Abstract
TNF has a critical mediator role in inflammation and is an important therapeutic target. We recently discovered that TNF production is regulated by neural signals through the vagus nerve. Activation of this “cholinergic antiinflammatory pathway” inhibits the production of TNF and other cytokines and protects animals from the inflammatory damage caused by endotoxemia and severe sepsis. Here, we describe a role for central muscarinic acetylcholine receptors in the activation of the cholinergic antiinflammatory pathway. Central muscarinic cholinergic activation by muscarine, the M1 receptor agonist McN-A-343, and the M2 receptor antagonist methoctramine inhibited serum TNF levels significantly during endotoxemia. Centrally administered methoctramine stimulated vagus-nerve activity measured by changes in instantaneous heart-rate variability. Blockade of peripheral muscarinic receptors did not abolish antiinflammatory signaling through the vagus nerve, indicating that peripheral muscarinic receptors on immune cells are not required for the cytokine-regulating activities of the cholinergic antiinflammatory pathway. The role of central muscarinic receptors in activating the cholinergic antiinflammatory pathway is of interest for the use of centrally acting muscarinic cholinergic enhancers as antiinflammatory agents.
Keywords: muscarinic receptors, systemic inflammation, TNF, vagus nerve
The overproduction of cytokines, including TNF, mediates tissue damage during the response to injury or pathogenic invasion (1). Physiological and molecular mechanisms that control TNF production maintain cytokine homeostasis and minimize or prevent damage during the host response to infection, injury, arthritis, and other disorders (1–3). Recently, we found (4–6) that electrical or pharmacological activation of the efferent vagus nerve inhibits the release of TNF and attenuates the development of endotoxin-induced shock in rodents. Stimulation of the efferent vagus nerve activates the release of acetylcholine. Although the vagus nerve is a “classical” cholinergic regulator of visceral functions in which peripheral muscarinic acetylcholine receptors have a major mediating role, the vagus-nerve cytokine-inhibiting activity (which is termed “the cholinergic antiinflammatory pathway”) requires signaling through nicotinic α7 subunit-containing receptors (4, 5).
Studies have implicated muscarinic receptor-dependent mechanisms in the central modulation and integration of vagal regulation of visceral functions. For example, vagus nerve control of glycogen synthesis in the liver, the Bezold–Jarish cardiovascular reflex and the regulation of exocrine pancreatic secretion are modulated centrally by brain muscarinic receptor mechanisms (7–10). Thus, we reasoned that brain muscarinic receptors could be involved in the central regulation of the vagus-nerve immunomodulatory function. Our results indicate that central cholinergic activation by selective muscarinic receptor ligands significantly inhibits systemic TNF in endotoxemic rats and activates the efferent vagus-nerve activity. In contrast, peripheral muscarinic receptors do not have a major role in mediating the inflammatory response to endotoxin and its inhibition by the cholinergic antiinflammatory pathway. These findings identify a pivotal role for central muscarinic cholinergic activation in the inhibition of the systemic inflammatory response during endotoxemia and indicate that the cholinergic antiinflammatory pathway represents a peripheral muscarinic-receptor-independent cholinergic function of the vagus nerve.
Results
Brain Muscarinic Receptors Mediate the Inhibition of Systemic TNF.
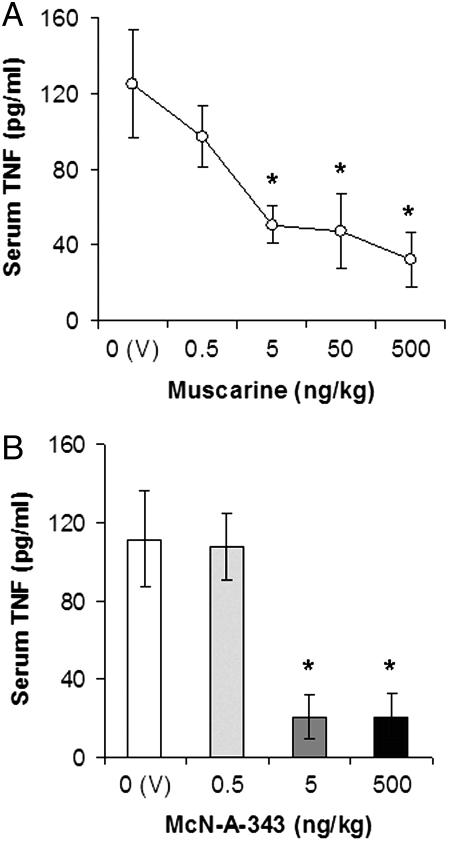
Five muscarinic receptor subtypes (M1–M5) with different synaptic locations and functions are found in the CNS and periphery (11). Except for the M5 subtype, muscarinic receptors (M1–M4) are widely distributed in the brain (12). To study the role of central muscarinic receptors in the inhibition of serum TNF, we administered intracerebroventricularly (i.c.v.) the prototype muscarinic receptor agonist muscarine (0.5–500 ng/kg) before endotoxin exposure. Muscarine dose-dependently inhibited serum TNF (Fig. 1A). Postsynaptically located M1 subtype muscarinic receptors have an important role in the processing of cholinergic neurotransmission in the brain (11, 12). We tested the TNF-suppressing efficiency of the M1 agonist McN-A-343 (0.5, 5, and 500 ng/kg, i.c.v.) and observed that it decreased endotoxin-induced serum TNF significantly (Fig. 1B). We have shown (6) that the tetravalent quanylhydrazone N,N′-bis (3,5-diacetylphenyl) decanediamide tetrakis (amidinohydrazone) tetrahydrochloride (CNI-1493; Chemical Abstract Service registry no. 164301-51-3) inhibits systemic TNF by central activation of the cholinergic antiinflammatory pathway. Therefore, we studied whether CNI-1493 interacted with muscarinic receptors. CNI-1493 (10 μM) inhibited specific binding to muscarinic receptors in an in vitro radioligand-binding assay, with highest specificity to the M1 subtype (data not shown), which indicated an interaction of the compound with these receptors. Together, these observations indicate that activation of central muscarinic receptors (and, specifically, the M1 subtype) inhibits systemic TNF release during endotoxemia.
Fig. 1.
Central administration of muscarinic receptor agonists inhibit systemic TNF in endotoxemic rats. (A) Central administration of muscarine inhibits serum TNF. Rats were injected (i.c.v.) with vehicle (V, saline) or muscarine 1 h before endotoxin (15 mg/kg, i.v.) administration. Serum TNF concentrations were analyzed by ELISA 1.5 h after endotoxin administration (n = 5–7 animals per group). (B) Central administration of the M1 agonist McN-A-343 suppresses serum TNF levels in endotoxemic rats. Animals were injected i.c.v. with vehicle (V) or McN-A-343 1 h before endotoxin (15 mg/kg, i.v.) administration. Serum TNF concentrations were analyzed by ELISA in blood, obtained 1.5 h after endotoxin administration (n = 5–7 animals per group; ∗, P < 0.05).
Central Administration of Methoctramine Decreases Systemic TNF in Endotoxemic Animals.
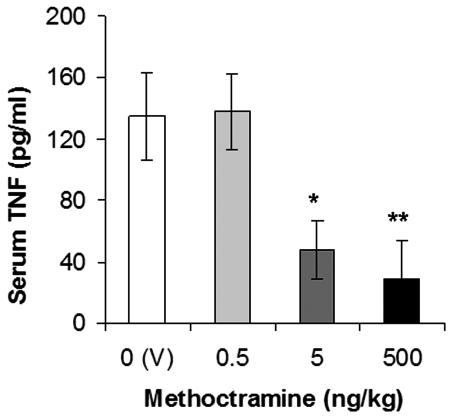
Next, we studied the antiinflammatory efficacy of an independent pharmacological pathway of central cholinergic activation. The release of acetylcholine in the cholinergic synapse is negatively regulated by the presynaptic M2 autoreceptor, and inhibition of this receptor by methoctramine and other specific antagonists enhances central cholinergic transmission (13, 14). We injected methoctramine (0.5, 5, and 500 ng/kg, i.c.v.) 1 h before endotoxin administration, and we observed a dose-dependent inhibition of serum TNF levels (Fig. 2). Together, these results (Figs. 1 and 2) identify a central cholinergic mechanism that is activated through muscarinic receptors to inhibit systemic TNF release.
Fig. 2.
Central treatment with the M2 antagonist methoctramine inhibits serum TNF levels during endotoxemia. Rats were injected (i.c.v.) with vehicle (V, saline) or methoctramine 1 h before endotoxin (15 mg/kg, i.v.) administration. Serum TNF concentrations were analyzed by ELISA 1.5 h after endotoxin administration (n = 5–7 animals per group; ∗, P < 0.05; ∗∗, P < 0.02).
Central Administration of Methoctramine Increases the High-Frequency Power Component of Heart-Rate Variability.
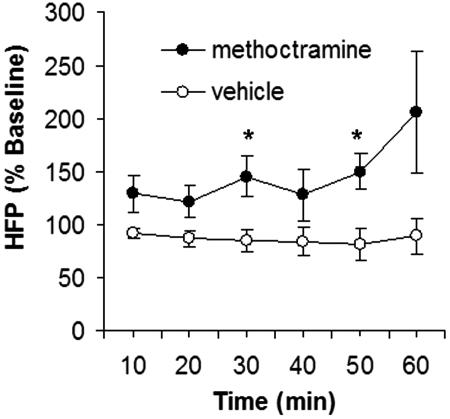
To determine the specificity of muscarinic receptor ligands on activating vagus-nerve signaling, we next measured the effect of methoctramine on vagus-nerve activity through determination of heart-rate variability. Heart-rate variability, which refers to variations in the R–R intervals on electrocardiogram (ECG) tracings, is a measure of the autonomic regulation of the cardiac function. Spectral analysis of ECG recordings enables assessment of the high-frequency power component of heart-rate variability, which correlates to vagus-nerve activity. Centrally administered methoctramine (500 ng/kg, i.c.v.) caused a significant increase in the high-frequency component of heart-rate variability (Fig. 3). No statistically significant change in the low-frequency power (a measure of sympathetic tone) was observed as a result of methoctramine treatment (data not shown). These data suggest that centrally acting methoctramine increases efferent vagus-nerve activity.
Fig. 3.
Central treatment with methoctramine increases efferent vagus-nerve activity. Animals were treated with vehicle (saline) or methoctramine (500 ng/kg) and ECG recorded for 60 min. High-frequency power (HFP) component of heart-rate variability was determined by spectral analysis (n = 5–7 animals per group; ∗, P < 0.02).
Peripheral Muscarinic Receptors Are Not Essential for the Regulation of the Systemic Inflammatory Response by the Cholinergic Antiinflammatory Pathway.
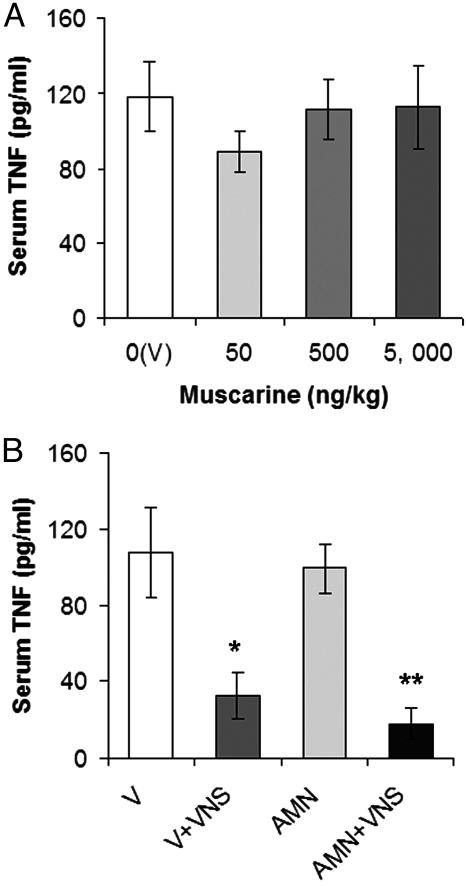
Although an essential role for the nicotinic α7 subunit in transmitting vagal antiinflammatory signaling in the periphery was shown in ref. 5, the role of muscarinic receptors in the systemic inflammatory response (and its regulation by the cholinergic antiinflammatory pathway) remained incompletely understood. Peripheral muscarinic receptors have an important mediating role in the vagus-nerve control of vital physiological functions. The presence of muscarinic receptors has been also reported on peripheral immune TNF-producing cells, including macrophages, monocytes, lymphocytes, and mast cells (15–17). To determine whether TNF release can be modulated by peripheral muscarinic receptor activation in vivo, we injected sublethal doses of muscarine i.v. before endotoxin administration in rats. Muscarine, which does not cross the blood–brain barrier, had no significant effect on serum TNF during endotoxemia (Fig. 4A). Activation of the cholinergic antiinflammatory pathway by electrical vagus-nerve stimulation (VNS; a standard approach used in refs. 4 and 6) attenuated serum TNF in endotoxemic rats (Fig. 4B). Peripheral muscarinic receptor blockade by the muscarinic antagonist atropine methyl nitrate (AMN, 1 mg/kg, i.v.), which does not cross the blood–brain barrier, did not prevent the suppression of TNF by VNS (Fig. 4B). These results indicate that the direct activation or inhibition of peripheral muscarinic receptors has no effect on systemic TNF release and its inhibition by the cholinergic antiinflammatory pathway.
Fig. 4.
Peripheral muscarinic receptors do not mediate the inflammatory response and its regulation by the cholinergic antiinflammatory pathway in endotoxemic rats. (A) Muscarine administered peripherally has no significant effect on serum TNF in endotoxemic rats. Vehicle (V, saline) or sublethal muscarinic doses (50–5,000 ng/kg i.v.) were injected 1 h before endotoxin (15 mg/kg, i.v.) administration. (B) Electrical VNS reduces serum TNF in endotoxemic rats, pretreated with vehicle (V, saline) or AMN (1 mg/kg, i.v.), compared with nonstimulated controls. Rats were pretreated with saline or AMN 30 min before VNS. Serum TNF concentrations were analyzed by ELISA 1.5 h after the administration of a lethal endotoxin dose (15 mg/kg) (n = 5–7 animals per group; ∗, P < 0.01; ∗∗, P < 0.001).
Discussion
Here, we provide insight into the central regulation of the recently discovered immunoregulatory function of the efferent vagus nerve (which is termed the cholinergic antiinflammatory pathway) and identify a primary role of brain muscarinic receptors in controlling systemic inflammation.
Muscarinic receptors are a family of G protein-coupled receptors that have a primary role in central cholinergic neurotransmission. Specific agonists, which activate postsynaptic muscarinic receptors, stimulate cholinergic signaling. Here, we show that central administration of the prototype muscarinic receptor agonist muscarine or the selective M1 agonist McN-A-343 causes a dose-dependent inhibition of endotoxin-induced systemic TNF levels. Central cholinergic transmission can also be activated by inhibition of the presynaptic M2 acetylcholine autoreceptor using selective antagonists. The presynaptic M2 autoreceptor negatively influences the release of acetylcholine in several brain regions, including the striatum, hippocampus, and cerebral cortex (18–20). A direct consequence of brain M2 autoreceptor inhibition is an elevation of acetylcholine release in the synaptic cleft. Methoctramine and other M2 receptor antagonists have been shown to enhance the release of acetylcholine in different brain structures (13, 21, 22). We demonstrate here that centrally administered M2 antagonist methoctramine significantly and dose-dependently decreases systemic TNF levels. These findings reveal a correlation between M1 agonist/M2 antagonist-activated central cholinergic neurotransmission and a lower magnitude of systemic inflammatory response (Fig. 5). Central administration of methoctramine increases vagal cholinergic outflow, as measured by the increased high-frequency power component of heart-rate variability. This observation suggests a role for the efferent vagus nerve in conveying the central signal to peripheral immune mechanisms (Fig. 5). The tetravalent guanylhydrazone CNI-1493, which is a central activator of the cholinergic antiinflammatory pathway (6), interacts with muscarinic receptors, and this finding also supports a mediating role for vagal mechanisms in transmitting CNS muscarinic cholinergic signaling to the periphery.
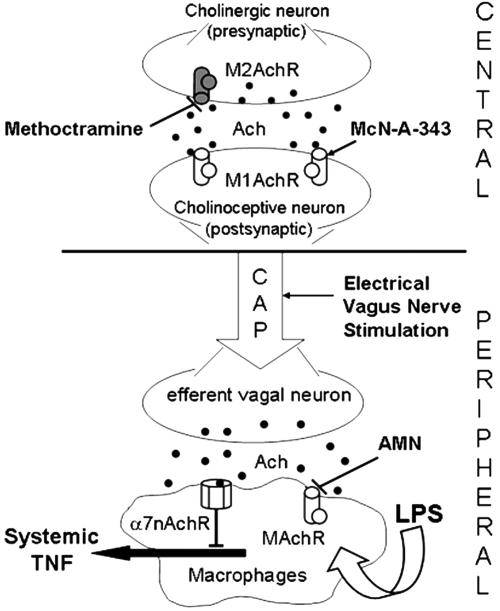
Fig. 5.
Central versus peripheral muscarinic cholinergic regulation of the systemic inflammatory response (proposed mechanism). In rodents, the endotoxin-induced systemic TNF release is inhibited by cholinergic signaling derived from efferent vagus-nerve fibers (4, 5). This cholinergic antiinflammatory pathway (CAP) is mediated through α7 nicotinic acetylcholine receptors (α7nAchR) on macrophages (5). Central muscarinic cholinergic activation by stimulation of M1 acetylcholine muscarinic receptors (MAchR, postsynaptic) or inhibition of M2 muscarinic acetylcholine receptors) M2AchR, presynaptic) inhibits systemic TNF release in endotoxemic rats and activates efferent vagus-nerve activity. Activation of the cholinergic antiinflammatory pathway by electrical vagus nerve stimulation reduces systemic TNF levels during endotoxemia through peripheral muscarinic receptor (MAchR)-independent and α7nAchR-dependent mechanisms (see text for details).
Reciprocal interconnections between brainstem autonomic nuclei and forebrain structures, including the hypothalamus, constitute a central autonomic network, which controls vagal functions, regulating visceral processes (23–27). Vagal preganglionic neurons originate from the dorsal motor nucleus of the vagus (DMV) in the brainstem medulla oblongata. Cardiovascular vagal efferents originate also from the medullar nucleus ambiguous. Muscarinic cholinergic mechanisms in the central autonomic network have been implicated in the regulation of vagus-nerve-regulated peripheral processes, including glycogen synthesis in the liver and the Bezold–Jarish cardiovascular reflex (7–9). Interestingly, Li et al. (10) have demonstrated with McN-A-343 that cholinergic inputs to the hypothalamus activate an M1 receptor-dependent mechanism, which regulates pancreatic exocrine secretion through the vagus nerve. It appears that muscarine and McN-A-343 do not cause a direct excitatory input on muscarinic receptors located on efferent preganglionic vagal neurons, because DMV neurons are virtually devoid of muscarinic receptor-binding sites (28).
This study reveals a role for muscarinic acetylcholine receptors in the central activation of the cholinergic antiinflammatory pathway, but we cannot exclude the fact that additional interactions occur between cholinergic and other neurotransmitter systems within the central autonomic network. A role for central cholinergic mechanisms in modulating peripheral inflammatory responses in other animal models has been suggested (29, 30), but the functional CNS-peripheral connection has not been elucidated. Our observations indicate that the cholinergic antiinflammatory pathway provides a functional connection. It is possible that sympathetic autonomic mechanisms, which are an integral part of the efferent circuitry of the inflammatory reflex (1), also contribute via central cholinergic activation to inhibit systemic TNF release.
Another aspect of this work was to study whether muscarinic receptors with a recognized role in mediating vital, efferent vagus-nerve functions in the periphery are involved in mediating vagal TNF inhibitory output. Muscarinic receptors are expressed on immune cells, including macrophages (15, 31, 32), which produce TNF during endotoxemia (33, 34). Vagal efferents, distributed throughout the reticuloendothelial system and other peripheral organs are positioned to rapidly transmit the antiinflammatory signal to these cells. Our data, showing a lack of significant effect of i.v. administered muscarine (which does not cross the blood–brain barrier) on systemic TNF release during endotoxemia, suggest that “immune” peripheral muscarinic receptors do not have an essential role in mediating the inflammatory response during endotoxemia. Blockade (by AMN) of peripheral muscarinic receptors on immune cells does not prevent the inhibition of TNF release by the activated (by electrical stimulation) cholinergic antiinflammatory pathway. This observation indicates that unlike other classical vagal cholinergic functions, the inhibition of TNF release does not involve signaling through muscarinic receptors (Fig. 5). The α7 subunit-containing nicotinic receptor is the only known acetylcholine receptor with a recognized role in mediating vagal antiinflammatory output in the periphery (5) (Fig. 5).
Thus, central, but not peripheral, muscarinic receptors are involved in regulating the inflammatory response during endotoxemia and its inhibition by the cholinergic antiinflammatory pathway. Activation of central muscarinic cholinergic transmission represents an experimental approach to achieve control over unrestrained systemic inflammation. The antiinflammatory effects of clinically used centrally acting cholinergic drugs in humans would be interesting to explore.
Materials and Methods
Animals.
Male Lewis rats (280–350 g; Charles River Laboratories) were used in the animal experiments. Animals were allowed to acclimate for at least 10 days before the corresponding experiment. Animals were housed in standard conditions (room temperature, 22°C; 12:12-h light/dark cycle) with access to regular chow and water. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols that were approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Endotoxemia and Drug Treatment.
Rats were anesthetized with urethane (1 g/kg, i.p.) and xylazine (10 mg/kg, intramuscular). Endotoxin (LPS, Escherichia coli 0111:B4; Sigma; 10 mg/ml in pyrogen-free saline) was sonicated for 30 min and injected in a dose of 15 mg/kg i.v. into the femoral vein. Compounds (Sigma) were injected either i.c.v. or i.v. (as indicated) 1 h before i.v. endotoxin administration in anesthetized animals. At 1.5 h after endotoxin exposure, animals were killed by CO2 asphyxiation, and blood was collected from the femoral vein.
i.c.v. Injections.
Rats were anesthetized and placed in a stereotaxic head frame (Stoelting) as described (6). The incisor bar was adjusted until the plane defined by the lambda and bregma was parallel to the base plate. A burr hole was made over the parietal bone, and the needle of a syringe (25 μl; Hamilton) was stereotaxically guided into the left lateral ventricle (0.8 mm posterior to bregma, 1.5 mm lateral to midline, and 4.0 mm below the surface of the parietal bone). The location of the needle in the ventricles according to these coordinates was confirmed by histological studies. Drugs were dissolved in saline and administered in a volume of 10 μl over 2 min to allow for diffusion and to control for backflow. The needle of the syringe was removed at 5 min after injecting the corresponding drug or vehicle. A piece of bone wax was used to cover the burr hole.
Radioligand-Binding Assay.
The in vitro interaction of CNI-1493 to muscarinic acetylcholine receptors was analyzed by a radioligand-binding assay performed by NovaScreen (Hanover, MD). The ability of CNI-1493 (10 μM, duplicate tests) to bind to muscarinic receptors was assessed as the percentage of inhibition of specific binding of radiolabeled muscarinic agonists and antagonists.
Heart-Rate Variability Determination and Spectral Analysis of Data.
Rats were anesthetized and positioned as described above. Two unipolar platinum electrodes were placed onto the anterior chest wall. The negative lead was placed in the right midclavicular line at the level of the suprasternal notch, and the positive lead was placed in the left midclavicular line at the level of the xiphoid process. The electrodes were attached to an amplifier (ECG 100C; Biopac Systems, Santa Barbara, CA) that transmitted the recorded electrical signal to a processing unit (MP150; Biopac Systems). The resulting ECG was processed offline by using acqknowledge software (Biopac Systems). Baseline ECGs were recorded for 10 min on each group. After injection of drug or vehicle, ECGs were recorded for a total of 60 min. This time was divided into 10-min epochs. Each epoch underwent spectral analysis by using fast Fourier transform to determine the high- and low-frequency power components of heart-rate variability. The range for low-frequency power was 0.4–1 Hz, and the range for high-frequency power was 1–6 Hz.
VNS.
Anesthetized animals were subjected to electrical VNS, or sham operation without stimulation. The left cervical vagus nerve was isolated from surrounding tissues and stimulated as described in ref. 4. In the sham-operated animals the left vagus nerve was visualized, but not isolated from surrounding tissue. Bipolar platinum electrodes (Plastics One, Roanoke, VA) connected to a stimulation module (STM 100A; Harvard Apparatus) applied constant voltage stimuli (5 V, 2 ms, and 1 Hz) for 20 min (10 min before and 10 min after LPS injection) in the indicated experiments. At 30 min before VNS, animals were injected i.v. with saline or AMN, as indicated.
TNF Analysis.
Blood was centrifuged at 1,500 × g for 15 min, and the supernatants were collected for TNF determination. Sera were used for TNF protein analysis by ELISA (R & D Systems) according to the manufacturer’s recommendations.
Statistical Analysis.
Values are presented as mean ± SEM. ANOVA followed by Student’s t test were performed on all data in the figures to compare mean values. Significant difference between groups was set at P < 0.05.
Acknowledgments
This work was supported in part by North Shore–Long Island Jewish General Clinical Research Center Grant M01 RR018535 and the National Institute of General Medical Sciences (K.J.T.).
Abbreviations
- i.c.v.
intracerebroventricular
- VNS
vagus-nerve stimulation
- AMN
atropine methyl nitrate
- CNI-1493
N,N′-bis (3,5-diacetylphenyl) decanediamide tetrakis (amidinohydrazone) tetrahydrochloride.
Footnotes
Conflict of interest statement: K.J.T. and V.A.P. are inventors on patents related to the content of this paper. K.J.T. is a founder and consultant to Critical Therapeutics, Inc.
References
- 1.Tracey K. J. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Tracey K. J. Fatal Sequence: The Killer Within. Washington, DC: Dana; 2005. pp. 128–204. [Google Scholar]
- 3.Pavlov V. A., Tracey K. J. Cell Mol. Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., Tracey K. J. Nature. 2000;405:458–461. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., et al. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 6.Bernik T. R., Friedman S. G., Ochani M., DiRaimo R., Ulloa L., Yang H., Sudan S., Czura C. J., Ivanova S. M., Tracey K. J. J. Exp. Med. 2002;195:781–789. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazu T., Matsushita H., Ishikawa K. Science. 1976;194:535–536. doi: 10.1126/science.9692. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita H., Ishikawa K., Shimazu T. Brain Res. 1979;163:253–261. doi: 10.1016/0006-8993(79)90353-6. [DOI] [PubMed] [Google Scholar]
- 9.Saito K., Yoshioka M., Kohya T., Kitabatake A. J. Auton. Nerv. Syst. 1994;49:64–68. doi: 10.1016/0165-1838(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Wu X., Zhu J., Yan J., Owyang C. J. Physiol. 2003;552:571–587. doi: 10.1113/jphysiol.2003.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wess J. Crit. Rev. Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 12.van der Zee E. A., Luiten P. G. Prog. Neurobiol. 1999;58:409–471. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 13.Stillman M. J., Shukitt-Hale B., Kong R. M., Levy A., Lieberman HR. Brain Res. Bull. 1993;32:385–389. doi: 10.1016/0361-9230(93)90204-o. [DOI] [PubMed] [Google Scholar]
- 14.Lachowicz J. E., Duffy R. A., Ruperto V., Kozlowski J., Zhou G., Clader J., Billard W., Binch H., III, Crosby G., Cohen-Williams M., et al. Life Sci. 2001;68:2585–2592. doi: 10.1016/s0024-3205(01)01056-6. [DOI] [PubMed] [Google Scholar]
- 15.Mita Y., Dobashi K., Suzuki K., Mori M., Nakazawa T. Eur. J. Pharmacol. 1996;297:121–127. doi: 10.1016/0014-2999(95)00722-9. [DOI] [PubMed] [Google Scholar]
- 16.Tayebati S. K., El-Assouad D., Ricci A., Amenta F. J. Neuroimmunol. 2002;132:147–155. doi: 10.1016/s0165-5728(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 17.Masini E., Fantozzi R., Blandina P., Brunelleschi S., Mannaioni P. F. Agents Actions. 1983;13:327–332. doi: 10.1007/BF01971484. [DOI] [PubMed] [Google Scholar]
- 18.Billard W., Binch H., III, Crosby G., McQuade R. D. J. Pharmacol. Exp. Ther. 1995;273:273–279. [PubMed] [Google Scholar]
- 19.Kitaichi K., Hori T., Srivastava L. K., Quirion R. Brain Res. Mol. Brain Res. 1999;67:98–106. doi: 10.1016/s0169-328x(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhank W., Basile A. S., Gomeza J., Volpicelli L. A., Levey A. I., Wess J. J. Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirion R., Wilson A., Rowe W., Aubert I., Richard J., Doods H., Parent A., White N., Meaney M. J. J. Neurosci. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stillman M. J., Shukitt-Hale B., Galli R. L., Levy A., Lieberman H. R. Brain Res. Bull. 1996;41:221–226. doi: 10.1016/s0361-9230(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 23.Loewy A. D. In: Central Regulation of Autonomic Functions. Loewy A. D., Spyer K. M., editors. London: Oxford Univ. Press; 1990. pp. 88–104. [Google Scholar]
- 24.Loewy A. D. Prog. Brain Res. 1991;87:253–268. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- 25.Benarroch E. E. Mayo Clin. Proc.; 1993. pp. 988–1001. [DOI] [PubMed] [Google Scholar]
- 26.Swanson L. W., Sawchenko P. E. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 27.Farkas E., Jansen A. S., Loewy A. D. Brain Res. 1997;764:257–261. doi: 10.1016/s0006-8993(97)00592-1. [DOI] [PubMed] [Google Scholar]
- 28.Hoover D. B., Hancock J. C., DePorter T. E. Brain Res. Bull. 1985;15:5–11. doi: 10.1016/0361-9230(85)90054-1. [DOI] [PubMed] [Google Scholar]
- 29.Miceli P. C., Jacobson K. Autonomic Neurosci. 2003;105:16–24. doi: 10.1016/S1566-0702(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 30.Langley R. J., Kalra R., Mishra N. C., Sopori M. L. J. Neuroimmunol. 2004;148:140–145. doi: 10.1016/j.jneuroim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Sato E., Koyama S., Okubo Y., Kubo K., Sekiguchi M. Am. J. Physiol. 1998;274:L970–L979. doi: 10.1152/ajplung.1998.274.6.L970. [DOI] [PubMed] [Google Scholar]
- 32.de la Torre E., Davel L., Jasnis M. A., Goton T., de Lustig E. S., Sales M. E. Breast Cancer Res. 2005;7:R345–R352. doi: 10.1186/bcr1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chensue S. W., Terebuh P. D., Remick D. G., Scales W. E., Kunkel S. L. Am. J. Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- 34.Grivennikov S. I., Tumanov A. V., Liepinsh D. J., Kruglov A. A., Marakusha B. I., Shakhov A. N., Murakami T., Drutskaya L. N., Förster I., Clausen B. E., et al. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]