Abstract
During the use of a phenotypic anti-human immunodeficiency virus type 1 (HIV-1) drug resistance assay in a large set of clinical virus isolates, we found a unique variant (CL-4) that exhibited a high level of nelfinavir (NFV) resistance and rather enhanced replication under subinhibitory concentrations of NFV (0.001 to 0.1 μM). Comparison of gag-pol sequences of the CL-4 variant and its predecessor virus isolates showed a stepwise accumulation of a total of 19 amino acid substitutions in protease (PR) and Gag p17 during 32-month NFV-containing antiretroviral therapy, while other Gag regions including the cleavage sites of the p55 precursor remained highly conserved. To understand the relationship between the genetic and phenotypic changes in CL-4, we constructed chimeric viruses using pNL4-3, replacing the PR, p24PR, or p17PR gene segment of CL-4 or its predecessor. A series of tissue culture infections with the chimeras in the absence or presence of increasing concentrations of NFV demonstrated that only the p17PR segment of CL-4 could confer the NFV-dependent replication enhancement phenotype on NL4-3. Our data suggest a novel adaptation mechanism of HIV-1 to NFV, in which coevolution of Gag and PR genes generates a variant that replicates more efficiently in the cellular environment in the presence of NFV than without the drug.
Human immunodeficiency virus type 1 (HIV-1) protease (PR) is essential for maturation of virus particles. During or after budding of virus particles from the plasma membrane, PR cleaves Gag and Gag-Pol precursor proteins into the multifunctional Gag proteins (matrix [MA; p17], capsid [CA; p24], and nucleocapsid [NC; p7]) and three enzymes (reverse transcriptase [RT], integrase, and PR), which are indispensable for de novo retroviral replication (13, 26). Protease inhibitors (PIs) are designed to bind into the active site of the PR homodimer, a functional entity of PR, and block its catalytic activity. Consequently, PI treatment of infected cells results in accumulation of morphologically immature, replication-incompetent viral particles, leading to prevention of de novo infection. Various PIs, such as indinavir, saquinavir (SQV), ritonavir, amprenavir, and nelfinavir (NFV), are now widely used clinically as the main drug in highly active antiretroviral therapy (HAART), in combination with other classes of anti-HIV-1 drugs.
When suppression of HIV-1 replication by PI-containing therapy is incomplete, variants with reduced sensitivity to the PI can emerge by accumulating nonsynonymous mutations in the PR gene (4, 14, 17, 20), causing a serious reduction in the clinical efficacy of HAART (1, 5, 10). PI resistance-associated mutations often affect the substrate specificity of PR (8) and can impair enzyme function, resulting in reduction of the replicative capacity of the variants (2, 7, 16-18, 28). In some in vivo infection cases, however, the impaired growth capacity recovers partially by accumulation of secondary mutations at the cleavage sites in the Gag-Pol precursor (6, 16, 21, 29) or at non-cleavage sites in Gag (11). Thus, under selective pressures of PIs, HIV-1 seems to evolve through stepwise accumulation of amino acid substitutions to increase the replicative advantages under the PI environment.
While the assumption is conceivable, no study has thus far addressed a variant(s) that replicates better in the presence of a PI than without the drug. Here, we report a remarkable HIV-1 NFV-resistant case, in which HIV-1 had evolved after prolonged administration of an NFV-containing regimen to display not only a high level of NFV resistance but also enhanced replication under subinhibitory concentrations of NFV. Molecular characterization of the variant suggested that coevolution of Gag and PR genes had provided the predecessor virus in the host the ability to replicate better in the presence of NFV than in the absence of the drug. Our data illustrate a novel mechanism, i.e., NFV-dependent replication enhancement, for HIV-1 adaptive evolution under the selective pressure of NFV.
MATERIALS AND METHODS
Clinical specimens and ethical considerations.
A Japanese homosexual man infected with HIV-1 had consulted the AIDS Clinical Center, International Medical Center of Japan, since 1997. Plasma samples and clinical isolates were obtained serially from the patient. The patient had been treated with various anti-HIV agents, including zidovudine, zalcitabine (ddC), and SQV, before treatment with lamivudine plus stavudine combined with NFV. The Institutional Ethics Committee approved this study (IMCJ-H13-80), and a written informed consent was obtained from the patient.
Virus isolates.
Clinical HIV-1 isolates CL-1, CL-2, CL-3, and CL-4 were obtained from the serial plasma samples obtained from our patient by using a CCR5-expressing HeLa/CD4+ cell clone 1-10 (MAGIC-5) (12). Briefly, MAGIC-5 cells grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) in a 48-well plate for 24 h were incubated with 1 ml of fresh plasma. The culture medium was changed every 2 or 3 days until a cytopathic effect was observed. Spread of HIV infection in the culture was confirmed by staining the cells with 5-bromo-4 chloro-3-indoyl-β-d-galactopyranoside (X-Gal) and measuring HIV-1 p24 antigen in the culture supernatant. The virus isolates were kept at −80°C until use.
Cells.
Peripheral blood mononuclear cells (PBMCs) obtained from HIV-1-seronegative healthy donors were stimulated with 1 μg of phytohemagglutinin (PHA)/ml for 72 h and grown in RPMI 1640 with 10% FCS and 10 U of interleukin-2 (Gibco-BRL, Grand Island, N.Y.) per ml for 24 h before infection. Transformed T-cell lines (MT-2 and PM-1 [15]) were maintained in RPMI 1640 with 10% FCS.
Drug susceptibility assay with MAGIC-5 cells.
NFV was kindly provided by the Japan Tobacco Co. (Tokyo, Japan). The drug susceptibility of the virus isolates to NFV was determined with MAGIC-5 cells (12). Briefly, MAGIC-5 cells (104) were infected with the diluted virus stock (300 blue cell-forming units [BFU]) in increasing concentrations of NFV (0, 0.001, 0.01, 0.1, and 1 μM) and incubated for 78 h. The culture supernatant was transferred to a new well containing MAGIC-5 cells without NFV and incubated for 48 h, fixed and stained with X-Gal, and counted under a microscope to assess the magnitude of de novo infection. The 50% inhibitory concentration (IC50) of NFV was calculated based on the dose-response curve. This experiment was performed in triplicate and repeated twice.
Sequence analyses of gag and pol genes.
Viral RNA was extracted from HIV-1 isolates with a High-Pure viral RNA kit (Boehringer, Mannheim, Germany), followed by RT-PCR with a One-Step RNA PCR kit (TaKaRa Shuzo, Otsu, Japan) to amplify the HIV-1 gag-pol DNA segment (2,341 bp). The first RT-PCR was conducted with a F641-R2982 primer pair (F641, 5′-GCCCGAACAGGGACTTGAAAGCG, pNL4-3 primer binding site at position 641 to 662; R2982, 5′-GATATCTAATCCCTGGTGTCT, pNL4-3 pol at position 2961 to 2982). The second PCR was performed with a F671-R2961 primer pair (F671, 5′-CCAGAGGAGATCTCTCGACGC, pNL4-3 noncoding positions 671 to 692; R2961, 5′-TCTTGTTTATACTAGGTATG, pNL4-3 pol position 2940 to 2961). The PCR products were purified with SUPREC-02 (TaKaRa Shuzo) and subjected to direct sequencing with an ABI Prism 377 automated DNA sequencer (Applied Biosystems, Foster City, Calif.). The primers used for the sequencing reaction were F671, F990 (5′-CCTTCAGACAGGATCAGAAG, pNL4-3 gag position 990 to 110), F1283 (5′-GCCCAGAAGTAATACCCATG, pNL4-3 gag position 1283 to 1302), F1741 (5′-ACAGAAACCTTGTTGGTCCA, pNL4-3 gag position 1741 to 1760), F2012 (5′-CTAGGAAAAAGGGCTGTTGG, pNL4-3 gag position 2012 to 2031), and DRPR3 (5′-AGCAGGAGACGATAGACAAGG, pNL4-3 gag position 2228 to 2248). Amino acid sequences were deduced with the Genetyx-Win program version 4.1 (Software Development, Tokyo, Japan).
Construction of gag-pro recombinant DNA clones.
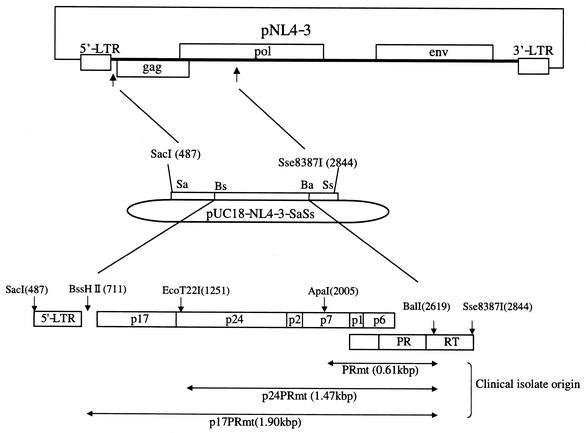
pUC18 containing the SacI-Sse8387I fragment (2,357 bp) of pNL4-3 (pUC18-NL4-3-SaSs) was constructed first to facilitate molecular cloning of the gag-pro segment. The DNA fragments amplified by PCR from the primary isolates were digested with BssHII and BalI (BssHII-BalI; 1,908 bp), and the fragment covering the entire gag and PR gene was cloned into pUC18-NL4-3-SaSs. A subclone designated p17PRmt-BsBa, with a sequence identical to that of each clinical isolate determined by the direct-sequencing method, was selected as a representative clone of the virus isolate. Subsequently, the EcoT22I-BalI fragment (1,372 bp) and the ApaI-Bal fragment (615 bp) of p17PRmt-BsBa covering the gag p24-PR and PR genes, respectively, were cloned into pUC18-NL4-3-SaSs. These two clones were designated p24PRmt-EcBa and PRmt-ApBa. Lastly, three pUC18-NL4-3-SaSs constructs carrying cloned p17PRmt-BsBa, p24PRmt-EcBa, and PRmt-ApBa were digested with BssHII and Sse8387I. Then, the digests (2,133 bp) were cloned back into pNL4-3 to generate full-length HIV-1 molecular clones of NL4-3PRmt, NL4-3p24PRmt, and NL4-3p17PRmt. The nucleotide sequences of the PCR-amplified fragments and around the recombinant sites of p17PRmt, p24PRmt, and PRmt were verified with an automatic sequencer.
Preparation of cell-free virus stocks of gag-pro recombinants by transfection.
HeLa cells (5 × 105 cells) were grown in DMEM with 10% FCS in a T25 flask for 24 h and transfected with 3 μg each of pNL4-3, pNL4-3PRmt, pNL4-3p24PRmt, and pNL4-3p17PRmt plasmid DNA using FuGENE 6 transfection reagent (Roche Diagnostics, Basel, Switzerland). The cells were incubated at 37°C for 24 h, washed once with DMEM, and cultured in 3 ml of DMEM containing 10% FCS. The culture supernatant containing the chimera virus was collected at 48, 72, and 96 h after transfection, respectively, filtered (0.45-μm pore size), analyzed for RT activity (27), and kept at −80°C until use.
Effects of NFV on HIV replication.
The method used to infect cells has been described previously (23-25). Briefly, PHA-stimulated PBMCs (2 × 105 cells), MT-2 cells (2 × 104 cells), and PM-1 cells (2 × 104 cells) were infected with 0.2 ml of cell-free supernatant containing HIV-1 (2 × 105 32P cpm of RT activity) in the absence or presence of NFV (0.1 and 1 μM) at 37°C for 16 h, washed once, and cultured in 0.2 ml of culture medium with the same concentration of NFV. In all infections, half of the culture medium volume was changed every 2 or 3 days, and the supernatant was kept at −80°C until use. Each experiment was carried out in duplicate and repeated three times.
Western blot analysis.
HeLa cells were transfected with 3 μg each of pNL4-3, pNL4-3p17PRmt, or pNL4-3PRmt plasmid DNA in the absence or presence of 0.1 μM NFV. The culture supernatant was harvested at 48 h after transfection and centrifuged at 37,800 × g for 90 min at 4°C to pellet virus particles. Transfected HeLa cells were washed once with phosphate-buffered saline and prepared for protein analysis as described previously (22). The virion pellet (6 × 105 cpm of RT activity) and cellular protein (25 μg of protein) resolved in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, Calif.) were fractionated with sodium dodecyl sulfate gradation gel (10 to 20%) electrophoresis (Bio-Rad Laboratories) and transferred to a nitrocellulose membrane (Millipore, Bedford, Mass.). The membrane was incubated with serum from an HIV-1-seropositive patient and hybridized with anti-protein A antibody conjugated with horseradish peroxidase (Amersham Pharmacia Biotech, Uppsala, Sweden). The immune complex was visualized with an ECL system (Amersham Pharmacia Biotech) according to the instructions provided by the manufacturer. The level of p24 in the loading sample was measured using Lumipulse Ortho HIV-1/2 (Fuji-Rebio, Tokyo, Japan).
Nucleotide sequence accession number.
The nucleotide sequence data reported here have been submitted to the DDBJ database under the accession numbers AB083565 through AB083568.
RESULTS
Identification of an HIV-1 variant CL-4 exhibiting NFV-dependent enhancement of replication.
We have recently established a rapid and simple assay for assessing the drug susceptibility of HIV-1 using the CCR5-expressing HeLa/CD4+ cell clone 1-10 (MAGIC-5) (12). In conducting the phenotypic anti-HIV-1 drug resistance assay using this system for a large set of clinical virus isolates, we noticed that some PI-resistant variants appeared to replicate better in the presence of the corresponding PI used in the treatment (data not shown). To better understand the underlying mechanism(s) of this observation, we selected a representative case treated with an NFV-containing regimen for the present study.
The clinical history and phenotypic drug resistance profile of the patient are summarized in Table 1. The nadir of the CD4-positive T-cell count was 68/μl, and the plasma HIV-1 RNA level was 2.1 × 105 copies/ml 8 months before commencement of treatment with the NFV-containing regimen. At the time of obtaining clinical isolate 1 (CL-1), the patient was being treated with ddC and SQV. Clinical isolates 2 (CL-2), 3 (CL-3), and 4 (CL-4) were obtained 11, 23, and 32 months after commencement of HAART, respectively. Although CD4 counts were increased to >100/μl, suppression of the viral load was incomplete during such treatment. Coinciding with a sustained high viral load, CL-3 exhibited high levels of resistance to SQV and NFV (increases in IC50s of SQV and NFV were more than 100-fold). After receiving 9 months of the same NFV-containing regimen, a variant CL-4 was isolated from the patient which was found to be extremely resistant to NFV, as evidenced by the significantly increased IC50 (from 107- to 600-fold). On the other hand, the IC50 of SQV remained similar during this period (from 156- to 128-fold).
TABLE 1.
Clinical data and time of isolation of clinical isolatesa
| Isolate | Regimen | Mos after NFV | CD4/μl | Viral load (copies/ml) | Fold resistance (IC50/μM)
|
|
|---|---|---|---|---|---|---|
| NFV | SQV | |||||
| —b | AZT | −8 | 68 | 2.1 × 105 | NT | NT |
| CL-1 | ddC, SQV | −2 | 84 | 8.5 × 104 | NT | NT |
| CL-2 | d4T, 3TC, NFV | 11 | 246 | 1.6 × 104 | NT | NT |
| CL-3 | d4T, 3TC, NFV | 23 | 192 | 1.9 × 104 | 107 (0.22) | 156 (0.59) |
| CL-4 | d4T, 3TC, NFV | 32 | 166 | 3.6 × 104 | 600 (>1) | 128 (0.48) |
The phenotypic drug resistance assay was performed using MAGIC-5 cells. Fold resistance was calculated by dividing the IC50 of the clinical isolate by the IC50 of NL4-3. AZT, zidovudine; ddC, zalcitabine; SQV, saquinavir; d4T, stavudine; 3TC, lamivudine; NFV, nelfinavir; NT, not tested.
—, no isolate employed.
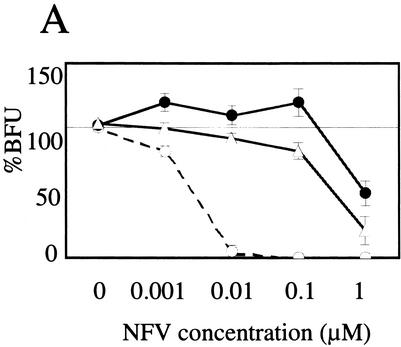
In the drug resistance assay system using MAGIC-5 cells (12), CL-3 displayed a typical dose-response curve to NFV, similar to that usually seen in most PI-resistant clinical isolates (Fig. 1A). In contrast, the dose-response curve of CL-4 to NFV was quite unique. Counts of blue cells derived from HIV-1 infection were consistently high in repeated experiments in the presence of 0.001 to 0.1 μM NFV, reaching up to 119% of that without NFV, suggesting that CL-4 replicates better in the presence of subinhibitory concentrations of NFV than in the absence of the drug in CCR5-expressing HeLa/CD4+ cells.
FIG. 1.
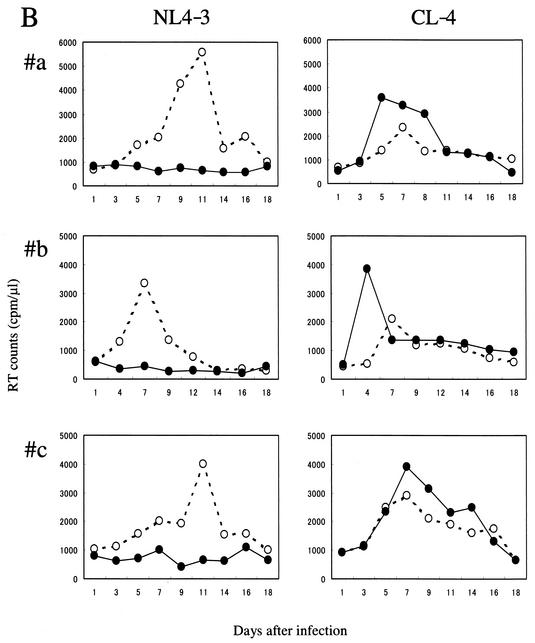
Identification of HIV-1 variant CL-4 that exhibits NFV-dependent enhancement of replication. (A) Effect of NFV on HIV-1 infectivity of MAGIC-5 cells (12). CCR5-expressing HeLa/CD4+ cells (MAGIC-5) were infected with 300 BFU of HIV-1 in the presence of the indicated concentrations of NFV for 78 h. The infectious titer was measured in culture supernatants of MAGIC-5 cells, and the percentages of BFU in NFV-treated cultures relative to those in NFV-free cultures were determined. Data are mean ± standard deviation values of six determinations. ○, NL4-3; ▵, CL-3; •, CL-4. (B) Effects of NFV on HIV-1 replication in PBMCs. PHA-stimulated PBMCs (2 × 105 cells) were infected with NL4-3 and CL-4 (2 × 105 32P cpm of RT activity) in the absence (○) or presence (•) of 0.1 μM NFV and cultured in the same concentration of NFV. Progeny virion production was monitored by RT activity (27) released into the culture medium at the indicated time points. Each experiment was carried out in duplicate using three different batches of donor PBMCs (panels a, b, and c).
To assess the effects of NFV on CL-4 replication in a more physiologically relevant system, PHA-stimulated PBMCs were infected with CL-4 and replication kinetics were monitored in the absence or presence of NFV (0.1 and 1.0 μM) by measuring RT activity released into the culture supernatant (Fig. 1B). In the absence of NFV, the kinetics of CL-4 replication were similar to those of the drug-sensitive control virus NL4-3, although the level of RT activity of CL-4 at the peak of infection (day 7) was about half that of NL4-3. In the presence of 0.1 μM NFV, replication of NL4-3 was completely suppressed, whereas variant CL-4 showed very efficient replication. In three repeated experiments, the replication kinetics of CL-4 was constantly fast, and the level of RT activity at the peak of infection was consistently higher in the presence of 0.1 μM NFV than without the drug (Fig. 1B, CL-4), suggesting that the subinhibitory concentration of NFV could potentiate replication of variant CL-4 in PBMCs, as well as in CCR5-expressing HeLa/CD4+ cells. Such enhancement of replication was not seen with other PIs, such as SQV and amprenavir, under the same experimental conditions. It was also not seen with variant CL-3, the predecessor virus isolate of CL-4 (data not shown), suggesting that the phenomenon is specific to CL-4 and NFV.
Genetic changes of gag-pro genes during HAART.
The above clinical data and phenotypic profiles of the clinical isolates suggested that PI-resistant HIV-1 variants emerged first, from which the CL-4 variant with the NFV-dependent replication enhancement phenotype had evolved. To assess the genetic changes in HIV-1 during antiretroviral therapy in our patient, we determined the nucleotide sequences of the gag-pro genes of CL-1, CL-2, CL-3, and CL-4 by direct sequencing of amplified DNA.
Comparison of the PR sequences showed a stepwise accumulation of amino acid substitutions during 32 months of treatment with an NFV-containing regimen (Table 2). With regard to PI resistance-associated mutations in the PR region, the CL-1 variant, which was isolated 2 months before the use of the NFV-containing regimen, already possessed a single mutation (Leu10→Ile), which might have developed during the preceding SQV-containing regimen. After 11 months of treatment with NFV, the patient harbored variant CL-2, which possessed four amino acid substitutions: L10I (Leu10→Ile), G48V, I54V, and V82A. CL-3 and CL-4 gained another substitution, M36I.
TABLE 2.
Amino acid substitutions in PRa
| Isolate | Resistance-associated mutations
|
Other mutations
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L10 | M36 | G48 | I54 | V82 | E35 | N37 | K43 | I62 | I72 | T74 | |
| CL-1 | I | — | — | — | — | — | — | — | — | — | — |
| CL-2 | I | — | V | V | A | — | — | — | — | V | S |
| CL-3 | I | I | V | V | A | — | — | — | V | V | S |
| CL-4 | I | I | V | V | A | D | S | T | V | V | S |
For each amino acid residue, the letter in the top lane indicates the amino acid associated with NL4-3. A dash indicates identity with NL4-3, and a single letter indicates the amino acid substitution. For example, CL-1 had an amino acid substitution at Leu10→Ile (L10I).
Interestingly, other substitutions (E35D, N37S, K43T, I62V, I72V, and T74S), whose contributions to PI resistance have not been described previously, also accumulated gradually in a manner dependent on the duration of NFV therapy. In particular, while CL-4 showed no further substitutions compared with CL-3 at the known drug resistance-associated mutation sites, three novel substitutions (E35D, N37S, and K43T) had accumulated in the N terminal of the PR.
Similarly, comparison of Gag sequences revealed several stepwise changes that occurred most remarkably in the Gag p17 peptide (Table 3). A total of nine amino acid substitutions (N47D, K55Q, M61R, G62R, F66S, V82I, S109N, Q117E, and N129D) accumulated gradually and sporadically through the p17 region of variant CL-4 during 32 months of NFV-containing antiretroviral therapy. In contrast, other regions of Gag were highly conserved during this period. This conservation was also noticed around the cleavage site of the Gag p55 precursor, and only a single substitution was found in CL-4 (Table 4).
TABLE 3.
Amino acid substitutions in Gag p17
| Isolate | Amino acid substitution in Gag p17a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CL-1 | N47 | K55 | M61 | G62 | F66 | V82 | S109 | Q117 | N129 | N130 |
| CL-2 | — | E | I | — | S | — | — | — | — | D |
| CL-3 | D | E | I | — | S | — | — | — | — | D |
| CL-4 | D | Q | I | R | S | I | N | E | D | — |
The letter and number in the top lane indicate the amino acid residue and its position in the Gag region associated with CL-1. A dash indicates identity with CL-1, and a single letter indicates the amino acid substitution. For example, CL-3 had an amino acid substitution at Asn47→Asp (N47D).
TABLE 4.
Amino acid substitutions at cleavage sites of Gag precursor
| Isolate | Amino acid substitutions at cleavage sites of Gag precursora
|
||||
|---|---|---|---|---|---|
| MA/CA | CA/p2 | p2/NC | NC/p1 | p1/p6 | |
| CL-1 | SQNF/PIVQ | ARVL/AEAM | GAIM/MQRG | RQAN/FLGK | PGNF/LQSR |
| CL-2 | ----/---- | ----/---- | ----/---- | ----/---- | ----/---- |
| CL-3 | ----/---- | ----/---- | ----/---- | ----/---- | ----/---- |
| CL-4 | ----/---- | --I-/---- | ----/---- | ----/---- | ----/---- |
Flanking amino acid residues at cleavage sites of Gag precursor are listed. Each amino acid is associated with CL-1. A dash indicates identity with CL-1. MA, matrix (p17); CA, capsid (p24); NC, nucleocapsid (p7).
Roles of genetic changes in conferring the biological phenotype of CL-4.
To assess the roles of the mutations described above in shaping the biological phenotype of CL-4, several recombinant molecular clones were constructed based on the pNL4-3 genetic background (Fig. 2). NL4-3PRmt, NL4-3p24PRmt, and NL4-3p17PRmt carried the cloned p1-p6-PR, p24-p2-p7-p1-p6-PR, and p17-p24-p2-p7-p1-p6-PR segments, respectively, from CL-4 or CL-3 virus isolates in the backbone of pNL4-3. They were used to assess the role of sporadic mutations in the corresponding regions of clinical isolates with respect to the genetic backbone of a drug-sensitive HIV-1.
FIG. 2.
Construction of pNL4-3-based gag-pro recombinants. The HIV-1 gag-pro DNA segment was amplified by RT-PCR from the CL-3 or CL-4 virus isolate and replaced with the BssHII-BalI fragment of pUC18-NL4-3-SaSs. Subsequently, the BssHII-Sse8387I fragment of pUC18-NL4-3-SaSs was cloned into pNL4-3 to reconstitute full-length HIV-1 molecular clones.
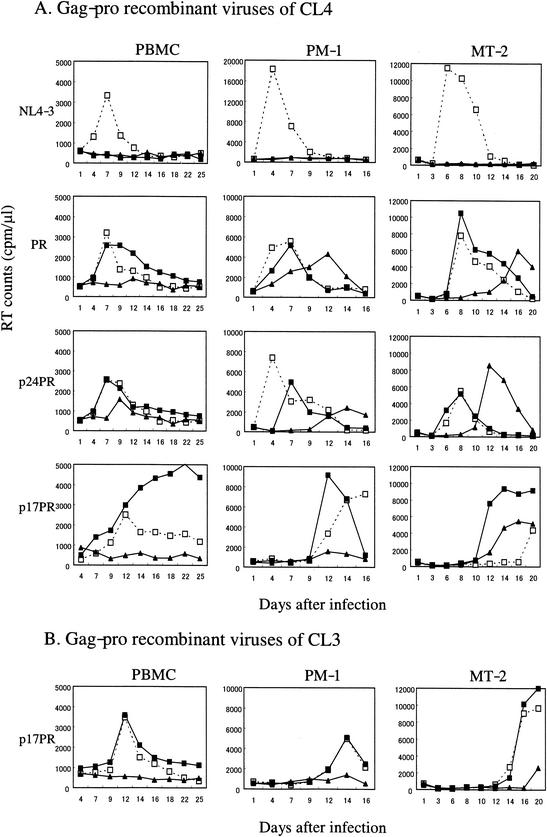
PHA-stimulated PBMCs, MT-2, and PM-1 cells were infected with an amount of virus corresponding to 2 × 105 cpm of RT activity (26), and virus replication was monitored in the absence or presence of 0.1 and 1 μM NFV (Fig. 3). In the absence of NFV, all recombinant viruses tested retained replication competence in all tissue culture infection systems tested. The replication kinetics in PBMCs were comparable among parental and each recombinant NL4-3, whereas those in PM-1 and MT-2 were significantly slower with the recombinant NL4-3 carrying the p17-PR segment of CL-3 or CL-4 than with others (Fig. 3). These data suggest the presence of intrinsic impairment of the Gag-PR segment of CL-3 and CL-4 that becomes apparent only on replication in transformed T-cell lines.
FIG. 3.
Effects of NFV on replication of gag-pro recombinant viruses in PBMCs, PM-1, and MT-2 cells. (A) Replication kinetics of NL4-3 and NL4-3PRmt, NL4-3p24PRmt, and NL4-3p17PRmt of CL-4 were examined with PBMCs, PM-1, and MT-2 in the absence (□) or presence of 0.1 μM (▪) and 1 μM (▴) NFV. (B) Replication kinetics of NL4-3p17PRmt of CL-3 were examined under the same conditions.
As expected, the parental NL4-3 did not grow at all in the presence of NFV, whereas all CL-4 recombinants retained replicative capacity under such an environment. The recombinant carrying the C-terminal portion of Gag and the entire PR (NL4-3PRmt of CL-4) grew efficiently in the presence of 0.1 μM NFV in all cells tested (Fig. 3A, PR), suggesting that mutations in these segments alone are sufficient to confer NFV resistance on NL4-3.
Of note was the recombinant carrying the entire gag and PR genes of CL-4 (NL4-3p17PRmt of CL-4). In the presence of 0.1 μM NFV, the recombinant replicated with significantly faster kinetics to higher titers than the same amount of virus did in the absence of the drug (Fig. 3A, p17PR). The NFV-dependent replication enhancement was observed in all cells tested in three repeated experiments. In contrast, the phenomenon was not seen in other recombinants of CL-4 (Fig. 3A, PR and p24PR), or in a recombinant carrying the entire gag-pro genes of CL-3 (Fig. 3B, p17PR). These data suggest that mutations in the Gag p17 segment of CL-4 are indispensable for generating the phenotype of the original CL-4 virus isolate.
Western blot analyses of the Gag processing pattern in the presence of NFV.
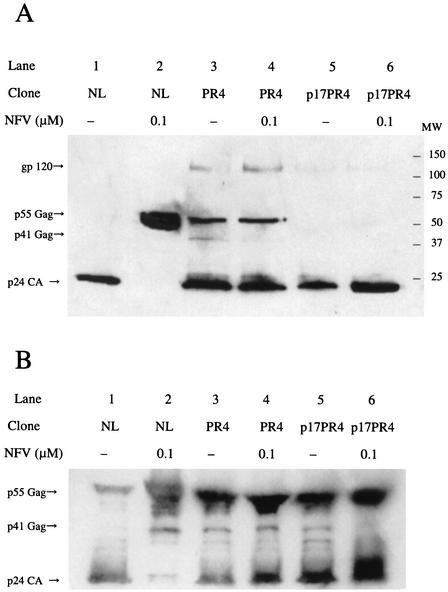
To obtain further insight into the role of NFV in modulating viral infectivity, the Gag processing pattern was assessed in the absence or presence of NFV by Western blot analysis (Fig. 4). After transfection of equal amounts of the parental and recombinant NL4-3 DNAs into HeLa cells, the cells were cultured with or without NFV for 48 h. Virions corresponding to 6 × 105 cpm of RT activity (Fig. 4A) and cell lysate containing 25 μg of protein (Fig. 4B) were loaded in each lane for electrophoresis.
FIG. 4.
Western blot analyses in the absence or presence of NFV. HeLa cells were transfected by full-length molecular clones and cultured in the absence or presence of NFV (0.1 μM). At 48 h posttransfection, virions in culture supernatants (A) and cells (B) were harvested and subjected to Western blot analysis. HIV Gag protein was visualized using serum from an HIV-1-seropositive patient. Lanes 1 and 2, pNL4-3; lanes 3 and 4, pNL4-3PRmt; lanes 5 and 6, pNL4-p17PRmt.
In the absence of NFV in virions (Fig. 4A), the Gag p55 precursor of the NL4-3 control was efficiently cleaved into the Gag p24 CA peptide (Fig. 4A, lane 1). In contrast, processing of the recombinant carrying the PR of CL4 was less efficient, as evidenced by the presence of p55 and p41 Gag uncleaved products (Fig. 4A, lane 3), suggesting that the CL-4 PR mutant altered the substrate specificity and that the NL4-3 Gag precursor was not an efficient substrate. Interestingly, the processing of the recombinant carrying the entire Gag and PR of CL4 was almost as efficient as that of the parental NL4-3 (Fig. 4A, lane 5), suggesting that mutations in CL-4 Gag compensate for the impairment of the PR mutant.
In the presence of NFV (0.1 μM), NL4-3 virions only had the p55 precursor (Fig. 4A, lane 2), confirming that the concentration of NFV used in the present replication experiment could completely block the PR function of the PI-sensitive clone. In contrast, processing of the recombinant carrying the PR of CL4 was not significantly affected by NFV (Fig. 4A, lane 4). The amount of p55 Gag precursor was completely cleaved in the recombinant virus carrying p17PR4 (Fig. 4A, lane 6).
The Western blot analysis of virions failed to reveal cleavage enhancement by NFV in p17PR4 carrying recombinant virus (Fig. 4A, lanes 5 and 6). Therefore, we further analyzed the cleavage pattern in the transfected HeLa cells. As expected, p41 Gag was efficiently cleaved in NL4-3 in the absence of NFV (Fig. 4B, lane 1). In contrast, such a cleavage was inhibited by NFV (Fig. 4B, lane 2). In PR4 carrying recombinant virus, p41 Gag was visible both in the absence and presence of NFV (Fig. 4B, lanes 3 and 4), suggesting that the cleavage efficiency was partially complicated but not affected by NFV. In the absence of NFV, the cleavage efficiency of the p17PR4-carrying recombinant virus was still impaired, as suggested by the presence of visible p41 Gag (Fig. 4B, lane 5). However, in the presence of NFV, p41 Gag was cleaved as efficiently as NL4-3 (wild type) (Fig. 4B, lane 6).
DISCUSSION
In the present study, (i) we identified a unique NFV-resistant variant of HIV-1 (CL-4) by applying our simple drug resistance assay system (12); (ii) we classified, based on sequence comparisons, the mutations potentially involved in conferring the CL-4 phenotype, which were determined by using the pNL4-3-based chimeric viruses harboring the gene segment responsible for conferring the CL-4 phenotype; and (iii) we assessed the roles of CL-4 Gag mutations in compensating Gag cleavage defects of the PR mutant. Based on the above analyses, we showed in the present study (i) the existence of an HIV-1 variant that replicated more efficiently under NFV than in the absence of this drug (Fig. 1); (ii) gradual accumulation of point mutations in the PR and Gag p17 during treatment with an NFV-containing regimen, but lack of any such change in most cleavage sites of the Gag precursor (Tables 2, 3, and 4); (iii) mutations in the CL4 Gag p6-PR segment alone are sufficient to confer NFV resistance, while those in the CL-4 Gag p17 are indispensable for generating the CL-4 phenotype (Fig. 3); and (iv) mutations in the CL-4 Gag can compensate for Gag cleavage defects caused by PR mutations (Fig. 4).
During acquisition of the CL-4 phenotype, three amino acid substitutions (E35D, N37S, and K43T), a single substitution (V to I), and seven substitutions (E55Q, G62R, V82I, S109N, Q117E, N129D, and D130N) accumulated in a stepwise fashion in the PR, the Gag CA/p2 cleavage site, and the Gag p17, respectively (Tables 2, 3, and 4). In contrast, other regions remained highly conserved. Our data suggest that some or all of these mutations, in concert with preexisting mutations, culminated in the formation of the CL-4 phenotype of HIV-1 during the 9-month NFV-based therapy. In particular, the substitutions in Gag p17 are essential, because only the p17PR segment of CL-4, but not of CL-3, or the p24PR segment or PR segment could confer the CL-4 phenotype of the drug-sensitive virus (Fig. 3). It is possible, however, that substitutions localized to the α-helix of the C-terminal domain of Gag p17 might interact with a p24 mutation and alter the exposure of the MA-CA cleavage site in the Gag precursor. Further studies involving site-directed mutagenesis are necessary to determine the precise set of mutations conferring the NFV-dependent replication enhancement phenotype.
The underlying molecular mechanism(s) of the NFV-dependent replication enhancement was not identified in the present study. It is possible that in the case of the mutant PR of CL-4, NFV acts as an allosteric effector and regulator of the enzyme function, instead of acting as a competitive inhibitor. Modulation of binding affinity to a substrate by binding of different low-molecular-weight ligands is commonly seen in multisubunit proteins for metabolic control and is an important mechanism for regulating enzyme activity. Although no study has reported such an allosteric feature for the HIV-1 PR homodimer, prolonged selective pressures of NFV during HAART, combined with a high level of tolerance of the HIV-1 PR to the sequence variation, might have generated the PR mutant possessing an allosteric binding site to NFV. In this context, mutations of p17 on the Gag-pol precursor of CL-4 could be critical in enhancing the cleavage of the Gag-pol polypeptide by the NFV-bound PR.
In this regard, Western blot analyses suggested that the cleavage efficiency of the Gag C-terminus p6 of this mutant can be enhanced in the presence of NFV, which is consistent with the above possibility. The possible allosteric effects of NFV on the CL-4 PR should be most effective for CL-4 Gag substrates, because only the chimera possessing the entire Gag of CL-4 exhibited a detectable NFV effect on virus replication (Fig. 3). Biochemical and structural studies of the CL-4 PR are necessary in order to assess each of these issues.
Several studies have suggested coevolution of gag and pro genes during treatment with PI-containing regimens (6, 16, 21, 29). It is conceivable that the HIV-1 PR and its substrates evolve coordinately to generate the correct processing products, thereby assuring production of infectious progeny virions. Most of the gag mutations for PI resistance reported so far are located around the cleavage sites of the Gag p55 precursor (6, 16, 21, 29), whereas the roles of mutations at the Gag non-cleavage sites have been poorly addressed. Because highly PI-resistant viruses often lack cleavage site mutations and their compensatory effects on the impaired PR function appear to be partial (16), and because mutations apart from the cleavage sites can affect the cleavability of the precursor in the three-dimensional structure, it is important to evaluate the roles of non-cleavage site mutations in PI resistance.
The present findings evoke an argument regarding continuation of incomplete HAART. When HAART results in incomplete suppression of virus replication due to emergence of variants resistant to the available drugs, some clinical trials currently recommend continuation of therapy, because any interruption could result in increased plasma viral load and low CD4 cell counts (3, 9, 19). In general, it is believed that continuation of HAART under such circumstances can still produce some clinical benefits because of the reduced replication capacity of the PI-resistant virus (9). The present findings, however, suggest that in some cases continuation of incomplete HAART may allow viral replication, resulting in the generation of variants of phenotypes similar to those described in the present study. Thus, phenotypic drug resistance assays appear to deserve more attention, particularly for patients who fail to respond to HAART but must continue treatment using the same regimen.
In conclusion, we have described in the present study a novel mechanism, NFV-dependent replication enhancement, for HIV-1 adaptive changes. Our results suggested that coevolution of Gag and PR genes was a key event for adaptation of HIV-1 to survive the strong pressure of NFV-containing therapy in this particular patient. The present findings have clinical implications that may have an impact on HAART.
Acknowledgments
We thank H. Mitsuya of the National Cancer Institute, National Institutes of Health, for helpful suggestions. We are also indebted to T. Kusagawa, Y. Hirabayashi, and S. Ida for continuous discussions throughout this study and to Y. Takahashi for technical support.
This study was supported by a grant-in-aid for AIDS research from the Ministry of Health, Labor, and Welfare of Japan (H12-AIDS-001), by the Organization of Pharmaceutical Safety and Research (01-4), and by the Japanese Foundation for AIDS Prevention.
REFERENCES
- 1.Aizawa, S., H. Gatanaga, S. Ida, A. Sakai, M. Tanaka, Y. Takahashi, Y. Hirabayashi, and S. Oka. 1999. Clinical benefits of resistance assay for HIV-specific protease inhibitors: when to check and in whom? AIDS 13:1278-1279. [DOI] [PubMed] [Google Scholar]
- 2.Bleiber, G., M. Munoz, A. Ciuffi, P. Meylan, and A. Telenti. 2001. Individual contributions of mutant protease and reverse transcriptase to viral infectivity, replication, and protein maturation of antiretroviral drug-resistant human immunodeficiency virus type 1. J. Virol. 75:3291-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condra, J. H., C. J. Petropoulos, R. Ziermann, W. A. Schleif, M. Shivaprakash, E. A. Emini, M. Cornelissen, G. Kampinga, F. Zorgdrager, and J. Goudsmit. 2000. Drug resistance and predicted virologic responses to human immunodeficiency virus type 1 protease inhibitor therapy. J. Infect. Dis. 182:758-765. [DOI] [PubMed] [Google Scholar]
- 4.Condra, J. H., D. J. Holder, W. A. Schleif, O. M. Blahy, R. M. Danovich, L. J. Gabryelski, D. J. Graham, D. Laird, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, T. Yang, J. A. Chodakewitz, P. J. Deutsch, R. Y. Leavitt, F. E. Massari, J. W. Mellors, K. E. Squires, R. T. Steigbigel, H. Teppler, and E. A. Emini. 1996. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J. Virol. 70:8270-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, D. Titus, T. Yang, H. Teppler, K. E. Squires, P. J. Deutsch, and E. A. Emini. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. [DOI] [PubMed] [Google Scholar]
- 6.Cotes, H. C., Z. L. Brumme, and P. R. Harrigan. 2001. Human immunodeficiency virus type 1 protease cleavage site mutations associated with protease inhibitor cross-resistance selected by indinavir, ritonavir, and/or saquinavir. J. Virol. 75:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croteau, G., L. Doyon, D. Thibeault, G. McKerche, L. Pilote, and D. Lamarre. 1997. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J. Virol. 71:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauber, D. S., R. Ziermann, N. Parkin, D. J. Maly, S. Mahrus, J. L. Harris, J. A. Ellman, C. Petropoulos, and C. S. Craik. 2002. Altered substrate specificity of drug-resistant human immunodeficiency virus type 1 protease. J. Virol. 76:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and G. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 10.Gatanaga, H., S. Aizawa, Y. Kikuchi, N. Tachikawa, I. Genka, S. Yoshizawa, Y. Yamamoto, A. Yasuoka, and S. Oka. 1999. Anti-HIV effect of saquinavir combined with ritonavir is limited by previous long-term therapy with protease inhibitors. AIDS Res. Hum. Retrovir. 15:1493-1498. [DOI] [PubMed] [Google Scholar]
- 11.Gatanaga, H., Y. Suzuki, H. Tsang, K. Yoshimura, M. F. Kavlick, K. Nagashima, R. J. Gorelick, S. Mardy, C. Tang, M. F. Summers, and H. Mitsuya. 2002. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J. Biol. Chem. 277:5952-5961. [DOI] [PubMed] [Google Scholar]
- 12.Hachiya, A., S. Aizawa-Matsuoka, M. Tanaka, Y. Takahashi, S. Ida, H. Gatanaga, Y. Hirabayashi, A. Kojima, M. Tatsumi, and S. Oka. 2001. Rapid and simple phenotypic assay for drug susceptibility of human immunodeficiency virus type 1 using CCR5-expressing HeLa/CD4+ cell clone 1-10 (MAGIC-5). Antimicrob. Agents Chemother. 45:495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz, R. A., and A. M. Skalka. 1994. The retroviral enzymes. Annu. Rev. Biochem. 63:133-173. [DOI] [PubMed] [Google Scholar]
- 14.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, Y. Xu, K. Real, B. M. Bernstein, A. J. Japour, E. Sun, and R. A. Rode. 2001. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J. Virol. 75:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammano, F., C. Petit, and F. Clavel. 1998. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J. Virol. 72:7632-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammano, F., V. Trouplin, V. Zennou, and F. Clavel. 2000. Retracing the evolutionary pathways of human immunodeficiency virus type 1 resistance to protease inhibitors: virus fitness in the absence and in the presence of drug. J. Virol. 74:8524-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Picado, J., A. V. Savara, L. Sutton, and R. T. D'Aquila. 1999. Replicative fitness of protease inhibitor-resistant mutants of human immunodeficiency virus type 1. J. Virol. 73:3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, V., C. Sabin, K. Hertogs, S. Bloor, J. Martinez-Picado, R. D'Aquila, B. Larder, T. Lutz, P. Gute, E. Weidmann, H. Rabenau, A. Phillips, and S. Staszewski. 2000. Virological and immunological effects of treatment interruptions in HIV-1 infected patients with treatment failure. AIDS 14:2857-2867. [DOI] [PubMed] [Google Scholar]
- 20.Molla, A., M. Korneyeva, Q. Gao, S. Vasavanonda, P. J. Schipper, H. M. Mo, M. Markowitz, T. Chernyavskiy, P. Niu, N. Lyons, A. Hsu, G. R. Granneman, D. D. Ho, C. A. Boucher, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1996. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat. Med. 2:760-766. [DOI] [PubMed] [Google Scholar]
- 21.Robinson, L. H., R. E. Myers, B. W. Snowden, M. Tisdale, and E. D. Blair. 2000. HIV type 1 protease cleavage site mutations and viral fitness: implications for drug susceptibility phenotyping assays. AIDS Res. Hum. Retrovir. 16:1149-1156. [DOI] [PubMed] [Google Scholar]
- 22.Sato, H., J. Orenstein, D. Dimitrov, and M. Martin. 1992. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology 186:712-724. [DOI] [PubMed] [Google Scholar]
- 23.Sato, H., T. Shiino, N. Kodaka, K. Taniguchi, Y. Tomita, K. Kato, T. Miyakuni, and Y. Takebe. 1999. Evolution and biological characterization of human immunodeficiency virus type 1 subtype E gp120 V3 sequences following horizontal and vertical virus transmission in a single family. J. Virol. 73:3551-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, H., Y. Tomita, K. Shibamura, T. Shiino, T. Miyakuni, and Y. Takebe. 2000. Convergent evolution of reverse transcriptase (RT) genes of human immunodeficiency virus type 1 subtypes E and B following nucleoside analogue RT inhibitor therapies. J. Virol. 74:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato, H., Y. Tomita, K. Ebisawa, A. Hachiya, K. Shibamura, T. Shiino, R. Yang, M. Tatsumi, K. Gushi, H. Umeyama, S. Oka, Y. Takebe, and Y. Nagai. 2001. Augmentation of HIV-1 subtype E (CRF01_AE) multiple-drug resistance by insertion of a foreign 11-amino-acid fragment into the reverse transcriptase. J. Virol. 75:5604-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegers, K., G. Rutter, H. Kottler, U. Tessmer, H. Hohenberg, and H. G. Krausslich. 1998. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J. Virol. 72:2846-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zennou, V., F. Mammano, S. Paulous, D. Mathez, and F. Clavel. 1998. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J. Virol. 72:3300-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Y. M., H. Imamichi, T. Imamichi, H. C. Lane, J. Falloon, M. B. Vasudevachari, and N. P. Salzman. 1997. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J. Virol. 71:6662-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]