Abstract
The activating transcription factor 1 (AP-1) family of proteins consists of a large number of inducible factors that are implicated in many biological processes, including cellular and viral gene expression, cell proliferation, differentiation, and tumorigenesis. Here, we investigated the role of the AP-1 family members c-Jun and c-Fos in transcriptional regulation of the JC virus (JCV) promoter in glial cells. DNA binding studies demonstrated the specific association of c-Jun with its DNA sequences corresponding to the AP-1 site within the JCV promoter. Functional analysis of the promoter showed that ectopic expression of c-Jun and c-Fos results in an additive activation of the JCV early and late promoters. Further functional assays indicated that the JCV AP-1 binding site is sufficient to confer responsiveness to both c-Jun/c-Fos- and UV-induced activation when transposed to a heterologous promoter. Analysis of c-Jun expression during the viral infection cycle by Western blotting revealed that c-Jun is posttranslationally modified by phosphorylation and its protein level is substantially increased at the late phases of infection cycle. Altogether, our findings indicate that AP-1 family members may play a role in the pathogenesis of JCV-induced disease in the human brain by modulating JCV gene transcription.
JC virus (JCV) causes a fatal demyelinating disease, progressive multifocal leukoencephalopathy, in immunocompromised patients (4). The promoter and enhancer elements present within the regulatory region of the JCV prototype Mad-1 strain consist of two 98-bp tandem repeats, each of which contains a partially characterized binding site for activating transcription factor 1 (AP-1) adjacent to the NF-1 binding element at nucleotide positions 56 to 63 and 154 to 161 (12, 24, 39). An additional AP-1-like site is also juxtaposed to an NF-1 site at positions 262 to 269 (39) (see Fig. 1A). Although earlier studies revealed the interaction of an AP-1 family member, c-Jun, with this binding site on the JCV promoter (1), its involvement in JCV gene expression remained unclear.
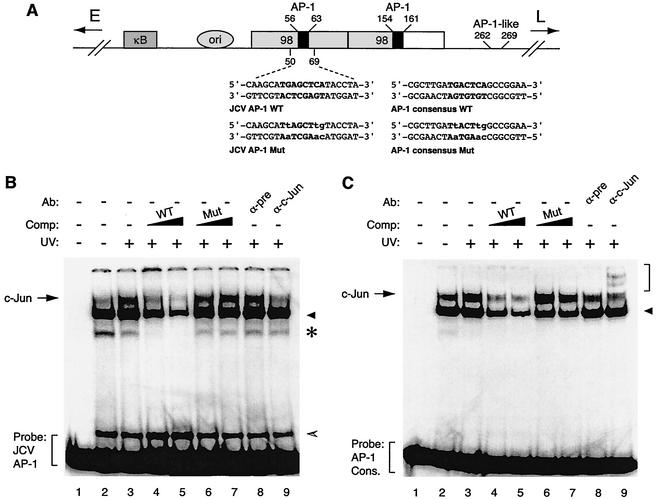
FIG. 1.
c-Jun specifically interacts with the potential c-Jun binding site of JCV in an electrophoretic mobility shift assay. (A) Structural organization of JCV Mad-1 regulatory region. The positions of the NF-κB motif (κB), the origin of viral DNA replication (ori), and the two 98-tandem repeats are depicted. The direction of the expression of early (E) and late (L) genes is indicated by the arrows. Positions of AP-1 binding sequences within JCV regulatory region are also indicated. Wild-type (WT) and mutant (Mut) oligonucleotides used in electrophoretic mobility shift assays as the JCV AP-1 binding site and AP-1 consensus binding site are shown. AP-1 binding sites are in bold, and base substitutions are in lowercase. (B) Competitive band shift and antibody supershift assays. Band shift assays were carried out as described previously (26, 29). A WT JCV AP-1 oligonucleotide spanning nucleotides 50 to 69 of the JCV regulatory region (40,000 cpm/lane) was end labeled and incubated with nuclear extracts (10 μg/lane) prepared from U-87MG cells either untreated (lane 2) or treated with UV light (250 nm, 40 J/m2) (lane 3). In addition, probe plus nuclear extract from UV-treated cells was also incubated with either unlabeled JCV AP-1 WT (lanes 4 and 5) or its mutant (lanes 6 and 7) variant competitor (25- and 150-fold molar excesses, respectively). Probe plus nuclear extract mixture was also incubated either with a preimmune (2 μg) (pre, lane 8) or an anti-c-Jun (KM-1, 2 μg; Santa Cruz) (lane 9) antibody. DNA-protein complexes were then resolved on a 6% polyacrylamide gel under native conditions and visualized by autoradiography as described previously (25, 26). (C) Interaction of c-Jun with AP-1 consensus (cons.) sequence in an electrophoretic mobility shift assay. Conditions were as described for panel B except that competitor WT and mutant oligonucleotides are shown as AP-1 consensus WT and AP-1 consensus Mut, respectively, in panel A. In panels B and C, the specific DNA-protein complexes are indicated by an arrow, and nonspecific complexes, where applicable, are indicated by either a solid arrowhead, a hatched arrowhead, or an asterisk. In panel C, a bracket indicates antibody supershifted complexes. Comp., competitor; Ab, antibody.
AP-1 was first defined as an inducible DNA binding protein specific to positive regulatory elements found within the regulatory regions of both simian virus 40 (SV40) and the metallothionein gene (2, 17, 18). Biochemical purification showed that AP-1 is not a single transcription factor but instead a series of related dimeric complexes of the Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra-1, and Fra-2) families (3, 42). Each family member is a phosphonuclear protein composed of three distinct functional domains, including a carboxy-terminal leucine zipper domain followed by an adjacent basic domain and an amino-terminal transactivation domain. AP-1 family members are induced by a wide variety of signals, including, but not limited to, UV and ionizing radiation, oxidative stress, neuronal depolarization, cytokines (tumor necrosis factor alpha, gamma interferon, and interleukin 1), and viral infection (6, 11, 13-15, 31, 32, 44). The AP-1 family members are collectively known as proto-oncogenes because of their high sequence homology to some retrovirus-encoded oncogenic proteins and their involvement in many cellular processes, including cell proliferation, survival, and apoptosis (5, 19, 31, 38, 42).
In light of earlier data on the activation of AP-1 upon various viral infections (10, 16, 20, 30, 43) and the fact that JCV promoter-enhancer elements contain AP-1 binding sites, we investigated the effect of AP-1 on JCV gene regulation and obtained experimental evidence that the AP-1 family members c-Jun and c-Fos positively regulate transcription from both JCV early and late promoters. On the other hand, we assessed the effect of JCV infection on c-Jun expression levels and showed that c-Jun protein levels are modulated and modified by phosphorylation during the viral infection cycle.
c-Jun interacts with potential AP-1 binding site from JCV promoter in vitro.
Upon induction by a variety of extracellular stimuli, the AP-1 family of transcription factors bind to their cognate DNA sequences and stimulate transcription of AP-1-responsive promoters (3, 23, 35). UV irradiation-mediated stress is known to be one of the potent inducers of the AP-1 family of transcription factors (3, 31). Since JCV promoter-enhancer elements contain several potential binding sites for AP-1 (39), we sought to investigate the possibility that AP-1 family members, upon induction, may physically interact with DNA target sequences present within the regulatory region of JCV. To test this possibility, we performed DNA band shift assays using both a double-stranded 32P-, end-labeled probe (JCV AP-1 WT [wild type]; sequences shown in Fig. 1A) and nuclear extracts prepared from U-87MG cells which had been treated with UV or left untreated prior to extract preparation. When the JCV AP-1 probe was incubated with nuclear extracts from untreated cells, we observed the formation of a major DNA-protein complex and also a considerably less intense band with slower migration (Fig. 1B, lane 2). In addition, the DNA-protein complex became more intense when the JCV AP-1 probe was incubated with nuclear extracts from UV-irradiated cells (Fig. 1B, compare band intensities in lanes 3 and 2), suggesting that appearance of this intense DNA-protein complex is likely due to induced AP-1 family members which interact with the probe. The specificity of this induced band is further investigated by competitive band shift experiments. Addition of the unlabeled JCV AP-1 oligonucleotide to the reaction mixture reduced the efficiency of the DNA-protein complex formation to basal levels (Fig. 1B, lanes 4 and 5). In contrast, unlabeled mutant JCV AP-1 oligonucleotide had no effect on the induced complex, indicating the specificity of the interaction between the JCV AP-1 sequence and the AP-1 family of transcription factors. The nature of this complex was further examined by antibody supershift assays. While addition of preimmune antisera (Fig. 1B, lane 8) to the reaction mixture failed to show any effect on the formation of the induced band, anti-c-Jun antibody completely prevented the formation of this band, further confirming the specificity of complex formation between the JCV AP-1 probe and AP-1. In parallel (Fig. 1C), we also performed competitive band shift and antibody supershift experiments using oligonucleotide probes containing the consensus sequences for the AP-1 binding site which served as a positive control for the experimental conditions used for Fig. 1B. Taken together, our results indicate that c-Jun, a member of the AP-1 family of transcription factors, specifically binds to JCV AP-1 DNA target sequences.
Transcriptional activation of JCV promoters by AP-1 family members.
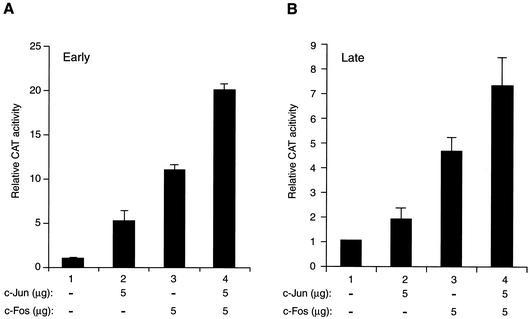
Our observations from band shift studies implied that AP-1 transcription factors may regulate transcription from JCV promoters by binding to their responsive DNA elements. To investigate this further, the human astrocytic glial cell line U-87MG, which efficiently supports JCV transcription, was transfected with a reporter construct containing the JCV early promoter, alone or together with expression plasmids expressing either c-Jun or c-Fos transgenes. Both c-Jun (Fig. 2A, bar 2) and c-Fos (bar 3) significantly increased the activity of the JCV early promoter (approximately 5- to 11-fold, respectively). Interestingly, the level of activation of the promoter by both transactivators was slightly higher (Fig. 2A, bar 4) than the sum of the activities obtained with c-Jun (bar 2) and c-Fos (bar 3) alone, suggesting that c-Jun and c-Fos have an additive effect on transcription from the JCV early promoter.
FIG. 2.
C-Jun and c-Fos activate transcription from both JCV promoters. (A and B) Reporter constructs (5 μg) containing the JCV regulatory region in either the early (pBLCAT3-Mad-1E) or late (pBLCAT3-Mad-1L) orientation were transfected into U-87MG cells by the calcium phosphate method either alone or in combination with expression plasmids for c-Jun (RSV-c-Jun) and c-Fos (RSV-c-Fos). Expression plasmid concentrations used in transfection assays are shown. At 48 h posttransfection, promoter activity for each transfectant was determined with 100 μg of cell lysate and presented as chloramphenicol acetyltransferase activity relative to the basal expression of the promoter. The pBLCAT3-Mad-1L and the pBLCAT3-Mad-1E reporter constructs have been described (8). Expression plasmids for RSV-c-Jun and RSV-c-Fos were kindly provided by B. E. Sawaya (Temple University).
A similar set of cotransfection experiments was carried out to assess the cooperative activity of c-Jun and c-Fos on the JCV late promoter (Fig. 2B). Consistent with their effect on the JCV early promoter activity, overexpression of c-Jun and c-Fos resulted in an additive activation of the viral late promoter in glial cells as well. Altogether, these results demonstrate that c-Jun and c-Fos enhance each other's effects on both JCV early and late promoters.
The JCV AP-1 binding site confers responsiveness to activation by both c-Jun/c-Fos and UV irradiation in a heterologous promoter context.
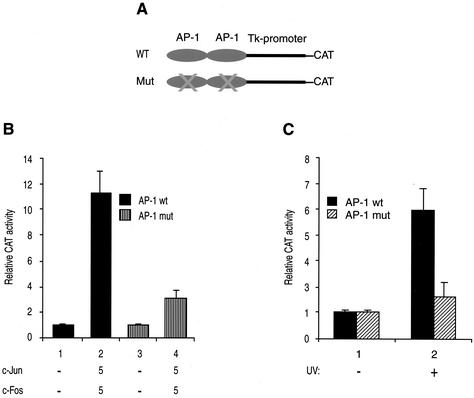
In the next series of experiments, we sought to determine whether the JCV AP-1 binding site confers responsiveness to its effectors when transposed to a heterologous promoter. To address this issue, U-87MG cells were transfected with either a WT reporter construct or its mutant version alone or together with expression plasmids expressing c-Jun or c-Fos transgenes. A schematic representation of a heterologous promoter containing either the WT JCV AP-1 binding site or its mutant version is shown in Fig. 3A. Compared to the observed activity for the mutant promoter (Fig. 3B, bar 4), the heterologous promoter containing WT JCV AP-1 binding sites as tandem repeats was significantly activated by c-Jun and c-Fos (7.3-fold) (bar 2), indicating that the JCV AP-1 binding site is sufficient to induce transcriptional activity by c-Jun/c-Fos when out of context.
FIG. 3.
AP-1 binding site of JCV confers responsiveness to c-Jun and c-Fos in a heterologous promoter. (A) Schematic representation of a heterologous promoter (pBLCAT2) (8) containing either WT JCV or mutant AP-1 binding sites. A tandem repeat of an oligonucleotide spanning nucleotides 50 to 69 of the JCV Mad-1 regulatory region and containing either WT JCV AP-1 binding site or its mutant variant was cloned upstream of the promoter at BamHI/HindIII sites. The sequences of cloned oligonucleotides for both WT JCV AP-1 oligonucleotide and its mutant variant are shown in Fig. 1A. (B) Induction of transcription by c-Jun and c-Fos. Both the WT and mutant reporter constructs (5 μg) shown in panel A were transfected, as described for Fig. 2A, into U-87MG cells alone or in combination with expression plasmids for c-Jun (RSV-c-Jun; 5 μg) or c-Fos (RSV-c-Fos; 5 μg), and transfectants were then processed as described for Fig. 2A. (C) JCV AP-1 binding sites are responsive to UV induction. Both the WT and mutant reporter constructs (5 μg) shown in panel A were also transfected into U-87MG cells as described for panel B, and transfectants were then either untreated or treated with UV and processed for chloramphenicol acetyltransferase activity as described for Fig. 2A.
In parallel, we also examined the transcriptional activity of the constructs illustrated in Fig. 3A when the cells were transfected and subsequently treated with short-wave UV light. Consistent with our observations from Fig. 3B, the WT construct responded strongly to UV treatment, in contrast to the mutant promoter (Fig. 3C, bars 2), indicating that the JCV AP-1 binding site is sufficient to mediate transcriptional activation not only by c-Jun/c-Fos but also by UV irradiation when transposed to a heterologous promoter.
c-Jun protein levels are modulated during the viral infection cycle.
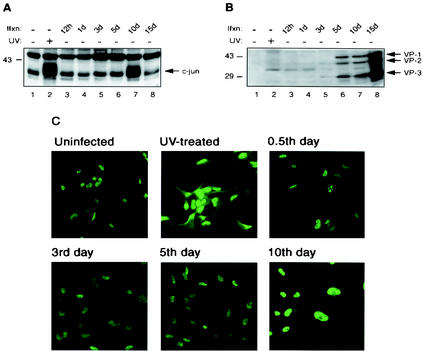
Our observations from transient-transfection assays strongly suggested the involvement of AP-1 family members in the regulation of transcription from JCV promoters. In addition, there are numerous reports demonstrating the stimulation of mitogen-activated protein kinase pathways by a number of different viral infections which lead to upregulation of c-Jun levels (10, 16, 20, 22, 30, 43). In light of previous reports and our observations in this communication, we have reason to speculate that perhaps the kinetics of c-Jun protein expression levels are modulated during the JCV infection cycle as well. To this end, SVG-A, a primary human fetal glial cell line that is transformed with replication-defective SV40 and serves as a convenient culture system for JCV infection (21), was infected with the hybrid JCV Mad-1/SVEΔ. JCV Mad-1/SVEΔ is a JCV-based virus in which a small portion of the SV40 regulatory region is inserted into the viral regulatory sequence in order to enhance the efficiency of viral infection (37). Protein extracts from the infected cells were analyzed by Western blotting for the detection of c-Jun using an anti-c-Jun (KM-1) monoclonal antibody which detects both phosphorylated and unphosphorylated forms of the protein. While the basal level of c-Jun protein expression remained relatively unaltered in the early and middle phases of the infection cycle (Fig. 4A, lanes 3 to 6), the total protein level for unphosphorylated and phosphorylated forms of c-Jun increased dramatically by day 10 postinfection (lane 7). In contrast, its level decreased to below normal as the infection cycle approached its termination point (Fig. 4A, compare lane 8 to lane 1). We also analyzed more data points for the increased level of c-Jun during the infection cycle and observed that c-Jun is detectable as early as day 9 postinfection by Western blot analysis (data not shown). Upregulation of c-Jun protein levels during late phases of viral infection cycle correlates with previously reported viral infection cases (10, 16, 20, 22, 30, 43), suggesting that there might be a common mechanism for such elevated levels of c-Jun protein in various viral infection cases. Figure 4A, lane 2, shows a positive control for upregulation of c-Jun by UV irradiation. In parallel, we analyzed the expression of JCV capsid proteins during viral infection by Western blotting (Fig. 4B) as a positive control for viral infection cycle shown in Fig. 4A.
FIG. 4.
Modulation of c-Jun expression levels during infection cycle. (A) Following infection of SVG-A cells by JCV Mad-1/SVEΔ (27, 37), nuclear extracts were prepared at the indicated time points and analyzed by Western blotting for the detection of c-Jun using an anti-c-Jun antibody (KM-1) (lanes 3 to 8). Viral infection studies were carried out as described previously (27). In lanes 1 and 2, nuclear extracts prepared from SVG-A cells that were either untreated (lane 1) or treated with UV (lane 2) were loaded as negative and positive controls, respectively. Ifxn, infection. (B) Whole-cell extracts were analyzed in parallel by Western blotting for detection of JCV capsid proteins (VPs) with a rabbit polyclonal antibody raised against SV40 capsid proteins (Lee Biomolecular Research, San Diego, Calif.) which is cross-reactive with JCV viral capsid proteins. This panel serves as a positive control for panel A in terms of progression of JCV infection. (C) Subcellular distribution of c-Jun during infection cycle was examined by indirect immunofluorescence microscopy. Infected cells were fixed with cold acetone at 0.5, 3, 5, and 10 days postinfection. Samples were incubated with anti-c-Jun (KM-1) primary antibody followed by incubation with a FITC-conjugated goat anti-mouse secondary antibody as described previously (27). Samples were then examined by a fluorescent microscope for detection of c-Jun. In parallel, SVG-A cells that were untreated or treated with UV were also processed as positive and negative controls, respectively.
In addition to analyzing c-Jun protein levels by Western blotting, we examined the modulation and subcellular distribution of c-Jun during viral infection by indirect immunofluorescence microscopy. SVG-A cells were infected with Mad-1/SVEΔ (37) and fixed with cold acetone when cells were at 0.5, 3, 5, and 10 days postinfection. Samples were incubated with anti-c-Jun (KM-1) primary antibody followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody and examination by fluorescent microscopy for the detection of c-Jun. In parallel, SVG-A cells treated with UV or left untreated were processed as positive and negative controls, respectively. As shown in Fig. 4C, although there was no apparent change in the subcellular distribution of c-Jun, which is exclusively nuclear, c-Jun protein levels considerably increased by day 10 postinfection. This observation correlates with both the level of c-Jun signal detected for UV-treated cells (positive control) and the c-Jun signal detected when cells are at day 10 postinfection by Western blot analysis (Fig. 4A, lane 7). To test the specificity of this immunostaining in parallel studies, uninfected and infected cells were reacted with a preimmune antiserum followed by incubation with FITC-conjugated secondary antibody. Preimmune antisera failed to detect c-Jun, indicating the specificity of immunostaining by anti-c-Jun antibody (data not shown).
c-Jun is phosphorylated during late phases of infection.
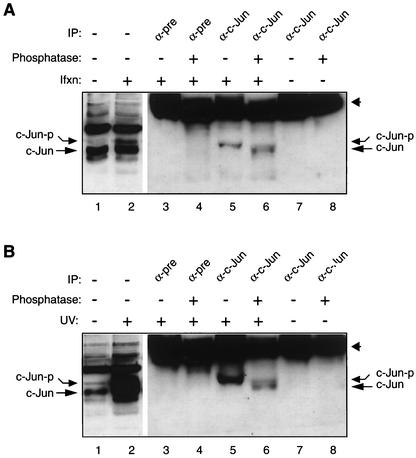
Many cellular regulatory proteins are known to undergo phosphorylation in response to a variety of stress stimuli, including viral infection. Phosphorylation has been shown to play a critical role in functional aspects of many regulatory proteins (33, 41). Consistent with this notion, Western blot analysis of nuclear extracts prepared from infected cells at late phases of infection (Fig. 4A, lane 7) showed a relatively slower-migrating band detected by anti-c-Jun antibody. This observation suggested that this slower migration pattern may be due to virus-induced posttranslational modification, perhaps phosphorylation of c-Jun (Fig. 4A, lane 7). To confirm this, c-Jun was immunoprecipitated with anti-c-Jun antibody from nuclear extracts prepared from SVG-A cells infected with Mad-1/SVEΔ when cells were at day 10 postinfection. Immunocomplexes were then either left untreated or treated with alkaline phosphatase and analyzed by Western blotting using the same anti-c-Jun antibody. Of note, this antibody immunoprecipitates only the phosphorylated form of c-Jun but detects both unphosphorylated and phosphorylated forms on Western blots. There is a clear downward shift in the migration pattern of the band corresponding to immunoprecipitated and subsequently alkaline phosphatase-treated c-Jun (Fig. 5A, lane 6) compared to that of the band corresponding to untreated c-Jun (lane 5). The downward-shifted band migrated to the same level as the unphosphorylated form of c-Jun, as observed in control lanes (Fig. 5A, compare the migration pattern of c-Jun in lane 6 with that in lanes 1 and 2). This faster migration of c-Jun (Fig. 5A, lane 6) is strongly suggestive of dephosphorylation of c-Jun by a phosphatase. Specificity of immunoprecipitation was confirmed by the lack of coprecipitation of c-Jun by normal antisera (Fig. 5A, lanes 3 and 4). In addition, the integrity of c-Jun antibody under treatment conditions was demonstrated (Fig. 5A, lanes 7 and 8). Lanes 1 and 2 show negative and positive controls for analysis of unphosphorylated and phosphorylated forms of c-Jun from nuclear extracts prepared from uninfected and infected cells, respectively. In parallel (Fig. 5B), we performed a similar alkaline phosphatase assay for c-Jun prepared from SVG-A cells treated with UV and used it as a positive control for panel A, since UV irradiation had already been shown to potently induce c-Jun phosphorylation (28, 41). Taken together, these findings clearly demonstrate that c-Jun is phosphorylated during the late phase of JCV infection.
FIG. 5.
Western blot analysis of alkaline phosphatase-treated c-Jun from infected cells. (A) c-Jun is phosphorylated during JCV infection. Nuclear extracts (100 μg) prepared from SVG-A cells infected with Mad-1/SVEΔ (day 10) were immunoprecipitated with either a preimmune (pre) (lanes 3 and 4) or an anti-c-Jun antibody (KM-1, which immunoprecipitates only the phosphorylated form of c-Jun in an immunoprecipitation assay) (lanes 5 and 6). Immunocomplexes were then either untreated (lanes 3 and 5) or treated with alkaline phosphatase (lanes 4 and 6) and analyzed by Western blotting using KM-1, which detects both phosphorylated and dephosphorylated forms of c-Jun on Western blots. Lanes 1 and 2 serve as negative and positive controls for analysis of nuclear extracts from uninfected and infected cells, respectively. In parallel, anti-c-Jun antibody alone was either untreated (lane 7) or treated with alkaline phosphatase (lane 8). Both lanes serve as controls for the integrity of anti-c-Jun antibody with or without phosphatase treatment. (B) Nuclear extracts from UV-treated SVG-A cells were treated with alkaline phosphatase as described for panel A. This panel serves as a positive control for panel A. Ifxn, infection; IP, immunoprecipitation; c-Jun-p, phosphorylated c-Jun. Arrowheads in both panels point to the position of the large fragment of antibody detected by the secondary antibody.
The AP-1 family of transcription factors is known to regulate transcription from many cellular and viral promoters and is implicated in many important cellular and viral processes, including cell proliferation, differentiation, and tumorigenesis. In addition, these factors were shown to be induced by a variety of extracellular stimuli, including UV light and viral infection (3, 42). Nucleotide sequence analysis of the JCV regulatory region revealed the presence of several DNA binding motifs for AP-1 which are scattered along the viral regulatory region and show little sequence divergence from that of the AP-1 consensus motif (1, 39). Initial characterization of these sites by DNase I footprinting assays showed that an AP-1 member, c-Jun, can protect these sites (1), but their contribution to JCV gene regulation remains unknown. In this report, we present evidence that the AP-1 transcription factors c-Jun and c-Fos positively regulate transcription from JCV promoters. In addition, we also demonstrate that c-Jun is posttranslationally modified by phosphorylation and its protein levels concomitantly increase during the late phase of viral infection.
Our observations from cotransfection studies demonstrated that AP-1 family members additively activate transcription from JCV early and late promoters. It is also interesting that the response of the JCV early promoter to c-Jun/c-Fos-mediated activation is considerably higher than that of the JCV late promoter (approximately threefold) (Fig. 2), indicating that the differential regulation of JCV promoters by these proteins may have important consequences in terms of JCV early gene expression during the initial stages of viral infection. In other words, AP-1 family members may contribute enormously to the expression of JCV early genes in the absence of viral regulatory proteins because respective family members were shown to be induced by a variety of extracellular stimuli, including viral infection (13, 40, 44). Such an assumption makes AP-1 a prime candidate for being one of the stimulatory factors involved in JCV early gene expression in the absence of viral regulatory proteins.
Many regulatory proteins are known to undergo posttranslational modifications in several ways, including phosphorylation (7). Phosphorylation has been shown to play a critical role in many aspects of cell function, including signal transduction, cell cycle progression, and gene transcription. Among many, a prominent example of such regulation by phosphorylation is observed in the function of stress-inducible proteins. For instance, the NF-κB family of proteins are retained in the cytoplasm in their inactive form by their inhibitor, IκB, which masks the nuclear localization signal of NF-κB. Upon exposure of cells to extracellular stimuli, such as viral infection and UV light, IκB is phosphorylated at its Ser-33 and -36 residues by an activated IκB kinase complex and undergoes degradation by ubiquitin-mediated proteolysis. Free NF-κB then translocates into the nucleus and transactivates many NF-κB-responsive genes (34). The AP-1 family member c-Jun was previously shown to undergo phosphorylation in response to a variety of stimulators, including, but not limited to, UV irradiation (32), tumor necrosis factor alpha (6), and viral infection (9, 36). We also demonstrated (Fig. 5A) that c-Jun undergoes phosphorylation in an infection cycle-dependent manner during the late phase of the JCV life cycle. Our findings in this respect correlate with previous observations from different viral infection cases (9, 20).
The biological significance of c-Jun phosphorylation by JCV infection is currently unknown. However, it is reasonable to suggest that c-Jun phosphorylation may represent a mechanism by which the virus manipulates cellular processes to promote its own replication. In addition, a subset of cellular genes activated by c-Jun may ensure efficient viral gene expression and DNA replication and thereby facilitate viral growth. Alternatively, activation of c-Jun could represent, in part, a spontaneous cellular defense mechanism against viral invasion, with the aim of abolishing virus replication by apoptosis. Experiments are under way to differentiate between these possibilities.
Acknowledgments
We thank E. O. Major and W. Atwood for providing cells and viruses and past and present members of the Center for Neurovirology and Cancer Biology for their insightful discussion and sharing of ideas and reagents. We thank Cynthia Schriver for editorial assistance.
This work was made possible by grants awarded by NIH to K.K. and M.S.
REFERENCES
- 1.Amemiya, K., R. Traub, L. Durham, and E. O. Major. 1992. Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC virus. A common characteristic of many brain-specific genes. J. Biol. Chem. 267:14204-14211. [PubMed] [Google Scholar]
- 2.Angel, P., M. Imagawa, R. Chiu, B. Stein, R. J. Imbra, H. J. Rahmsdorf, C. Jonat, P. Herrlich, and M. Karin. 1987. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49:729-739. [DOI] [PubMed] [Google Scholar]
- 3.Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 4.Berger, J. R., and M. Concha. 1995. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1:5-18. [DOI] [PubMed] [Google Scholar]
- 5.Bossy-Wetzel, E., L. Bakiri, and M. Yaniv. 1997. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 16:1695-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner, D. A., M. O'Hara, P. Angel, M. Chojkier, and M. Karin. 1989. Prolonged activation of Jun and collagenase genes by tumor necrosis factor-alpha. Nature 337:661-663. [DOI] [PubMed] [Google Scholar]
- 7.Brown, V. D., and B. L. Gallie. 2002. The B-domain lysine patch of pRB is required for binding to large T antigen and release of E2F by phosphorylation. Mol. Cell. Biol. 22:1390-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, N. N., and K. Khalili. 1995. Transcriptional regulation of human JC polyomavirus promoters by cellular proteins YB-1 and Pur alpha in glial cells. J. Virol. 69:5843-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke, P., S. M. Meintzer, C. Widmann, G. L. Johnson, and K. L. Tyler. 2001. Reovirus infection activates JNK and the JNK-dependent transcription factor c-Jun. J. Virol. 75:11275-11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, P., S. M. Gibson, S. Widmann, C. Garrington, T. P. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devary, Y., R. A. Gottlieb, L. F. Lau, and M. Karin. 1991. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol. Cell. Biol. 11:2804-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisque, R. J., and F. A. White.1992. The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. Humana Press Inc., Totowa, N.J.
- 13.Glenn, G. M., and W. Echart. 1990. Transcriptional regulation of early-response genes during polyomavirus infection. J. Virol. 64:2193-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldgaber, D., H. W. Harris, T. Hla, T. Maciag, R. J. Donnelly, J. S. Jacobsen, M. P. Vitek, and D. C. Gajdusek. 1989. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA 86:7606-7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrlich, P., H. Ponta, and H. J. Rahmsdorf. 1992. DNA damage-induced gene expression: signal transduction and relation to growth factor signaling. Rev. Physiol. Biochem. Pharmacol. 119:187-223. [DOI] [PubMed] [Google Scholar]
- 16.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 20:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, W., A. Haslinger, M. Karin, and R. Tjian. 1987. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature 325:368-372. [DOI] [PubMed] [Google Scholar]
- 18.Lee, W., P. Mitchell, and R. Tjian. 1987. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 49:741-752. [DOI] [PubMed] [Google Scholar]
- 19.Le-Niculescu, H., E. Bonfoco, Y. Kasuya, F. X. Claret, D. R. Green, and M. Karin. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell. Biol. 19:751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 21.Major, E. O., A. E. Miller, P. Mourrain, R. G. Traub, E. de Widt, and J. Sever. 1985. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc. Natl. Acad. Sci. USA 82:1257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean, T. L., and S. L. Bachenheimer. 1999. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 24.Raj, G. V., and K. Khalili. 1995. Transcriptional regulation: lessons from the human neurotropic polyomavirus, JCV. Virology 213:283-291. [DOI] [PubMed] [Google Scholar]
- 25.Safak, M., G. L. Gallia, S. A. Ansari, and K. Khalili. 1999. Physical and functional interaction between the Y-box binding protein YB-1 and human polyomavirus JC virus large T antigen. J. Virol. 73:10146-10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safak, M., G. L. Gallia, and K. Khalili. 1999. Reciprocal interaction between two cellular proteins, Purα and YB-1, modulates transcriptional activity of JCVCY in glial cells. Mol. Cell. Biol. 19:2712-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safak, M., B. Sadowska, R. Barrucco, and K. Khalili. 2002. Functional interaction between JC virus late regulatory agnoprotein and cellular Y-box binding transcription factor, YB-1. J. Virol. 76:3828-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sassone-Corsi, P. 1994. Goals for signal transduction pathways: linking up with transcriptional regulation. EMBO J. 13:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with "mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.See, R., and Y. Shi. 1998. Adenovirus E1B 19,000-molecular-weight protein activates c-Jun N-terminal kinase and c-Jun-mediated transcription. Mol. Cell. Biol. 18:4012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 32.Shaulian, E., M. Schreiber, F. Piu, M. Beeche, E. F. Wagner, and M. Karin. 2000. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 103:897-907. [DOI] [PubMed] [Google Scholar]
- 33.Steegenga, W., A. J. van der Eb, and A. G. Jochemsen. 1996. How phosphorylation regulates the activity of p53. J. Mol. Biol. 263:103-113. [DOI] [PubMed] [Google Scholar]
- 34.Thanos, D. 1995. NF-κB: a lesson in family values. Cell 80:529-532. [DOI] [PubMed] [Google Scholar]
- 35.Tokiwa, G., I. Dikic, S. Lev, and J. Schlessinger. 1996. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science 273:792-794. [DOI] [PubMed] [Google Scholar]
- 36.Tyler, K. L., P. Clarke, R. L. DeBiasi, D. Kominsky, and G. J. Poggioli. 2001. Reoviruses and the host cell. Trends Microbiol. 9:560-564. [DOI] [PubMed] [Google Scholar]
- 37.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170:353-361. [DOI] [PubMed] [Google Scholar]
- 38.van Straaten, F., R. Muller, T. Curran, C. Van Beveren, and I. M. Verma. 1983. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc. Natl. Acad. Sci. USA 80:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaz, B., P. Cinque, M. Pickhardt, and T. Weber. 2000. Analysis of the transcriptional control region in progressive multifocal leukoencephalopathy. J. Neurovirol. 6:398-409. [DOI] [PubMed] [Google Scholar]
- 40.Vogt, P. 2001. Jun, the oncogene. Oncogene 20:2365-2377. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm, D., H. van Dam, I. Herr, B. Baumann, P. Herrlich, and P. Angel. 1995. Both ATF-2 and c-Jun are phosphorylated by stress-activated protein kinases in response to UV irradiation. Immunobiology 193:143-148. [DOI] [PubMed] [Google Scholar]
- 42.Wisdom, R. 1992. AP-1: one switch for many signals. Exp. Cell Res. 253:180-185. [DOI] [PubMed] [Google Scholar]
- 43.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 44.Zullo, J., C. D. Stiles, and R. L. Garcea. 1987. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc. Natl. Acad. Sci. USA 84:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]