and chased with 100 µg/ml unlabeled methionine. At various times, samples were taken and quickly frozen in a dry ice–ethanol bath. Total RNA was isolated, separated by electrophoresis and blotted as described above. Nylon membranes were incubated in En3Hance (NEN) and submitted to autoradiography.
Table 3. mRNA half-life (min) of PAB1, ACT1 and CATpG mRNAs.
| Strain | PAB1 | ACT1 | CATpG |
|---|---|---|---|
| RRP43 | 15 | 17 | 37 |
| rrp43-1 | 25 | 39 | 52 |
| rrp43-2 | 21 | 32 | 65 |
| rrp43-3 | 15 | 41 | 62 |
Immunoblot analysis
Yeast strains YCO47, YCO48, YCO49 and YCO50 (Table 2) were incubated at 25°C to an OD600 of ∼0.8 and shifted to 37°C for various times. Cells were collected by centrifugation, suspended in PBS (29) and disrupted by vortexing with glass beads. Extracts were cleared by centrifugation, fractionated by SDS–PAGE and transferred to PVDF membranes (Bio-Rad). Membranes were incubated with an anti-HA antibody (Santa Cruz Biotechnology), followed by incubation with anti-mouse IgG linked to horseradish peroxidase (HRP) (Amersham Biosciences). The translation factor eIF2α served as an internal control and was detected with a rabbit antiserum (provided by John McCarthy, University of Manchester), followed by incubation with anti-rabbit IgG linked to HRP (Amersham Biosciences). Blots were developed using the ECL system as described by the supplier (Amersham Biosciences).
RESULTS
Amino acid substitutions in the central region of Rrp43p cause a temperature-sensitive (ts) phenotype
Previous studies on Rrp43p were based on carbon source conditional strains, which contained the RRP43 gene under control of the regulated GAL1 promoter (14,27). Since this promoter is repressed by glucose and induced by galactose, the conditional strain must be maintained in galactose-containing medium, resulting in an excess of Rrp43p in the cell. Analysis of mRNA from this type of conditional strain showed that it was not suitable for mRNA stability assays (data not shown). In addition, we intended to study the effects of Rrp43p deficiency on RNA metabolism with subtle changes that could reveal its specific function. Therefore, we sought to generate RRP43 mutants with temperature-sensitive phenotypes using an in vitro mutagenesis technique (see Materials and Methods; Fig. 2B) that allows for in vivo recombination of mutagenic PCR products with a co-transformed linear vector (35). Approximately 1700 transformants were screened and three strains (rrp43-1, rrp43-2 and rrp43-3) showing stable temperature-sensitive phenotypes were isolated (see Materials and Methods; Fig. 2). To confirm that the ts phenotype was associated with mutations in the plasmid-borne copy of RRP43, plasmids pCFUS-rrp43-1, pCFUS-rrp43-2 and pCFUS-rrp43-3 were isolated from strains rrp43-1, rrp43-2 and rrp43-3, respectively, and retransformed into strain YCO43 (Table 2), which contains plasmid YCp111GAL-RRP43. The resulting strains were cured of YCp111GAL-RRP43 and tested for growth at 37°C. None of the strains containing the plasmids isolated from strains rrp43-1, rrp43-2 and rrp43-3 was able to grow at 37°C (data not shown). Since the temperature-sensitive phenotype could be caused by rapid degradation of the mutant proteins at the non-permissive temperature, we inserted the HA epitope tag at the N-terminus of the wild-type and mutant proteins to examine their stability by immunoblot analysis. The HA-tagged proteins are functional since they complement the chromosomal deletion of RRP43 at the permissive temperature (Fig. 2D). Interestingly, equivalent levels of wild-type and mutant RRP43 proteins were detected following incubation of cells at 37°C for up to 12 h (Fig. 2E), revealing that the temperature-sensitive phenotype is caused by inactivation rather than by rapid degradation of the mutant proteins at 37°C.
Sequence analysis was performed with plasmids pCFUS-rrp43-1, pCFUS-rrp43-2 and pCFUS-rrp43-3 recovered from the mutant strains to identify the amino acid substitutions. rrp43-1 contains a unique amino acid substitution that replaces Val212 with Ala, whereas in rrp43-2 three mutations were detected, Cys230→Tyr, Ile274→Thr and Cys276→Tyr, and rrp43-3 has two amino acids replaced, Ser162→Phe and Ala246→Thr. It is interesting to note that all amino acid substitutions are located in the central region of Rrp43p between amino acids 162 and 276, suggesting that this region is important either for the thermostability of the protein or for its interaction with the exosome complex.
Temperature-sensitive mutants of Rrp43p show rRNA processing defects
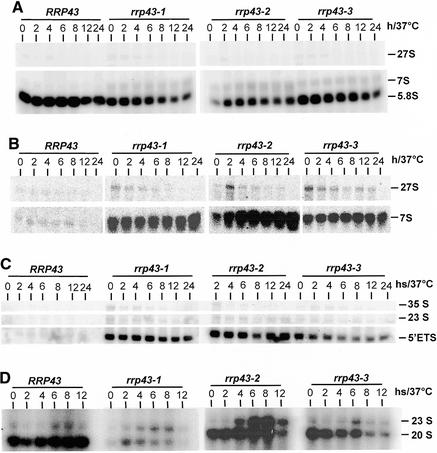
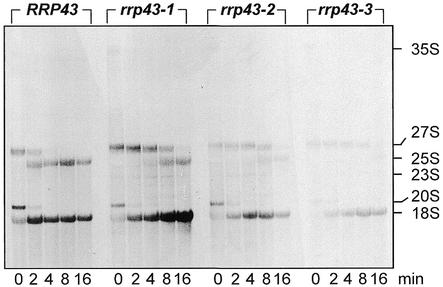
By creating ts mutations in RRP43, it was expected that pre-rRNA processing would be affected since a requirement for Rrp43p in rRNA synthesis has already been demonstrated (14,21). Therefore, we initially analyzed rRNA processing in the ts strains for various times at the restrictive temperature. Northern hybridization analyses detected both a moderate decrease in 5.8S rRNA levels and an increase in 7S pre-rRNA levels in the rrp43 ts mutants (Fig. 3A and B). These strains also showed a strong accumulation of the 5′ ETS region upstream from cleavage site A0 (Fig. 3C). Accumulation of the 5′ ETS has also been reported for yeast strains defective for the exosome subunits Rrp6p, Rrp40p and Csl4p and the RNA helicase Dob1p/Mtr4p (4,11,17,34,42), indicating that degradation of the spacer sequences is a very complex process. The three mutant strains showed slower processing of the 27S pre-rRNA and under-accumulation of the mature 25S rRNA as observed by [methyl-3H]methionine pulse–chase labeling of pre-rRNA (Fig. 4), which is consistent with the accumulation of the 27S pre-rRNA observed in the mutant strains by northern blot analysis. A modest accumulation of the 35S pre-rRNA and of the aberrant 23S pre-rRNA indicates that processing at the sites A0 and A1 may be impaired in the mutant strains (Fig. 3D) (4). The 23S pre-rRNA has been considered an aberrant precursor, containing the 5′ ETS, the 18S rRNA and part of ITS1, that is formed by direct cleavage of the 35S pre-rRNA at site A3 (43); however, under the conditions used in this work, the 23S pre-rRNA can be detected even in the control strain, although at only a low level (Fig. 3D). Northern hybridization with probe 2 detected both the 20S pre-RNA, which is the immediate precursor of the 18S rRNA, and the 23S pre-rRNA (Fig. 3D). By comparing the 23S:20S ratio, a relative increase in the aberrant 23S pre-rRNA is observed in the mutant strains. This increase is more striking in strain rrp43-2. In strain rrp43-3, the levels of 20S pre-rRNA decreased following longer incubation at the restrictive temperature, but no corresponding increase in 23S pre-rRNA was detected, suggesting a possible destabilization of the 20S pre-rRNA. In strain rrp43-2, on the other hand, the higher 23S:20S ratio is clearly caused by an increase in the aberrant 23S pre-rRNA concentration. These results suggest that the mutants may show allele-specific defects. An apparent lower level of [methyl-3H]methinone labeling was observed in the case of strains rrp43-2 and rrp43-3. This may be another indication of allele-specific phenotypes and is not surprising, because allele-specific defects have been described for other mutant strains, such as the ts conditional mutants of NOP1 (44). The aberrant 23S pre-rRNA is barely detectable in the pulse–chase labeling with [methyl-3H]methinone in the mutant strains, probably because cells were shifted to the non-permissive temperature for only 4 h, whereas for steady-state analysis, samples were analyzed for up to 12 h. All of the pre-rRNA processing defects detected in the rrp43 ts strains are consistent with the previous reports about RRP43 function (14,27).
Figure 3.
Northern blot analysis of pre-rRNA processing in rrp43 mutant strains. Total RNA was extracted from cells incubated at 37°C for different time intervals and hybridized against the probes indicated in Figure 1. (A) Hybridization against probe 3 detected similar amounts of 5.8S rRNA in the control strain YCO44 at all time points, whereas mutants show a decrease in 5.8S concentration and an increase in 7S pre-rRNA after shifting cells to 37°C. (B) Hybridization against probe 4 detected a large amount of the 7S pre-rRNA in all of the mutant strains as compared to the control strain, but rrp43-2 cells accumulate a higher amount of this pre-rRNA. Unprocessed 27S pre-rRNA is observed with probe 4 in the mutant strains. (C) The excised 5′ ETS accumulates in rrp43 ts mutants as detected by hybridization with probe 1. The control strain rapidly processes the 5′ ETS, whereas mutant strains accumulate this excised spacer sequence even at the permissive temperature (time 0). (D) Hybridization against probe 2, which is complementary to the 3′ end of the 20S pre-rRNA. Synthesis of this pre-rRNA is not affected in the control strain over time at 37°C whereas the mutant strains, especially rrp43-2, show an increased amount of the aberrant 23S pre-rRNA relative to the amount of the 20S pre-rRNA following incubation at 37°C.
Figure 4.
Pulse–chase labeling of rRNA in rrp43 ts strains. Pulse–chase labeling with [methyl-3H]methionine was performed after incubating cells at 37°C for 4 h. RNA samples were collected at the indicated time intervals. An aliquot of 40 µg of total RNA was loaded in each lane and the agarose gel was stained with ethidium bromide to check that equal amounts of RNA loaded (not shown). Accumulation of 25S and 18S rRNAs is affected in the ts strains after incubation for 4 h at 37°C.
mRNA shows a longer half-life in rrp43 ts strains
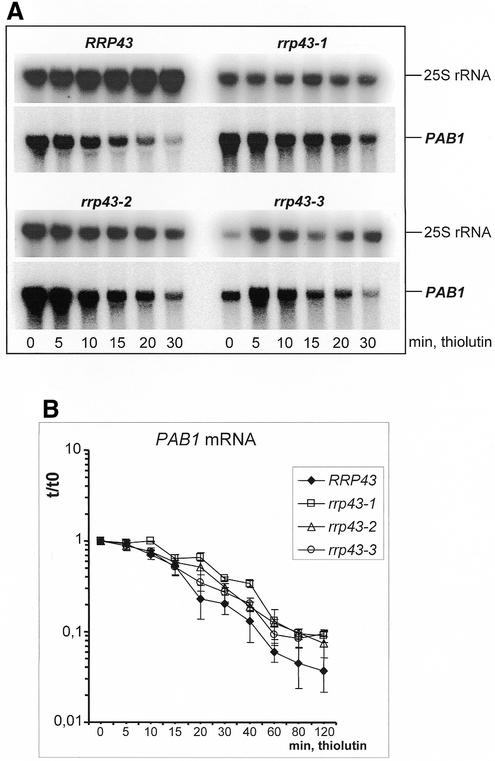
It has been demonstrated previously that Rrp43p is part of both nuclear and cytoplasmic exosome complexes. Moreover, the exosome subunits Rrp4p, Rrp41p/Ski6p and Csl4p/Ski4p, which are components of the cytoplasmic complex, are required for mRNA degradation (10,18,19,26). These findings raised the possibility that the cytoplasmic fraction of Rrp43p also participates in mRNA degradation. To test this hypothesis, we analyzed the stability of several mRNAs in the ts strains isolated in this work. These were grown at 25°C to early log phase and then transferred to 37°C for 4 or 8 h. Subsequently, cells were collected and resuspended in pre-warmed YNB-glucose medium containing 20 µg/ml of the RNA polymerase II inhibitor thiolutin (40). Samples were collected at the moment of thiolutin addition (t0) and after various times of incubation at 37°C. RNA was extracted and analyzed by northern blotting using probes specific to the PAB1, ACT1, MFA2 and CATpG mRNAs. The relative mRNA levels at each time point were obtained by correcting the amount of the test mRNA to the amount of 25S rRNA. Although ts mutants contain less 25S rRNA than the control strain, the 25S rRNA was used as an internal control, because its amount does not change significantly during the time frame in which mRNA half-lives were analyzed. Similar results were obtained when the scR1 RNA, which is transcribed by RNA polymerase III, was used as an internal control. Each of the mRNAs tested showed a significant stabilization in the rrp43 mutant strains relative to the control strain (Figs 5 and 6). Figure 5B shows the graph of PAB1 mRNA decay, which includes data from five independent experiments. Figure 6B shows ACT1 and CATpG decay based on seven and eight independent experiments, respectively. Stability of the MFA2 mRNA was also analyzed and this mRNA showed a longer half-life in all of the mutant strains, although the analysis was done only three times (data not shown). The half-lives of the mRNAs tested, shown in Table 3, were estimated from the average values plotted in the graphs of Figures 5 and 6. The half-life of the PAB1 mRNA was 15 min in the control strain and increased to 21 and 25 min in strains rrp43-2 and rrp43-1, respectively. In strain rrp43-3, the half-life of the PAB1 mRNA is very similar to the control strain in the beginning of the assay. However, following longer times of incubation at the non-permissive temperature, the relative amount of the PAB1 mRNA in rrp43-3 cells is very similar to the other two mutants (Fig. 5B), which indicates a stabilization of this mRNA in each of the three mutants. The half-life of the ACT1 mRNA increased from 20 min in the control strain to 32 min in rrp43-2 and ∼40 min in strains rrp43-1 and rrp43-3. In order to further characterize mRNA decay in the mutant strains isolated in this work, we analyzed the stability of the heterologous CAT (chloramphenicol acetyltransferase) gene containing a poly(G) segment at the 3′-UTR. A CATpG cassette was inserted into a plasmid under the control of the strong constitutive PGK1 promoter and the resulting plasmid transformed into the strain YCO44 (control) and the ts mutants. The poly(G) segment impairs 5′→3′ mRNA degradation (18,45) and increases the half-life of the CAT mRNA from a few minutes (4 min) (39) up to 37 min in the control strain (Fig. 6). Interestingly, the CATpG mRNA half-life was even longer in the rrp43 mutant strains, increasing from 37 min in the control strain to 52 min in rrp43-1, to 65 min in rrp43-2 and to 62 min in rrp43-3 cells. Since the poly(G) segment impairs 5′→3′ mRNA degradation, we also performed northern blot analysis with a probe specific to the poly(G). This analysis detected a degradation intermediate, corresponding to the poly(G) fragment, at a significantly higher concentration in the mutant strains (Fig. 6C). Accumulation of this intermediate and the longer half-lives of the PAB1 and ACT1 mRNAs provide strong evidence that Rrp43p is required for proper mRNA decay in yeast.
Figure 5.
Analysis of PAB1 mRNA stability in rrp43 ts mutants. (A) Northern blot analysis of PAB1 mRNA and of 25S rRNA. Following incubation at 37°C for 8 h, thiolutin was added to cultures to a final concentration of 20 µg/ml and cell samples were collected at the times indicated. Total RNA was extracted and analyzed by northern blotting using probes complementary to the PAB1 mRNA. Subsequently, the membranes were stripped of the PAB1 probe and re-hybridized using a probe complementary to the mature 25S rRNA. The 25S rRNA served as an internal control to normalize the amount of total RNA loaded in each lane. (B) Graph showing the relative amount of PAB1 mRNA in each time point. Blots of five independent experiments were quantitated using a phosphorimager. PAB1 mRNA shows a longer half-life in the mutant strains.
Figure 6.
Analysis of ACT1 and CATpG stability in rrp43 ts strains. (A) Northern blot analysis of the ACT1 and CATpG mRNAs. The 25S rRNA was used as an internal control. Cells were incubated at 37°C for 4 h, at which time thiolutin was added (0), and samples were collected at the times indicated. Membranes were hybridized against the ACT1 and CATpG probes, which were subsequently stripped off, followed by rehybridization with a 25S rRNA probe. (B) Graphs showing the decay of the ACT1 and CATpG mRNAs. Blots of seven and eight independent experiments were quantitated using a phosphorimager to determine the ACT1 and CATpG mRNAs half-lives, respectively. Both mRNAs show a longer half-life in the ts strains after a period of 4 h at 37°C. (C) Northern blot of a polyacrylamide gel to analyze CATpG degradation intermediates. The probe used was an end-labeled oligonucleotide complementary to the poly(G) insertion of CATpG. A degradation intermediate of CATpG mRNA accumulates in the ts strains after incubation at 37°C.
In vivo interactions of Rrp43p with other proteins
Isolation of the exosome by chromatography from yeast cells results in purification of the entire complex (10,14), making it difficult to identify pair-wise subunit interactions. Rrp43p interacts with Nip7p both in the two-hybrid and in co-purification assays. Nip7p is also involved in rRNA maturation, but is not an exosome subunit (25,26). In addition, Rrp43p deficiency affects processing of the 18S and 25S rRNA precursors, indicating that it may interact with more factors of the rRNA processing pathway (27). In order to understand Rrp43p interactions within the exosome complex and to identify novel factors that interact with this protein, we performed a yeast two-hybrid screen (28) using strain YCO45 that carries plasmid pBTM-RRP43, encoding a lexA–Rrp43p fusion as bait for two-hybrid interactions. A cDNA encoding the Rrp46p exosome subunit was identified among the clones that showed a positive two-hybrid interaction, as determined by growth on medium lacking histidine and β-Gal activity (data not shown). The remaining clones contained cDNAs encoding a proteasome subunit and the 90 kDa subunit of TFIIIB, which are not expected to be involved in RNA metabolism. The specificity of Rrp43p interactions with these proteins needs to be confirmed by other methods and will be addressed elsewhere.
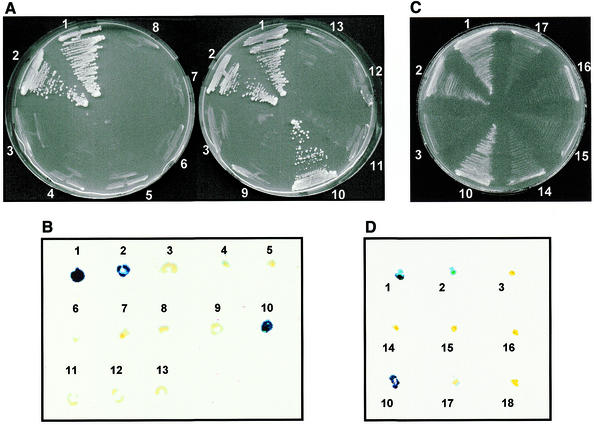
Since Rrp46p was the only exosome subunit isolated from the pACT-cDNA library screen, we decided to test directly for Rrp43p interactions with individual exosome subunits. PCR-amplified ORFs of the genes RRP4, RRP40, RRP41/SKI6, RRP42, RRP44/DIS3, RRP45, RRP46, MTR3, CSL4/SKI4 and RRP6 were cloned into the plasmid pGAD-C (31), generating pGAD-RRP4 through pGAD-RRP46, pGAD-MTR3, pGAD-CSL4 and pGAD-RRP6 (Table 1). The complete coding sequences of all ORFs were verified by DNA sequence analysis and the plasmids were transformed into strain YCO45. The resulting strains were tested for growth on selective (–His) plates and for β-Gal activity. The results showed that Rrp43p interacts strongly only with Rrp46p (Fig. 7). The remaining exosome subunits did not show interactions with Rrp43p under the conditions tested (Fig. 7).
Figure 7.
Analysis of Rrp43p interactions with other exosome subunits. The yeast two-hybrid system was used to determine interactions between Rrp43p and other exosome subunits. The interaction assays were performed in strain YCO45, which contains both the yeast HIS3 and the E.coli lacZ genes as markers for two-hybrid protein interactions. Strain YCO45 (containing plasmid pBTM-RRP43) was transformed with a second plasmid containing the GAL4 activation domain fused to the sequences encoding the other exosome subunits. (A and B) Test of Rrp43p interactions with other exosome subunits as determined by His+ phenotype (growth on YNB –His plate) and lacZ expression (β-Gal activity), respectively. Only Rrp46p interacts strongly with Rrp43p in this assay. (C and D) Analysis of Rrp46p interactions with ts mutants of rrp43. The assay was performed as described above. 1 and 2, strains L40-41 and L40-61 (positive controls); 3, YCO46 (negative control). Numbers ranging from 4 to 13 indicate strain YCO45 transformed with pGAD-C containing the indicated exosome subunit: 4, RRP4; 5, RRP40; 6, RRP41; 7, RRP42; 8, RRP44; 9, RRP45; 10, RRP46; 11, RRP6; 12, CSL4; 13, MRT3. Numbers 14–16 indicate strain L40, carrying pACT-RRP46 and pBTM116 containing the indicated RRP43 allele: 14, rrp43-1; 15, rrp43-2; 16, rrp43-3. 17, negative control that contains plasmids pACT-RRP46 and pBTM116; 18, negative control that contains pACT-RRP46 and YCplac22.
Amino acid substitutions in the central region of Rrp43p severely affected its function; therefore, it was relevant to test whether these mutations affected the Rrp43p–Rrp46p interaction. For this reason, the ORFs encoding the rrp43-1, rrp43-2 and rrp43-3 alleles were cloned into the vector pBTM116 and tested for interactions with Rrp46p (pGAD-RRP46) in the L40 strain. The results shown in Figure 7 demonstrated that the Rrp43p ts mutants do not interact with Rrp46p, either at 25 (data not shown) or 30°C. Most likely these mutations either cause changes in Rrp43p structure, in such a way that the interaction is no longer possible, or they lie in the domains responsible for interaction with Rrp46p.
DISCUSSION
Central amino acid substitutions inactivate Rrp43p activity at 37°C
Rrp43p function has been inferred from its co-purification with the exosome complex and from experiments using strains in which it was conditionally expressed. These studies have shown that Rrp43p deficiency impairs formation of the 5.8S 3′ end and degradation of the excised 5′ ETS (10,14,27) and affects processing of the 18S and 25S rRNA precursors (27). The finding that Rrp43p specifically interacts with Nip7p, a protein also involved in rRNA maturation but not an exosome subunit, suggests that it is involved in more than one step of pre-rRNA processing. A role for Rrp43p in RNA metabolism is further supported by the fact that it shows sequence similarity to bacterial RNase PH. Furthermore, a fraction of Rrp43p is found in the cytoplasm (10,26). In order to further characterize the role of Rrp43p in RNA metabolism, in this work we isolated rrp43 ts mutants and analyzed their effects in vivo on pre-rRNA processing, mRNA degradation and interactions with the exosome.
Three rrp43 ts alleles were isolated, which bear one or more amino acid substitutions in the central region of Rrp43p, ranging from Ser162 to Cys276. In the case of mutant rrp43-2, two of the five cysteine residues found in RRP43 are replaced by tyrosine, which may disrupt disulfide bonds, causing structural changes in the protein. However, rrp43-1 has only the Val212Ala substitution, and shows the same phenotype with respect to temperature sensitivity. Moreover, the temperature-sensitive phenotype of the mutant strains is not caused by rapid turnover of the mutant proteins, since no reduction of the HA-tagged mutant protein levels was detected for up to 12 h of incubation under non-permissive conditions. The HA is a small tag and does not affect protein function since HA-tagged proteins complement the chromosomal deletion of RRP43 at the permissive temperature. In addition, the HA tag does not affect the conditional phenotypes of the mutant strains at 37°C. The presence of the mutant proteins after 12 h of incubation at 37°C indicates that they remain stable under non-permissive conditions. Similar results have been reported for conditional mutant alleles of NOP2, which are stable for up to 24 h at the non-permissive temperature, as determined by immunoblot analysis (46).
Since it has already been shown that Rrp43p participates in rRNA processing, we intended to analyze the effects of the amino acid changes on Rrp43p function. Formation of the 5.8S rRNA 3′ end and degradation of the 5′ ETS are the steps that appear to require the function of an intact exosome, since inactivation of individual subunits blocks these steps (4,14,23,27). Accordingly, the ts mutants showed a moderate effect in 5.8S rRNA synthesis, with accumulation of unprocessed 7S pre-rRNA. In addition, slower processing of the 27S pre-rRNA was also observed in the rrp43 ts mutants, which is consistent with previous studies (27). The slower processing of the 27S pre-rRNA in the mutants is clearly detected by pulse–chase labeling, where it can be visualized up to 8 (rrp43-1 and rrp43-2) or 16 min (rrp43-3) after the chase with methionine. Steady-state analysis by northern blot detected a higher 27S pre-rRNA concentration in the mutants, relative to the control strain. However, the band intensity decreases over time at 37°C. Although at a lower rate, the 27S pre-rRNA continues to be processed following incubation at 37°C since there is formation of 5.8S rRNA even after 24 h at 37°C. The molecular mechanism by which the mutations in Rrp43p impair 27S pre-rRNA processing is not known. Therefore, it is not possible to explain why the concentration of this pre-rRNA decreases in the ts strains over time at the restrictive temperature. Apparently, a fraction of the 27S pre-rRNA is destabilized and degraded prior to cleavage at sites C1 and C2. Previous studies based on carbon source-regulated depletion of Rrp43p detected a decrease in 5.8S formation and 7S pre-rRNA accumulation only after 4–6 h of GAL1 promoter repression (14,27). The ts strains used in this work show these defects even at the permissive temperature (Fig. 3), demonstrating that apparently the mutants provide a more sensitive system for the analysis of Rrp43p function in rRNA processing.
The control strain shows background formation of an aberrant 23S pre-rRNA, but all of the mutants, especially rrp43-2, showed a higher 23S:20S ratio (Fig. 3). This pre-rRNA has been considered an aberrant precursor that is formed by direct cleavage of the 35S pre-rRNA at site A3 (see Fig. 1) (43) when cleavages at sites A0, A1 and A2 are blocked. Therefore, its appearance has been linked to defects in the pathway leading to 18S rRNA biosynthesis. However, the aberrant 23S pre-rRNA has also been detected in strains deficient for factors specifically involved in 25S and 5.8S rRNA biosynthesis. In this case, its presence has been considered a secondary effect of general inhibition of 35S processing, since rRNA synthesis and ribosome biogenesis are highly concerted events. It is unclear whether the increased relative amount of the aberrant 23S pre-rRNA in the ts mutant strains is a secondary effect, because they appear to show allele-specific defects (Figs 3 and 4), as has been observed for conditional ts mutants of NOP1 (44). Nevertheless, we show here that the ts mutants of Rrp43p show a significant relative increase in the 23S pre-rRNA, though to different extents, which is consistent with the proposal that the exosome, together with Dob1p/Mtr4p, degrades aberrant pre-rRNA processing intermediates (4).
Rrp43p is involved in mRNA decay
As mentioned previously, the findings that several exosome subunits are involved in mRNA degradation and that a fraction of Rrp43p is found in the cytoplasm suggested a role for Rrp43p in mRNA decay (47; reviewed in 2,24). The analyses performed in this work show that the half-lives of three native S.cerevisiae mRNAs and of the heterologous reporter mRNA CATpG are significantly increased in rrp43 ts strains, following inactivation of Rrp43p. For example, the half-life of the ACT1 mRNA increased 2-fold in the mutant strains relative to the half-life observed in the control strain. All three mRNAs tested showed a similar half-life increase in the mutant strains. The half-life values of native mRNAs obtained here were slightly different from those obtained in other strain backgrounds (48,49). This could be explained by the RNA polymerase II inhibition method utilized (thiolutin) and by the incubation at 37°C. Secondary effects of cell incubation at 37°C for long time periods have been reported (13), but no effect was detected on the control strain YCO44 (RRP43) subjected to the same conditions. Previous studies on mRNA degradation involving exosome subunits were based on an assay that detects a poly(G)-containing intermediate that is stabilized in strains with defects in Rrp4p, Ski6p/Rrp41p and Csl4p/Ski4p (18,19). Similar experiments performed in this work demonstrated the stabilization of an mRNA degradation intermediate in the mutant strains, which supports the hypothesis that Rrp43p can participate directly in mRNA decay. This hypothesis is also supported by the stabilization of the full-length ACT1 and PAB1 mRNAs in the rrp43 ts strains. Since a fraction of Rrp43p is found in the cytoplasm, we further suggest that it participates in mRNA decay in this cell compartment. Rrp43p inactivation may not have produced a strong effect on mRNA degradation because the main cytoplasmic mRNA turnover pathway involves 5′→3′ degradation, whereas 3′→5′ degradation, which involves all of the exosome subunits, is more active in the nucleus (47). Further experiments are required to characterize a possible Rrp43p role in nuclear pre-mRNA degradation.
Rrp43p interacts strongly with Rrp46p, but not with other exosome subunits
The exosome processes rRNA, snRNA, snoRNAs, pre-mRNAs and mRNAs, and its function in all of these processes must be regulated. Therefore, it is expected that the exosome must interact with many factors in order to distinguish and properly process the different substrates. It has already been demonstrated that Rrp43p interacts with Nip7p (26) and that the proteins Ski2p, Ski3p and Ski8p interact with the cytoplasmic exosome (18). Following Rrp4p characterization as a 5.8S rRNA processing factor (50), the exosome has been purified from yeast cell extracts as a protein complex co-purifying with ProtA–Rrp4p, which is also involved in other cellular events (10,14). However, it is still unknown how interactions within the complex occur, specifically, what components directly interact. In addition, it is not known whether all of the subunits have a catalytic function or if some may have only a structural function. Most probably, each enzyme of the exosome complex acts preferentially on a different substrate (51), and the study of protein–protein interactions should shed some light on these questions. For example, experiments with the two-hybrid system were recently used to investigate protein–protein interactions within the U6 snRNP complex and contributed to the identification of proteins that interact with U6 RNA (52). In this work, the two-hybrid system analyses revealed that Rrp43p interacts strongly with Rrp46p. The rrp43 ts mutant proteins did not show any interaction with Rrp46p under the conditions tested, suggesting that the ts phenotype is caused by disruption of the Rrp43p–Rrp46p interaction, although we cannot exclude the possibility that Rrp43p possesses a catalytic activity that is defective in the ts strains. Nevertheless, the finding that Rrp43p interacts with the exosome through Rrp46p fits the model of a multiple enzyme complex, where each subunit would be able to reach its preferred substrate (24,53).
Acknowledgments
ACKNOWLEDGEMENTS
We thank David Goldfarb for strains and plasmids and Hamza El-Dorry and the members of his laboratory, especially Nathalie Cella, for interesting discussions. We would like to thank Ari J. S. Ferreira and Tereza C. Lima Silva for helping with DNA sequencing analysis. We also thank Fabio Siviero for helping with computer programs. We are grateful to Rogério Meneghini for his support, especially during the initial phase of this work. This work was supported by FAPESP (98/06671-2 to C.C.O. and 00/02788-4 to N.I.T.Z.). F.A.G. was a recipient of a CNPq fellowship.
REFERENCES
- 1.Caponigro G. and Parker,R. (1996) Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol. Rev., 60, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P. and Tollervey,D. (2000) mRNA stability in eukaryotes. Curr. Opin. Genet. Dev., 10, 193–198. [DOI] [PubMed] [Google Scholar]
- 3.Woolford J.L. Jr, and Warner,J.R. (1991) The ribosome and its synthesis. In Broach,J.R., Pringle,J.R. and Jones,E.W. (eds), The Molecular Biology of the Yeast Saccharomyces: Genome Dynamics, Proteins Synthesis and Energetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 587–626.
- 4.Allmang C., Mitchell,P., Petfalsky,E. and Tollervey,D. (2000) Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res., 28, 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venema J. and Tollervey,D. (1995) Processing of pre-ribosomal RNA in Saccharomyces cerevisae. Yeast, 11, 1629–1650. [DOI] [PubMed] [Google Scholar]
- 6.Geerlings T.H., Vos,J.C. and Raué,H.A. (2000) The final step in the formation of 25S rRNA in Saccharomyces cerevisiae is performed by 5′-to-3′ exonucleases. RNA, 6, 1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry Y., Wood,H., Morrissey,J.P., Petfalski,E., Kearsey,S. and Tollervey,D. (1994) The 5′ end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J., 13, 2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C.L. and Stevens,A. (1993) Yeast cells lacking 5′→3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol. Cell. Biol., 13, 4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens A., Hsu,C.L., Isham,K.R. and Larimer,F.W. (1991) Fragments of the internal transcribed spacer1 of pre-rRNA accumulate in Saccharomyces cerevisiae lacking 5′→3′ exoribonuclease 1. J. Bacteriol., 173, 7024–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allmang C., Petfalsky,E., Podtelejnikov,A., Mann,M., Tollervey,D. and Mitchell,P. (1999) The yeast exosome and human PM-Scl related complexes of 3′-5′ exonucleases. Genes Dev., 13, 2148–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkard K.T. and Butler,J.S. (2000) A nuclear 3′-5′ exonuclease involved in mRNA degradation interacts with poly(A) polymerase and the hnRNA protein Npl3p. Mol. Cell. Biol., 20, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hoof A., Lennertz,P. and Parker,R. (2000) Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5.8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J., 19, 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hoof A., Lennertz,P. and Parker,R. (2000) Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol. Cell. Biol., 20, 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell P., Petfalsky,E., Shevchenko,A., Mann,M. and Tollervey,D. (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′-5′ exoribonucleases. Cell, 91, 457–466. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer R., Allmang,C., Raijmakers,R., van Aarssen,Y., Egberts,W.V., Petfalski,E., van Venrooij,W.J., Tollervey,D. and Pruijn,G.J.M. (2001) Three novel components of the human exosome. J. Biol. Chem., 276, 6177–6184. [DOI] [PubMed] [Google Scholar]
- 16.Estévez A.M., Kempf,T. and Clayton,C. (2001) The exosome of Trypanosoma brucei. EMBO J., 20, 3831–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chekanova J.A., Shaw,R.J., Wills,M.A. and Belostotsky,D.A. (2000) Poly(A) tail-dependent exonuclease AtRrp41p from Arabidopsis thaliana rescues 5.8S rRNA processing and mRNA decay defects of the yeast ski6 mutant and is found in an exosome-sized complex in plant and yeast cells. J. Biol. Chem., 275, 33158–33166. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs-Anderson J.S. and Parker,R. (1998) The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J., 17, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Hoof A., Staples,R.R., Baker,R.E. and Parker,R. (2000) Function of the ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell. Biol., 20, 8230–8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C.-Y., Gherzi,R., Ong,S.-E., Chan,E.L., Raijmakers,R., Pruijn,G.J.M., Stoecklin,G., Moroni,C., Mann,M. and Karin,M. (2001) AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell, 107, 451–464. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee D., Gao,M., O’Connor,J.P., Raijmakers,R., Pruijn,G., Lutz,C.S. and Wilusz,J. (2002) The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J., 21, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z. and Kiledjian,M. (2001) Functional link between the mammalian exosome and mRNA decapping. Cell, 107, 751–762. [DOI] [PubMed] [Google Scholar]
- 23.Briggs M.W., Burkard,K.T.D. and Butler,J.S. (1998) Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8S rRNA 3′ end formation. J. Biol. Chem., 273, 13255–13263. [DOI] [PubMed] [Google Scholar]
- 24.van Hoof A. and Parker,R. (1999) The exosome: a proteasome for RNA? Cell, 99, 347–350. [DOI] [PubMed] [Google Scholar]
- 25.Zanchin N.I.T., Roberts,P., DeSilva,A., Sherman,F. and Goldfarb,D.S. (1997) Saccharomyces cerevisiae Nip7p is required for efficient 60S ribosome subunit biogenesis. Mol. Cell. Biol., 17, 5001–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanchin N.I.T. and Goldfarb,D.S. (1999) Nip7p interacts with Nop8p, an essential nucleolar protein required for 60S ribosome biogenesis, and the exosome subunit Rrp43p. Mol. Cell. Biol., 19, 1518–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanchin N.I.T. and Goldfarb,D.S. (1999) The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucleic Acids Res., 27, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien C.T., Bartel,P.L., Sternglanz,R. and Fields,S. (1991) The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl Acad. Sci. USA, 88, 9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J., Maniatis,T. and Fritsch,E.F. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Bartel P.L., Chien,C.T., Sternglanz,R. and Fields,S. (1993) Analysing protein-protein interactions using two-hybrid system. In Hartley,D.A. (ed.), Cellular Interactions in Development: A Practical Approach. Oxford University Press, Oxford, UK, pp. 153–179.
- 31.James P., Halladay,J. and Craig,E.A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics, 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niedenthal R.K., Riles,L., Johnston,M. and Hegemann,J.H. (1996) Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast, 12, 773–786. [DOI] [PubMed] [Google Scholar]
- 33.Gietz R.D. and Sugino,A. (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene, 74, 527–534. [DOI] [PubMed] [Google Scholar]
- 34.Sherman F., Fink,G.R. and Hicks,J.B. (1986) Laboratory Course Manual for Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Mulhrad D., Hunter,R. and Parker,R. (1992) A rapid method for localized mutagenesis of yeast genes. Yeast, 8, 79–82. [DOI] [PubMed] [Google Scholar]
- 36.Chen D.C., Yang,B.C. and Kuo,T.T. (1992) One step transformation of yeast in stationary phase. Curr. Genet., 21, 83–84. [DOI] [PubMed] [Google Scholar]
- 37.Hollenberg S.M., Sternglanz,R., Cheng,P.F. and Weintraub,H. (1995) Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol. Cell. Biol., 15, 3813–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vojtek A.B. and Hollenberg,S.M. (1995) Ras-Raf interaction: two-hybrid analysis. Methods Enzymol., 255, 331–342. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira C.C. and McCarthy,J.E.G. (1995) The relationship between eukaryotic translation and mRNA stability: a short open reading frame strongly inhibits translational initiation and greatly accelerates mRNA degradation in the yeast Saccharomyces cerevisiae. J. Biol. Chem., 270, 8936–8943. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez A., Tipper,D.J. and Davies,J. (1973) Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob. Agents Chemother., 3, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decker C.J. and Parker,R. (1993) A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev., 7, 1632–1643. [DOI] [PubMed] [Google Scholar]
- 42.de la Cruz J., Kressler,D., Tollervey,D. and Linder,P. (1998) Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J., 17, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russel I.D. and Tollervey,D. (1992) NOP3 is an essential yeast protein which is required for pre-rRNA processing. J. Cell Biol., 119, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tollervey D., Lehtonen,H., Jansen,R., Kern,H. and Hurt,E.C. (1993) Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome biogenesis. Cell, 72, 443–457. [DOI] [PubMed] [Google Scholar]
- 45.Vreken P. and Raue,H.A. (1992) The rate-limiting step in yeast PGK1 mRNA degradation is an endonucleolytic cleavage in the 3′-terminal part of the coding region. Mol. Cell. Biol., 12, 2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong B., Wu,K., Brockenbrough,J.S., Wu,P. and Aris,J. (2001) Temperature sensitive nop2 alleles defective in synthesis of 25S rRNA and large ribosomal subunits in Saccharomyces cerevisiae. Nucleic Acids Res., 29, 2927–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bousquet-Antonelli C., Presutti,C. and Tollervey,D. (2000) Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell, 102, 765–775. [DOI] [PubMed] [Google Scholar]
- 48.Herrick D., Parker,R. and Jacobson,A. (1990) Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol. Cell. Biol., 10, 2269–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulhrad D., Decker,C.J. and Parker,R. (1994) Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to de-capping followed by 5′ to 3′ digestion of the transcript. Genes Dev., 8, 855–866. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell P., Petfalsky,E. and Tollervey,D. (1996) The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev., 10, 502–513. [DOI] [PubMed] [Google Scholar]
- 51.Allmang C., Kufel,J., Chanfreau,G., Mitchell,P., Petfalsky,E. and Tollervey,D. (1999) Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J., 18, 5399–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayes A.E., Verdone,L., Legrain,P. and Beggs,J.D. (1999) Characterization of Sm-like proteins in yeast and their association with U6 snRNA. EMBO J., 18, 4321–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell P. and Tollervey,D. (2000) Musing on the structural organization of the exosome complex. Nature Struct. Biol., 7, 843–846. [DOI] [PubMed] [Google Scholar]