Abstract
Poliovirus (PV) can establish persistent infections in human neuroblastoma IMR-32 cells. We previously showed that during persistent infection, specific mutations were selected in the first extracellular domain of the PV receptor (CD155) of these cells (N. Pavio, T. Couderc, S. Girard, J. Y. Sgro, B. Blondel, and F. Colbère-Garapin, Virology 274:331-342, 2000). These mutations included the Ala 67 → Thr substitution, corresponding to a previously described allelic form of the PV receptor. The mutated CD155Thr67 and the nonmutated IMR-32 CD155 (CD155IMR) were expressed independently in murine LM cells lacking the CD155 gene. Following infection of the cells with PV, we analyzed the death of cells expressing these two forms of CD155. Levels of DNA fragmentation, caspase activity, and cytochrome c release were lower in LM-CD155Thr67 cells than in LM-CD155IMR cells. Thus, the level of apoptosis was lower in cells expressing mutated CD155 selected during persistent PV infection in IMR-32 than in cells expressing the wild-type receptor.
Poliovirus (PV), the etiological agent of paralytic poliomyelitis, belongs to the Picornaviridae family (for a review, see reference 14). It causes paralysis due to the destruction of motor neurons (15), a consequence of PV replication (20). The virion is composed of a single-stranded RNA molecule of positive polarity surrounded by an icosahedral capsid composed of four proteins, VP1 to VP4. A deep surface depression, called the canyon, surrounds each fivefold axis of symmetry. This depression contains the site responsible for binding to the cell receptor.
The human PV receptor, CD155, is a glycoprotein belonging to the immunoglobulin superfamily (40, 50). Furthermore, it is related to the nectin family of adhesion molecules, which are expressed at intercellular junctions (27, 45, 53, 63). CD155 is predicted to contain three extracellular immunoglobulin-like domains in the order V-C2-C2, followed by a transmembrane region and a cytoplasmic tail. The binding site for PV has been located in the V-like immunoglobulin domain (domain 1) (5, 11, 12, 36, 41, 52). In human epithelial HeLa cells, two different bases (G and A) have been found at nucleotide position 199 in the mRNA encoding CD155, resulting in the presence of an Ala or a Thr residue at amino acid position 67 within domain 1 of CD155; this difference in the CD155 molecules of HeLa cells may be due to an allelic difference (40, 50). Both these CD155 forms have been found in humans (A. Lundstöm, T. Pöyry, O. Vaarala, O. Ilonen, T. Hovi, M. Roivanen, and T. Hyypiä, Abstr. 6th International Symposium on Positive-Strand RNA Viruses, abstr. P1-44, 2001). The cellular role of CD155 is still unclear, but it has been reported to bind specifically to vitronectin, a multifunctional adhesive glycoprotein (43), and it is overexpressed in human colorectal carcinoma (49) and in malignant gliomas (33). Furthermore, the expression of CD155 and vitronectin production are associated with structures of the central nervous system involved in the differentiation of motor neurons during embryonic development (34, 48). Finally, it was recently shown that the cytoplasmic tail of CD155 interacts with Tctex-1, a light chain of the dyneins, which are microtubule-based molecular motors (54, 55).
Although PV was long considered to be an exclusively lytic virus, it has been shown that PV can establish persistent infections in cell cultures of neural origin (18, 57). Moreover, PV can persist in the mouse central nervous system after the onset of paralysis (24). These results are particularly interesting given that it has been suggested that the persistence of PV could be involved in postpolio syndrome (22). This syndrome occurs after several years of clinical stability and is characterized notably by new muscular atrophies that progress slowly (3, 25).
During persistent infection in human neuroblastoma IMR-32 cells, specific mutations were selected that affected domain 1 of CD155 (58). These mutations included the Ala 67 → Thr substitution, corresponding to a switch from one allelic form of the PV receptor to the other. The latter was not found to be expressed in IMR-32 cells. The two forms of the PV receptor, mutated CD155Thr67 and nonmutated CD155IMR, were expressed independently in murine LM cells lacking the CD155 gene. We found that stable transformant expressing the CD155 mutant selected during persistent PV infection displayed a delay in PV-induced cell lysis (58). A role for CD155 in PV-induced cell lysis was previously suggested by Morrison et al. (52), who observed that cells expressing some forms of CD155 generated by site-directed mutagenesis affecting residues in domain 1 did not display cytopathic effects during PV infection.
The picornaviruses, such as coxsackievirus B3 (17), Theiler's murine encephalomyelitis virus (65), hepatitis A virus (16), and PV (see references below), are able to kill cells by apoptosis in a variety of situations. Apoptosis is an active process of cell death that occurs in response to various stimuli, including viral infections (59). It involves a number of distinct morphological and biochemical features, such as cell shrinkage, plasma membrane blebbing, chromatin condensation, and internucleosomal DNA cleavage (59). These changes are mediated in particular by a family of proteases called caspases (cysteine proteases with aspartate specificity) (26). Following an apoptotic stimulus, caspases are activated by proteolysis and function by cleaving cellular proteins, including other members of the caspase family. It has also been shown that mitochondria are often involved in the control of caspase activation in the apoptotic cascade (23, 51). Apoptosis mediated by mitochondria is accompanied by prominent changes in this organelle, such as the efflux into the cytosol of several proteins, particularly cytochrome c, that are normally sequestered in the intermembrane space.
During paralytic poliomyelitis, it has been shown that PV multiplication and central nervous system injury are associated with apoptosis in a mouse model (31). In vitro, PV can induce apoptosis in HeLa cells (1, 2, 64), in the CaCo-2 enterocyte-like cell line (4), and in the U937 promonocyte cell line (47). It has also been shown that expression of viral proteases 2A (32) and 3C (8) may induce apoptosis, possibly via the cleavage of translation and transcription factors. However, the possibility cannot be excluded that other virus-encoded proteins and/or other elements involved in PV-cell interaction trigger apoptosis.
In this study, we further analyzed the PV-induced death of cells expressing the nonmutated form of the PV receptor, CD155IMR, or the mutated form, CD155Thr67, selected during persistent PV infection in IMR-32 cells.
The level of cell death is lower in PV-infected LM cells expressing CD155Thr67 than in LM cells expressing CD155IMR.
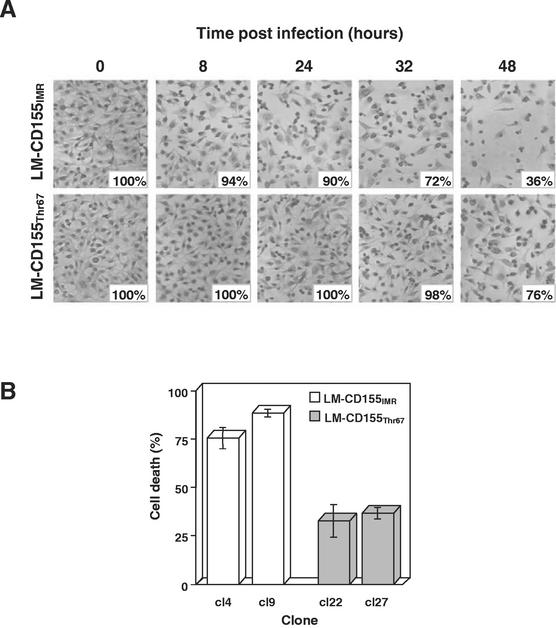
The previously described (58) LM cells expressing CD155IMR or CD155Thr67 were cloned. We then analyzed cell death following PV infection in two clones for each cell line (clones LM-CD155IMR/cl4 and LM-CD155IMR/cl9 and clones LM-CD155Thr67/cl22 and LM-CD155Thr67/cl27). Cell clones were infected with the PV-1/Sabin strain at a multiplicity of infection (MOI) of 10 50% tissue culture infectious doses (TCID50) per cell, and the kinetics of the onset of the cytopathic effect (the rounding and detachment of cells from the plate) were analyzed, after hematoxylin and eosin staining, over a period from 0 to 48 h postinfection (p.i.). Light microscopy showed that more LM-CD155Thr67 cells than LM-CD155IMR cells continued to adhere to the plate during the course of infection, as illustrated in Fig. 1A for one clone of each cell line. To quantify the total number of dead cells, we took into account both adherent and detached cells and we assessed cell death in infected cultures at 48 h p.i. by incubating cells with fluorescein diacetate (FDA; Molecular Probes) as previously described (9). FDA is cleaved by cytoplasmic esterases only in living cells, giving rise to a fluorescent de-esterified form. The percentage of cells that were living was determined by flow cytometric analysis with a Beckman Coulter XL3C cytometer (Villepinte, France). The levels of cell death were similar for both clones of each cell line, and the level was lower by a factor of about 2.5 in cells expressing the mutated form, CD155Thr67, than in those expressing the nonmutated form, CD155IMR (Fig. 1B).
FIG. 1.
Cell death in LM-CD155IMR and LM-CD155Thr67 cells infected with PV. (A) Kinetics of the cytopathic effect in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells. Cells were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell and stained with hematoxylin and eosin at the times indicated. The number of cells that continued to adhere to the plate in PV-infected cultures was determined by counting cells from an area corresponding to 1,000 cells in mock-infected cultures; the percentages of adherent cells in infected cultures with respect to those in mock-infected cultures are indicated. Magnification, ×150. (B) Cell death in LM-CD155IMR and LM-CD155Thr67 cells. Two clones for each cell line were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. At 48 h p.i., both adherent and detached cells were incubated with FDA, which is cleaved by cytoplasmic esterases only in living cells, giving rise to a fluorescent de-esterified form. The percentages of cells that were living were determined by flow cytometry. Cell death is expressed as the mean percentage of FDA-negative cells in the total population in three independent experiments. Error bars indicate the standard errors of the means.
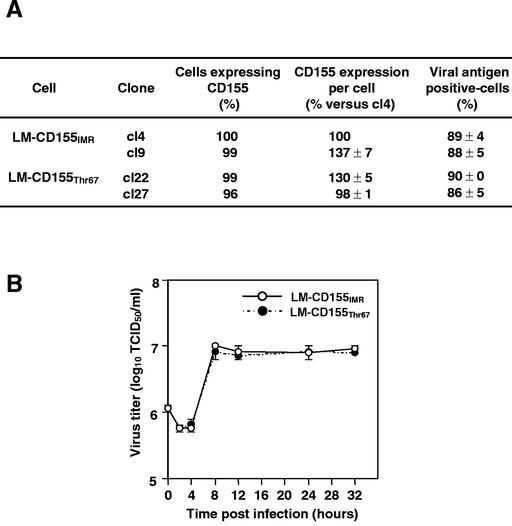
We checked that the lower percentage of dead cells among PV-infected CD155Thr67 LM-cells was not due simply to differences in PV receptor expression by determining the level of CD155 expression at the cell surface before PV infection by using flow cytometry, following labeling with monoclonal antibody 404.19 directed against CD155 (kindly supplied by M. Lopez, Marseille, France) (46). Some differences between cells were observed in the mean level of CD155 expression per cell (Fig. 2A). However, the differences did not seem to affect the percentage of infected cells quantified by flow cytometry following labeling with monoclonal antibody C3 directed against PV capsid protein VP1 (13) (Fig. 2A). Moreover, these differences did not affect the level of cell death, as the percentages of dead cells were similar for LM-CD155IMR/cl4 and LM-CD155IMR/cl9 and for LM-CD155Thr67/cl22 and LM-CD155Thr67/cl27 (Fig. 1B). Most subsequent experiments were performed with only one clone of each cell line (i.e., with LM-CD155IMR/cl4 and with LM-CD155Thr67/cl27) for which levels of CD155 expression were similar. The level of CD155 expression at the cell surface was regularly checked before performing experiments.
FIG. 2.
CD155 expression and viral growth in LM-CD155IMR and LM-CD155Thr67 cells. (A) CD155 expression and percentage of PV-infected LM-CD155IMR and CD155Thr67 cells. The level of CD155 expression at the cell surface was determined for two clones for each cell line before PV infection by using flow cytometry after immunofluorescence labeling with monoclonal antibody 404.19 directed against CD155 (46). Cells positive for viral antigen (both adherent and detached) at 8 h p.i. with PV-1/Sabin at a MOI of 10 TCID50 per cell were quantified by flow cytometry after immunofluorescence labeling with monoclonal antibody C3 directed against PV capsid protein VP1 (13). Results are presented as the means of two separate experiments. Standard errors of the means are indicated, except for the percentages of cells expressing CD155, for which they are lower than 0.5. (B) Single-cycle growth curves of PV in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells. Cell cultures were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. Cells and supernatants were harvested at the times indicated, and total virus yields were determined by TCID50 assay. Each point represents the mean of two separate experiments. Error bars indicate the standard errors of the means.
We then determined the kinetics of binding of PV to LM-CD155IMR/c4 and LM-CD155Thr67/cl27 cells and the time course of virus production during a single cycle of growth. Binding experiments were performed with [35S]methionine-labeled PV-1/Sabin at 4°C for from 0 to 90 min, as previously described (21), and no differences in PV-1/Sabin binding were observed between the two cell lines (data not shown), as previously reported for PV-1/Mahoney (19). The profiles of the single-cycle growth curves were also identical for the two cell lines (Fig. 2B), indicating that the decrease in the level of cell death in LM-CD155Thr67 cells resulted neither from a defect in virus adsorption to the PV receptor nor from the inhibition of virus replication. Altogether, these results indicate that cell death in both PV-infected LM-CD155IMR and LM-CD155Thr67 cells occurs at a later time period than that of viral yield and that the level of cell death is lower in LM-CD155Thr67 cells than in LM-CD155IMR cells.
Apoptosis levels are lower in PV-infected LM-CD155Thr67 cells than in LM-CD155IMR cells.
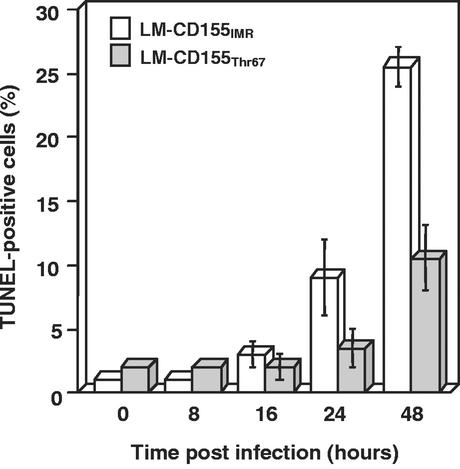
We investigated whether the lower level of cell death in LM-CD155Thr67 cells resulted from a decrease in the efficiency of the apoptotic process following PV infection by comparing DNA fragmentation in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cultures from 0 to 48 h p.i. by using terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) as previously described (31). In both cell lines, the percentage of TUNEL-positive cells (Fig. 3) paralleled the changes in cell death observed after cell layer staining (Fig. 1A); at 24 and 48 h p.i., the percentage of cell death in LM-CD155Thr67/cl27 cells was half of that in LM-CD155IMR/cl4 cells (Fig. 3). TUNEL-positive cells were also quantified for the other two clones (i.e., LM-CD155IMR/cl9 and LM-CD155Thr67/cl22) at the time at which the rate of cell death was maximal (48 h p.i.), and similar results were obtained (data not shown). Furthermore, these data were confirmed by means of an enzyme-linked immunosorbent assay (Cell Death Detection ELISA plus; Roche), carried out according to the manufacturer's instructions for both clones of each cell line at 48 h p.i. (data not shown).
FIG. 3.
Kinetics of DNA fragmentation in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells infected with PV. Cell cultures were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. At the times indicated, adherent cells were labeled for DNA fragmentation by TUNEL with biotin-16-dUTP and CY3-conjugated streptavidin as previously described (31). The percentage of TUNEL-positive cells was determined by counting 1,000 cells (by epifluorescence) under a Leica microscope. Results are presented as the means of two separate experiments. Error bars indicate the standard errors of the means.
We checked that the observed differences in DNA fragmentation between the LM-CD155IMR and LM-CD155Thr67 cell lines were not due to intrinsic differences in the apoptotic pathways used by these cell lines by treating LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells with two different proapoptotic reagents in the absence of virus: tumor necrosis factor (TNF), which induces apoptosis in response to a signal from the death domain of TNF receptor R1 (68), and a chemical agent, staurosporine, which triggers the mitochondrion-mediated apoptotic pathway (62). After staining with FDA as described above, no significant difference in the levels of cell death triggered by these reagents was observed between the two cell lines following 16 h of treatment (Table 1). We then checked that the lower level of cell death in LM-CD155Thr67 cells versus LM-CD155IMR cells was specific for PV infection. Both cell lines were infected with another picornavirus, the mengovirus, at a MOI of 10 TCID50 per cell for 18 h, and the percentages of cell death, analyzed after staining with FDA, were identical in both cell lines (Table 1). As PV infection results in the inhibition of macromolecular synthesis (35), we then verified that there was no difference in cellular protein shutoff between infected LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells. Cells were infected with PV-1/Sabin and labeled by incubation for 1 h with 30 μCi of [35S]methionine (1,175 Ci/mmol; ICN Pharmaceuticals, Inc.)/ml at 1 h 30 min, 2 h 30 min, 5 h, and 8 h p.i., as previously described (10). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of cell extracts showed that cellular protein shutoff levels were similar for the two cell lines (data not shown).
TABLE 1.
Cell death in LM-CD155 cell lines following TNF or staurosporine treatment or mengovirus infection
| Cell | Percentage of cell deatha after treatment or infection with:
|
||
|---|---|---|---|
| TNF (0.3 μM) | Staurosporine (1 μM) | Mengovirus (MOI = 10) | |
| LM-CD155IMR | 82 ± 4 | 71 ± 1 | 75 ± 2 |
| LM-CD155Thr67 | 75 ± 1 | 72 ± 1 | 74 ± 3 |
Cell death was determined after staining with FDA following incubation (16 h) with TNF or staurosporine or infection (18 h) with mengovirus at an MOI of 10 TCID50 per cell.
Thus, these data show that PV induces apoptosis in LM cells expressing CD155 and suggest that the lower level of cell death of LM-CD155Thr67 cells than of LM-CD155IMR cells results from a specific decrease in the level of the apoptotic process following PV infection.
Levels of caspase activation and cytochrome c release are lower in PV-infected LM-CD155Thr67 cells than in LM-CD155IMR cells.
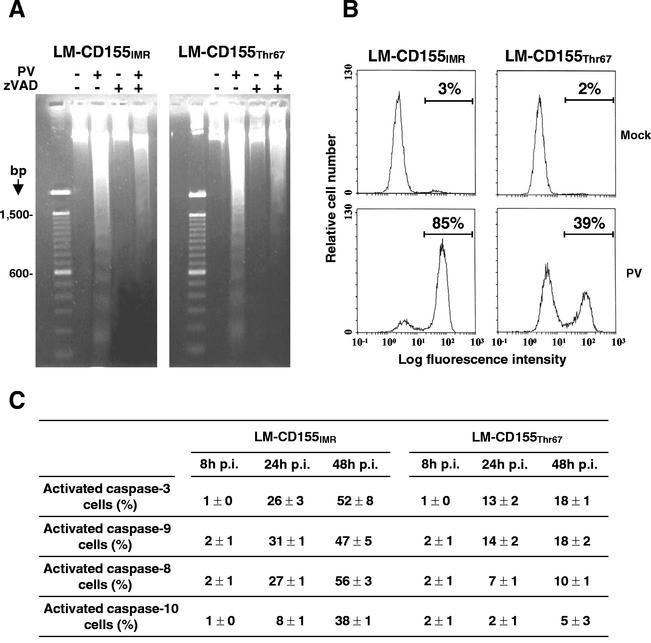
Apoptotic death involves a cascade of proteolytic events, most of which are executed by caspases (26). We investigated the involvement of caspases in PV-induced apoptosis in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells by studying the effect of the irreversible and cell-permeative pancaspase inhibitor zVAD-fmk [benzylocarbonyl-Val-Ala-Asp-(OMe) fluoro-methyl ketone; Bachem] on apoptosis in PV-infected cell cultures. The inhibitor was used at a concentration of 100 μM, which has been shown to inhibit caspases completely in cultured mammalian cells (61) without affecting PV growth (data not shown). Cells were infected by incubation with PV-1/Sabin for 48 h in the presence or absence of the inhibitor. We then analyzed DNA fragmentation by agarose gel electrophoresis. PV-infected LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells displayed substantial oligonucleosome DNA fragmentation in the absence of zVAD-fmk, and no DNA laddering was observed in mock-infected cells (Fig. 4A). DNA fragmentation was strongly inhibited in the presence of caspase inhibitor in both LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells infected with PV (Fig. 4A). Although this method is not quantitative, it demonstrates the apoptotic nature of PV-induced cell death in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells and the involvement of caspases.
FIG. 4.
Caspase activation in LM-CD155IMR and LM-CD155Thr67 cells infected with PV. (A) DNA laddering in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells infected with PV. Cell cultures were incubated with or without zVAD-fmk (100 μM) for 90 min and then not infected or infected by incubation with PV-1/Sabin at a MOI of 10 TCID50 per cell for 48 h in the presence or absence of zVAD-fmk (10 μM). DNA was then extracted from total cells (adherent and detached) and analyzed by electrophoresis in a 1.8% agarose gel after ethidium bromide staining. The first lane of each gel corresponds to a 100-bp DNA ladder marker (Gibco BRL). (B) Flow cytometric analysis of caspase activity in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells infected with PV. Cell cultures were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. At 48 h p.i., total cells were labeled using a CaspaTag fluorescein caspase (VAD) activity kit and analyzed by flow cytometry. Histograms of the relative cell number (y axes) versus the fluorescein intensity (x axes) show two peaks, corresponding to caspase-negative cells (first peak) and caspase-positive cells (second peak). The percentages of cells with activated caspases are indicated. One experiment out of two with similar results is shown. (C) Caspase-3, caspase-9, caspase-8, and caspase-10 activity in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells infected with PV. Cell cultures were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. At 24 and 48 h p.i., total cells were labeled with CaspaTag fluorescein kits for caspase-3 (DEVD) activity, caspase-9 (LEHD) activity, caspase-8 (LETD) activity, and caspase-10 (AEVD) activity and analyzed by flow cytometry. The results are expressed as the percentages of cells with activated caspases in the total population and are the means of two independent experiments. Standard errors of the means are indicated.
Following infection with PV-1/Sabin and incubation for 48 h, we quantified caspase activation in LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cells by determining global caspase activity by using flow cytometry with a CaspaTag fluorescein caspase (VAD) activity kit (Intergen) according to the manufacturer's instructions. This kit makes use of a carboxyfluorescein derivative of zVAD-fmk which irreversibly binds to activated caspases. At 48 h p.i., most of the LM-CD155IMR/cl4 cells displayed caspase activation, whereas less than half of the LM-CD155Thr67/cl27 cells had activated caspases (Fig. 4B).
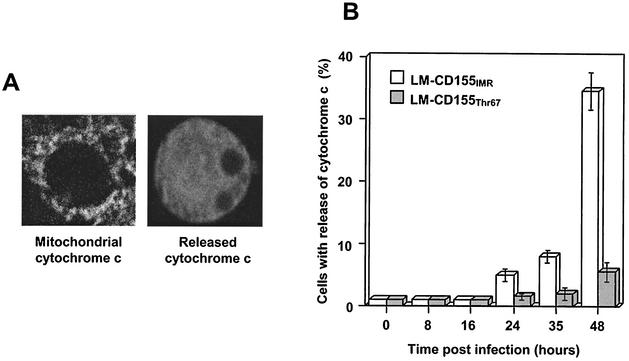
In many instances, mitochondria play a central role in caspase activation via the translocation of cytochrome c from mitochondria to the cytosol, where it forms a caspase-activating complex by interaction with Apaf-1 (apoptosis protease-activating factor 1) and pro-caspase-9 (44). This event triggers caspase-9 activation and initiates the apoptotic cascade by processing executive caspase-3. To investigate whether mitochondrial dysfunction was involved in the PV-induced apoptosis, we analyzed the location of cytochrome c in adherent cells of both cell lines following PV-1/Sabin infection. Cytochrome c was detected by immunofluorescence with a specific monoclonal antibody (Pharmingen). Cytochrome c labeling is granular in the cytoplasm of healthy cells, whereas it is diffuse in cells in which cytochrome c has been released into the cytosol (Fig. 5A). We determined the percentage of cells displaying cytochrome c release. LM-CD155IMR/cl4 cells displaying cytochrome c release exceeded 30% at 48 h p.i., whereas LM-CD155Thr67/cl27 cells showed only low levels of cytochrome c release (7%) at this time point (Fig. 5B). Thus, PV-induced apoptosis seems to involve mitochondrial dysfunction and this dysfunction was less severe in cells expressing mutated CD155.
FIG. 5.
Cytochrome c release in LM-CD155IMR and LM-CD155Thr67 cells infected with PV. LM-CD155IMR/cl4 and LM-CD155Thr67/cl27 cell cultures were infected with PV-1/Sabin at a MOI of 10 TCID50 per cell. At the times indicated, adherent cells were stained by immunofluorescence with a specific monoclonal antibody against cytochrome c and a secondary, fluorescein-conjugated antibody. (A) Distribution of cytochrome c as observed by confocal microscopy. In mock-infected cells (left panel), cytochrome c displays a dotted pattern, consistent with its location within the mitochondria. In PV-infected cells (right), the translocation of cytochrome c into the cytosol results in a diffuse staining pattern. Magnification, ×3,200. (B) Kinetics of cytochrome c release in PV-infected cells. The percentages of cells with released cytochrome c (as shown in panel A) were determined by counting 1,000 cells by epifluorescence. Results are presented as the means of two separate experiments. Error bars indicate the standard errors of the means.
As the mitochondrial pathway of caspase activation involves caspase-9, which in turn activates caspase-3 (44), we then studied specifically the activity of these two proteases in cells infected for 8, 24, and 48 h. Caspase-9 and caspase-3 activities were assessed by flow cytometry with a CaspaTag fluorescein caspase-9 (LEHD) activity kit and a CaspaTag fluorescein caspase-3 (DEVD) activity kit (Intergen), respectively, according to the manufacturer's instructions. Both caspase-9 and caspase-3 activation was observed only at the time points 24 and 48 h p.i., and their activity levels in LM-CD155Thr67/cl27 cells were only one-third to one-half those in LM-CD155IMR/cl4 cells (Fig. 4C). The caspase-9 activation suggests that the mitochondrial pathway is involved in the PV-induced apoptosis of LM cells.
However, the activation of the mitochondrial pathway does not exclude the involvement of the death receptor pathway (extrinsically-mediated pathway) (38) in PV-induced apoptosis. This latter pathway is activated by ligand binding to the membrane receptor, leading to the formation of the death-inducing signaling complex, which allows caspase-8 and/or caspase-10 autoactivation followed by caspase-3 activation (38, 39). Thus, we also checked for activation of caspase-8 and caspase-10 by flow cytometry with a CaspaTag fluorescein caspase-8 (LETD) activity kit and a CaspaTag fluorescein caspase-10 (AEVD) activity kit (Intergen), respectively, according to the manufacturer's instructions. The results showed that caspase-8 and caspase-10 were both activated (Fig. 4C). However, while caspase-8 activation paralleled that of caspase-3 and caspase-9, caspase-10 activation was delayed. Thus, it appeared that the two main apoptotic signaling pathways are simultaneously initiated in response to PV infection. However, this observation does not exclude a possible cross talk between the two apoptotic cascades. Indeed, it has been shown that caspase-8 can generate a truncated form of the Bid protein which then translocates to mitochondria and initiates the mitochondrial pathway (38). Moreover, some caspases activated by the mitochondrial pathway could also activate caspase-8 in a feedback loop (67).
The results reported here show that PV infection triggers apoptosis in LM cells expressing CD155, as shown by DNA fragmentation, caspase activity, and mitochondrial dysfunction. Interestingly, although viral growth was identical in the two cell lines, the level of PV-induced apoptosis was lower in cells expressing mutated CD155Thr67 selected during persistent PV infection of IMR-32 cells than in cells expressing nonmutated CD155IMR. It has been shown that expression of 2A or 3C PV protease is sufficient to induce cell apoptosis (8, 32). Thus, CD155 may be an additional cellular factor involved in the modulation of PV-induced apoptosis.
Several mechanisms could be proposed to account for the modulation of PV-induced apoptosis by CD155. One attractive mechanism is the modulation of an apoptotic signal triggered by the PV-CD155 interaction. The triggering of apoptosis by the binding of a virus to a receptor, or by a postbinding entry step, has been shown for other viruses such as reovirus (66), Sindbis virus (37), and human immunodeficiency virus (7). CD155 is related to the proteins of the nectin family, and although there is accumulating evidence that adhesion molecules participate not only in cell adhesion but also in a wide variety of processes which transduce signals in the cell (6), no data are presently available supporting the notion that a signal could be transduced via CD155. Nevertheless, a signal could be transduced via another molecule, interacting with CD155. One possible candidate for such a molecule is CD44, the major receptor for hyaluronic acid (28, 30, 60). Indeed, although CD44 is not required for PV attachment and replication, several data seem to indicate a physical association between CD155 and CD44 (28, 30, 60). It has also been shown that CD44 may be involved in the transduction of multiple signals and, notably, of apoptosis (29, 56). Another mechanism for the modulation of PV-induced apoptosis that cannot be excluded involves an interaction between CD155 and cellular factors involved in the apoptotic pathway. Such an interaction could occur via the CD155 intracytoplasmic domain or, more probably, via a membrane cofactor, because mutation Thr67 is located in extracellular domain 1 of CD155.
In both LM-CD155IMR and LM-CD155Thr67 cells, PV-induced apoptosis occurred late in the viral cycle, as previously observed in the CaCo-2 enterocyte-like cell line and in the U937 promonocyte cell line (4, 47). In HeLa cells, it has been shown that nonpermissive PV infection may promote an early apoptotic reaction whereas, upon productive PV infection, apoptosis appeared to be blocked by a distinct antiapoptotic function of PV (1, 2, 42, 64). Agol et al. (2) proposed a model in which, during productive PV infection in HeLa cells, the commitment of cells switches in the middle of the viral cycle from apoptosis towards an antiapoptotic state. In the context of this model, our results seem to indicate that in LM cells expressing CD155, PV-induced apoptosis would not be fully inhibited, making it possible for a late DNA fragmentation to occur.
We are presently investigating PV-induced signaling pathways in LM-CD155Thr67 and LM-CD155IMR cells, as well as the possible apoptosis inhibition pathway responsible for the late apoptosis observed in this model. The role of PV-CD155 interaction in PV-induced apoptosis is to be assessed, particularly with UV-inactivated PV. Furthermore, as the CD155Thr67 form was selected during persistent infection in IMR-32 neuroblastoma cells, it would also be interesting to determine which CD155 forms are expressed in patients developing poliomyelitis and postpolio syndrome.
Acknowledgments
A.-S.G. and Y.S. contributed equally to this work.
We thank Marc Lopez for generously providing monoclonal antibody 404.19 and Nicolas Escriou for mengovirus. We thank Nicole Pavio, with whom we initiated this work. We also thank Monique Dubois-Dalcq for her interest in our work. We are grateful to Francis Delpeyroux and to Yves Gaudin for critical reading of the manuscript.
This work was supported by grants from the Association Française contre les Myopathies to B.B. and F.C.-G. (contracts 6932, 7290, and 8143) and from the Association pour la Recherche Contre le Cancer to B.M. (contract 4080).
REFERENCES
- 1.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T. Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:343-353. [DOI] [PubMed] [Google Scholar]
- 2.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, L. I. Romanova, L. V. Sladkova, and E. A. Tolskaya. 2000. Competing death programs in poliovirus-infected cells: commitment switch in the middle of the infectious cycle. J. Virol. 74:5534-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agre, J. C., A. A. Rodriguez, and K. B. Sperling. 1989. Symptoms and clinical impressions of patients seen in a post-polio clinic. Arch. Phys. Med. Rehabil. 70:367-370. [PubMed] [Google Scholar]
- 4.Ammendolia, M. G., A. Tinari, A. Calcabrini, and F. Superti. 1999. Poliovirus infection induces apoptosis in CaCo-2 cells. J. Med. Virol. 59:122-129. [PubMed] [Google Scholar]
- 5.Aoki, J., S. Koike, I. Ise, Y. Satoyoshida, and A. Nomoto. 1994. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J. Biol. Chem. 269:8431-8438. [PubMed] [Google Scholar]
- 6.Aplin, A. E., A. K. Howe, and R. L. Juliano. 1999. Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 11:737-744. [DOI] [PubMed] [Google Scholar]
- 7.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barco, A., E. Feduchi, and L. Carrasco. 2000. Poliovirus protease 3C(pro) kills cells by apoptosis. Virology 266:352-360. [DOI] [PubMed] [Google Scholar]
- 9.Bartkowiak, D., S. Hogner, H. Baust, W. Nothdurft, and E. M. Rottinger. 1999. Comparative analysis of apoptosis in HL60 detected by annexin-V and fluorescein-diacetate. Cytometry 37:191-196. [DOI] [PubMed] [Google Scholar]
- 10.Bellocq, C., K. M. Kean, O. Fichot, M. Girard, and H. Agut. 1987. Multiple mutations involved in the phenotype of a temperature-sensitive small-plaque mutant of poliovirus. Virology 157:75-82. [DOI] [PubMed] [Google Scholar]
- 11.Belnap, D. M., B. M. McDermott, D. J. Filman, N. Q. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernhardt, G., J. Harber, A. Zibert, M. de Crombrugghe, and E. Wimmer. 1994. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology 203:344-356. [DOI] [PubMed] [Google Scholar]
- 13.Blondel, B., O. Akacem, R. Crainic, P. Couillin, and F. Horodniceanu. 1983. Detection by monoclonal antibodies of an antigenic determinant critical for poliovirus neutralization present on VP1 and on heat inactivated virions. Virology 126:707-710. [DOI] [PubMed] [Google Scholar]
- 14.Blondel, B., G. Duncan, T. Couderc, F. Delpeyroux, N. Pavio, and F. Colbère-Garapin. 1998. Molecular aspects of poliovirus biology with a special focus on the interactions with nerve cells. J. Neurovirol. 4:1-26. [DOI] [PubMed] [Google Scholar]
- 15.Bodian, D., and H. A. Howe. 1955. Emerging concept of poliomyelitis infection. Science 122:105-108. [DOI] [PubMed] [Google Scholar]
- 16.Brack, K., W. Frings, A. Dotzauer, and A. Vallbracht. 1998. A cytopathogenic, apoptosis-inducing variant of hepatitis A virus. J. Virol. 72:3370-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carthy, C. M., D. J. Granville, K. A. Watson, D. R. Anderson, J. E. Wilson, D. Yang, D. W. C. Hunt, and B. M. McManus. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colbère-Garapin, F., C. Christodoulou, R. Crainic, and I. Pelletier. 1989. Persistent poliovirus infection of human neuroblastoma cells. Proc. Natl. Acad. Sci. USA 86:7590-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colbere-Garapin, F., S. Jacques, A. S. Drillet, N. Pavio, T. Couderc, B. Blondel, and I. Pelletier. 2001. Poliovirus persistence in human cells in vitro. Dev. Biol. 105:99-104. [PubMed] [Google Scholar]
- 20.Couderc, T., C. Christodoulou, H. Kopecka, S. Marsden, L. F. Taffs, R. Crainic, and F. Horaud. 1989. Molecular pathogenesis of neural lesions induced by poliovirus type 1. J. Gen. Virol. 70:2907-2918. [DOI] [PubMed] [Google Scholar]
- 21.Couderc, T., J. Delpeyroux, H. Le Blay, and B. Blondel. 1996. Mouse adaptation determinants of poliovirus type 1 enhance viral uncoating. J. Virol. 70:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalakas, M. C. 1995. The post-polio syndrome as an evolved clinical entity. Definition and clinical description. Ann. N. Y. Acad. Sci. 753:68-80. [DOI] [PubMed] [Google Scholar]
- 23.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 24.Destombes, J., T. Couderc, D. Thiesson, S. Girard, S. G. Wilt, and B. Blondel. 1997. Persistent poliovirus infection in mouse motoneurons. J. Virol. 71:1621-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diard, C., J. F. Ravaud, and J. P. Held. 1994. French survey of postpolio sequelae. Risk factors study and medical social outcome. Am. J. Phys. Med. Rehabil. 73:264-267. [DOI] [PubMed] [Google Scholar]
- 26.Earnshaw, W., L. Martins, and S. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 27.Eberle, F., P. Dubreuil, M. G. Mattei, E. Devilard, and M. Lopez. 1995. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159:267-272. [DOI] [PubMed] [Google Scholar]
- 28.Fagan, E., G. Yousef, J. B. H. Garelick, G. Mann, A. Wolstenholme, B. Portmann, T. Harrison, J. F. Mowbray, A. Mowat, A. Zuckerman, and R. Williams. 1990. Persistence of hepatitis A virus in fulminant hepatitis and after liver transplantation. J. Med. Virol. 30:131-136. [DOI] [PubMed] [Google Scholar]
- 29.Foger, N., R. Marhaba, and M. Zoller. 2000. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur. J. Immunol. 30:2888-2899. [DOI] [PubMed] [Google Scholar]
- 30.Freistadt, M. S., and K. E. Eberle. 1997. Physical association between CD155 and CD44 in human monocytes. Mol. Immunol. 34:1247-1257. [DOI] [PubMed] [Google Scholar]
- 31.Girard, S., T. Couderc, J. Destombes, D. Thiesson, F. Delpeyroux, and B. Blondel. 1999. Poliovirus induces apoptosis in the mouse central nervous system. J. Virol. 73:6066-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstaub, D., A. Gradi, Z. Bercovitch, Z. Grosmann, Y. Nophar, S. Luria, N. Sonenberg, and C. Kahana. 2000. Poliovirus 2A protease induces apoptotic cell death. Mol. Cell. Biol. 20:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gromeier, M., S. Lachmann, M. R. Rosenfeld, P. H. Gutin, and E. Wimmer. 2000. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. USA 97:6803-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gromeier, M., D. Solecki, D. D. Patel, and E. Wimmer. 2000. Expression of the human poliovirus receptor/CD155 gene during development of the central nervous system: implications for the pathogenesis of poliomyelitis. Virology 273:248-257. [DOI] [PubMed] [Google Scholar]
- 35.Haller, A. A., and B. L. Semler. 1995. Translation and host cell shutoff, p. 113-133. In H. A. Rotbart (ed.), Human enterovirus infections. ASM Press, Washington, D.C.
- 36.He, Y. N., V. D. Bowman, S. Mueller, C. M. Bator, J. Bella, X. H. Peng, T. S. Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2000. Interaction of the poliovirus receptor with poliovirus. Proc. Natl. Acad. Sci. USA 97:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jan, J.-T., and D. E. Griffin. 1999. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J. Virol. 73:10296-10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann, S. H., and M. O. Hengartner. 2001. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 11:526-534. [DOI] [PubMed] [Google Scholar]
- 39.Kischkel, F. C., D. A. Lawrence, A. Tinel, H. LeBlanc, A. Virmani, P. Schow, A. Gazdar, J. Blenis, D. Arnott, and A. Ashkenazi. 2001. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276:46639-46646. [DOI] [PubMed] [Google Scholar]
- 40.Koike, S., H. Horie, I. Ise, A. Okitsu, M. Yoshida, N. Iizuka, K. Takeuchi, T. Takegami, and A. Nomoto. 1990. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 9:3217-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koike, S., I. Ise, and A. Nomoto. 1991. Functional domains of the poliovirus receptor. Proc. Natl. Acad. Sci. USA 88:4104-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama, A. H., H. Irie, F. Ueno, M. Ogawa, A. Nomoto, and A. Adachi. 2001. Suppression of apoptotic and necrotic cell death by poliovirus. J. Gen. Virol. 82:2965-2972. [DOI] [PubMed] [Google Scholar]
- 43.Lange, R., X. Peng, E. Wimmer, M. Lipp, and G. Bernhard. 2001. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology 285:218-227. [DOI] [PubMed] [Google Scholar]
- 44.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 45.Lopez, M., F. Eberle, M. G. Mattei, J. Gabert, F. Birg, F. Bardin, C. Maroc, and P. Dubreuil. 1995. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155:261-265. [DOI] [PubMed] [Google Scholar]
- 46.Lopez, M., F. Jordier, F. Bardin, L. Coulombel, C. Chabannon, and P. Dubreuil. 1997. Identification of a new class of Ig superfamily antigens expressed in hemopoiesis, p. 1081. In T. K. Kishimoto, A. E. G. von dem Borne, S. M. Goyert, D. Y. Mason, M. Miyasaka, M. Moretta, K. Okumura, S. Shaw, T. A. Springer, K. Sugamura, and H. Zola (ed.), Leucocyte typing, vol. VI. White cell differentiation antigens. Garland Publishing, New York, N.Y.
- 47.Lopez-Guerrero, J. A., M. Alonso, F. Martin-Belmonte, and L. Carrasco. 2000. Poliovirus induces apoptosis in the human U937 promonocytic cell line. Virology 272:250-256. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Moralez, J. R., J. A. Barbas, E. Marti, P. Bovolenta, D. Edgar, and A. Rodriguez-Tébar. 1997. Vitronectin is expressed in the ventral region of the neural tube and promotes the differentiation of motor neurons. Development 124:5139-5147. [DOI] [PubMed] [Google Scholar]
- 49.Masson, D., A. Jarry, B. Baury, P. Blanchardie, C. Laboisse, P. Lustenberger, and M. G. Denis. 2001. Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendelsohn, C. L., E. Wimmer, and V. R. Racaniello. 1989. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence and expression of a new member of the immunoglobulin superfamily. Cell 56:855-865. [DOI] [PubMed] [Google Scholar]
- 51.Mignotte, B., and J. L. Vayssiere. 1998. Mitochondria and apoptosis. Eur. J. Biochem. 252:1-15. [DOI] [PubMed] [Google Scholar]
- 52.Morrison, M. E., Y. J. He, M. W. Wien, J. M. Hogle, and V. R. Racaniello. 1994. Homolog-scanning mutagenesis reveals poliovirus receptor residues important for virus binding and replication. J. Virol. 68:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison, M. E., and V. R. Racaniello. 1992. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J. Virol. 66:2807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller, S., X. Cao, R. Welker, and E. Wimmer. 2002. Interaction of the poliovirus receptor CD155 with the dynein light chain Tctex-1 and its implication for poliovirus pathogenesis. J. Biol. Chem. 277:7897-7904. [DOI] [PubMed] [Google Scholar]
- 55.Ohka, S., and A. Nomoto. 2001. Recent insights into poliovirus pathogenesis. Trends Microbiol. 9:501-506. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto, I., Y. Kawano, D. Murakami, T. Sasayama, N. Araki, T. Miki, A. J. Wong, and H. Saya. 2001. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J. Cell Biol. 155:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavio, N., M.-H. Buc-Caron, and F. Colbère-Garapin. 1996. Persistent poliovirus infection of human fetal brain cells. J. Virol. 70:6395-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavio, N., T. Couderc, S. Girard, J. Y. Sgro, B. Blondel, and F. Colbère-Garapin. 2000. Expression of mutated receptors in human neuroblastoma cells persistently infected with poliovirus. Virology 274:331-342. [DOI] [PubMed] [Google Scholar]
- 59.Roulston, A., R. Marcellus, and P. E. Branton. 1999. Virus and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 60.Shepley, M. P., and V. R. Racaniello. 1994. A monoclonal antibody that blocks poliovirus attachment recognizes the lymphocyte homing receptor CD44. J. Virol. 68:1301-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slee, E. A., H. Zhu, S. C. Chow, M. MacFarlane, D. W. Nicholson, and G. M. Cohen. 1996. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem. J. 315:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tafani, M., D. A. Minchenko, A. Serroni, and J. L. Farber. 2001. Induction of the mitochondrial permeability transition mediates the killing of HeLa cells by staurosporine. Cancer Res. 61:2459-2466. [PubMed] [Google Scholar]
- 63.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tolskaya, E. A., L. Romanova, M. S. Kolesnikova, T. A. Ivannikova, E. A. Smirnova, N. T. Raikhlin, and V. I. Agol. 1995. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J. Virol. 69:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsunoda, I., C. I. B. Kurtz, and R. S. Fujinami. 1997. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology 228:388-393. [DOI] [PubMed] [Google Scholar]
- 66.Tyler, K. L., P. Clarke, R. L. DeBiasi, D. Kominsky, and G. J. Poggioli. 2001. Reoviruses and the host cell. Trends Microbiol. 9:560-564. [DOI] [PubMed] [Google Scholar]
- 67.Viswanath, V., Y. Wu, R. Boonplueang, S. Chen, F. F. Stevenson, F. Yantiri, L. Yang, M. F. Beal, and J. K. Andersen. 2001. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J. Neurosci. 21:9519-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]