Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8 [HHV-8]) is a gamma-2-herpesvirus responsible for Kaposi's sarcoma as well as primary effusion lymphoma (PEL). KSHV is a lymphotropic virus that has pirated many mammalian genes involved in inflammation, cell cycle control, and angiogenesis. Among these is the early lytic viral G protein-coupled receptor (vGPCR), a homologue of the human interleukin-8 (IL-8) receptor. When expressed, vGPCR is constitutively active and can signal via mitogen- and stress-activated kinases. In certain models it activates the transcriptional potential of NF-κB and activator protein 1 (AP-1) and induces vascular endothelial growth factor (VEGF) production. Despite its importance to the pathogenesis of all KSHV-mediated disease, little is known about vGPCR activity in hematopoietic cells. To study the signaling potential and downstream effects of vGPCR in such cells, we have developed PEL cell lines that express vGPCR under the control of an inducible promoter. The sequences required for tetracycline-mediated induction were cloned into a plasmid containing adeno-associated virus type 2 elements to enhance integration efficiency. This novel plasmid permitted studies of vGPCR activity in naturally infected KSHV-positive lymphocytes. We show that vGPCR activates ERK-2 and p38 in PEL cells. In addition, it increases the transcription of reporter genes under the control of AP-1, NF-κB, CREB, and NFAT, a Ca2+-dependent transcription factor important to KSHV lytic gene expression. vGPCR also increases the transcription of KSHV open reading frames 50 and 57, thereby displaying broad potential to affect viral transcription patterns. Finally, vGPCR signaling results in increased PEL cell elaboration of KSHV vIL-6 and VEGF, two growth factors involved in KSHV-mediated disease pathogenesis.
Kaposi's sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 is a gamma-2 herpesvirus that was discovered in 1994 when fragments of its genome were detected in an AIDS-related Kaposi's sarcoma (KS) lesion, a multifocal tumor of endothelial cell origin (13). Since then, KSHV has been found invariably in KS lesions of all epidemiologic types, and infection with KSHV precedes and predicts the development of KS in human immunodeficiency virus-infected patients (9, 76). KS remains the most common AIDS-related malignancy. Moreover, “endemic KS” is the most prevalent cancer in several sub-Saharan African countries, regardless of HIV coinfection. Consistent with the lymphotropic nature of gammaherpesviruses, KSHV has also been found in lymph nodes, peripheral blood B cells, and primary effusion lymphoma (PEL), a non-Hodgkin's lymphoma predominantly associated with AIDS but occurring in non-HIV-positive individuals as well (11). KSHV is also associated with a more aggressive behavior in another B-cell proliferative disorder called multicentric Castleman's disease (MCD) and an associated plasmablastic lymphoma (22, 70).
Although the pathogenesis of KSHV-mediated disease is not fully understood, it is clear that KSHV has pirated many human genes involved in inflammation, cell cycle regulation, and angiogenesis (12, 14, 60, 74, 78). Among these is a viral G-protein-coupled receptor (vGPCR) that is homologous to the human interleukin-8 (IL-8) receptors CXCR1 and CXCR2 as well as to the GPCR encoded by herpesvirus saimiri (ECRF3) (12). Although ECRF3, CXCR1, and CXCR2 are agonist dependent, vGPCR signals constitutively, as do other GPCRs associated with human disease (16, 71). Early work with COS-1 cells showed that vGPCR has activity in the phosphoinositide-inositol triphosphate-protein kinase C (PKC) pathway and leads to transcriptional activation of genes with PKC-responsive promoters containing an AP-1-binding motif. It was also shown that in these cells vGPCR activates the stress-activated kinases JNK and p38 in a Gαq-coupled manner (5, 8, 10). It is now clear that in endothelial cells, vGPCR can also couple to Gαi G proteins to activate a phosphatidylinositol 3′-kinase (PI3K)-Akt axis as well as the NF-κB transcription factors. Such signaling potential may explain the vGPCR-mediated survival advantage in primary endothelial cells and the increased elaboration of various inflammatory cytokines and adhesion molecules (17, 45, 51). Furthermore, via hypoxia-inducible factor 1a, vGPCR mediates the secretion of vascular endothelial growth factor (VEGF), thereby inducing the proliferation of human umbilical vein endothelial cells and blood vessel formation, both of which are crucial events in the pathogenesis of KS (6, 12, 69). Other functional studies show that vGPCR can transform fibroblasts and that when expressed under the control of a CD4 (primarily T-cell) promoter in transgenic mice, vGPCR causes vascular lesions with a KS-like histology. This mouse model emphasizes the potent paracrine effects that vGPCR can have on surrounding nonhematopoietic cells despite its expression from very few infiltrating lymphocytes (80). Unfortunately, little is known about the signaling properties of vGPCR in the hematopoietic cells relevant to KSHV-mediated disease. Given the importance of cellular context to most signaling proteins and pathways, understanding of the contribution of vGPCR to KSHV-mediated pathobiology requires study of such cells.
Here we present the derivation of KSHV-positive PEL cell lines engineered to express vGPCR in a controllable manner. A single plasmid was designed to incorporate the sequences necessary for tight tetracycline inducibility along with the inverted terminal repeat (ITR) sequences from adeno-associated virus type 2 (AAV-2) to maximize the probability of stable integration (29, 30). During latent AAV-2 infection, the AAV Rep protein binds DNA in or near the ITRs and mediates site-specific integration of the AAV genome into chromosome 19 of the human genome. However, even in the absence of Rep, the hairpin structure of the ITRs is thought to enhance random stable integration (25, 42, 55). Taking advantage of this property, we established inducible cell lines with one round of transfection followed by clonal selection for tight vGPCR inducibility. A PEL cell line was chosen as the parental line to express vGPCR in the context of natural KSHV infection and the presence of the entire viral genome. This allowed us to create a model for assessing vGPCR-mediated effects on other KSHV genes. Since vGPCR is an early lytic gene, latently infected PEL cell lines express very low levels. Therefore, using this inducible system, we can selectively induce vGPCR expression without treating the cells with agents that induce the lytic viral cycle. We have studied the effect of vGPCR on mitogen-activated protein kinase (MAPK) and stress-associated protein kinase (SAPK), transcription factor activation, and the regulation of various KSHV genes, including open reading frame (ORF) 50 and ORF 57, whose products are intimately involved in the regulation of KSHV transcription patterns. In addition, we show that vGPCR expression in PEL cells increases the production of KSHV viral IL-6 (vIL-6) and vascular endothelial growth factor (VEGF), two cytokines vital to the pathobiology of all KSHV-mediated disease (3, 7, 44, 49).
MATERIALS AND METHODS
Plasmids and transfections.
pTRUF2-Tet was constructed by initially cloning all the tetracycline regulatory elements into the cloning vector pSL301 (Invitrogen). The entire cassette was then transferred from pSL-301 to pTRUF2 (82). In brief, pTet-tTS was digested with XhoI and HindIII and the resulting 2,098-bp tTS insert was blunted and ligated into the EcoRV site of pSL-301 to make pSL301-tTS. Next, pTet-On was digested with XhoI and PvuII and the resulting 2,398-bp rtTA fragment was blunted and ligated into the SmaI site of pSL301-tTS to make pSL301-tTS-rtTA. Next, a simian virus 40 (SV40) pA tail was excised from pTRE by digestion with BamHI and PvuII; the fragment was blunted and ligated into the StuI site of pSL301-tTS-rtTA to create pSL301-tTS-rtTA-pA. Next, the tetracycline-responsive promoter was excised from pTRE by digestion with XhoI and EcoRI, blunted, and ligated into the NruI site of pSL301-tTS-rtTA-pA to create pSL301-tTS-rtTA-pA-TRE, which was then digested with PflMI and AflII to remove the entire tetracycline-responsive cassette; the 5,886-bp fragment was blunted and ligated into the MfeI site of pTRUF2 to create pTRUF2-Tet (12,168 bp). pTRUF2-Tet-vGPCR was made by digesting pcDNA3.1-SacA, a plasmid derived from a genomic clone, with XbaI and EcoRI to excise the vGPCR ORF (12). The vGPCR fragment was blunted and ligated into the blunted single NsiI site in pTRUF2-Tet. pTet-TS, pTet-On, and pTRE were from Clontech. Restriction enzymes were from Roche; T4 polymerase and the Klenow fragment used for blunting were from Fermentas. Luciferase reporter constructs to detect transcription factor activation included pNFκB-Luc, pAP1 (PMA)-TA-Luc, and pTA-Luc, (all from Clontech). A dual-plasmid system using pFR-Luc and pFA2-CREB was obtained from Stratagene, as was the control plasmid pFC2-dbd. The vIL-6 [K2P(−827)-luc] and ORF 50 (RpΔ1-luc) reporter constructs were a kind gift from Ren Sun. K2P(−827)-luc consists of nucleotides (nt) 18712 to 17876 cloned upstream of luciferase in pGL3-basic, as recently described (18). RpΔ1-luc uses nt 71594 to 69554 cloned into pGL2-basic. The ORF 57 luciferase reporter (p57P-luc) was a gift from Charles Wood, University of Nebraska. It consists of nt 81556 to 82080 cloned into pGL3-basic and has been fully described (21). Sequences were analyzed for transcription factor-binding elements by using MatInspector V2.2 (available online at http://transfac.gbf.de/TRANSFAC) (77).
Transfections were performed on exponentially growing cells by electroporation (Bio-Rad Gene Pulser II) at settings of 270 mV and 975 μF in 0.8-cm cuvettes (Invitrogen), using 8 × 106 cells resuspended in 0.8 ml RPMI 1640 per cuvette. The cuvettes were prechilled on ice, and after transfection, the cells were quickly plated in full culture medium as described below.
Cell line derivation and culture conditions.
BC-3, an Epstein-Barr virus (EBV)-negative, KSHV-positive PEL line, was derived as described above and maintained in RPMI 1640 plus 40 mg of gentamicin (Invitrogen) per ml with 10% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.) at 37°C under 5% CO2 (6). BC-3.6 and BC-3.14 were established by electroporation of BC-3 with 10 μg of pTRUF2-Tet-vGPCR. The cells were allowed to recover for 48 h in full culture medium, and then 1 mg of G418 (Invitrogen) per ml was added. After 2 weeks under antibiotic selection, single cells were placed on irradiated fibroblast feeder layers (1 × 104 to 2 × 104 cells/well; 5,000 rads of cobalt γ-irradiation) in 96-well plates by limiting dilution. After 2 weeks, wells with a single green fluorescent protein-positive colony were expanded and those with two or more were discarded. After reaching sufficient numbers, 48 lines were each screened for intact tetracycline inducibility-related protein expression by transfection, as described above, with 10 μg of pTRE-Luc (Clontech). Transfectants were split into two samples, one of which received 2 μg of doxycycline (Sigma) per ml. At 48 h, lysates were prepared and luciferase assays were performed. Lines with low background and high luciferase induction were used for vGPCR binding assays to assess the inducibility of vGPCR expression. Lines BC-3.6 and BC-3.14 are used interchangeably for all experiments presented here.
Binding assay.
BC-3 and its derivative cell lines, while growing in exponential phase, were subjected to various doses of doxycycline for 48 h. Aliquots (2 × 106 cells) were then washed in cold phosphate-buffered saline and resuspended in 200 μl of binding buffer (RPMI 1640 with 10 mg of bovine serum albumin [Sigma] per ml and 25 mM HEPES). Samples were incubated in duplicate for 2 h with 0.1 ng of125I-GROα (2,200 Ci/mMol) (Amersham) in a total of 400 μl of binding buffer, after which they were centrifuged through a 0.75-ml sucrose cushion (20% sucrose, 140 mM NaCl, 40 mM Tris [pH 7.6], 0.4% BSA) at 3,000 rpm (500 × g) for 5 min. Approximately 0.8 ml of supernatant was discarded, and the tubes were centrifuged again. The remainder of the supernatant was carefully removed, the tips of the tubes were cut off, and the contents were counted for radioactivity in a γ counter.
Luciferase assays.
For single-plasmid transfection experiments, the cells were split into two samples after transfection, plated in 24-well plates, and exposed to doses of doxycycline and GROα/IP-10 as shown in the figures. After 48 h, lysates were prepared using 1× cell culture lysis reagent as specified by the manufacturer (Promega). Assays were performed with 10 μl of lysate and 50 μl of beetle luciferin in a microtiter plate luminometer (Dynex Tech., Chantilly, Va.), using a 10-s read time. The protein concentration was used for normalization and was determined by the Bradford method with Bio-Rad DC protein assay reagent after dilution of samples and standards 1:1 in phosphate-buffered saline.
Western blot analysis and antibodies.
BC-3.6 cells were plated in full growth medium at 5 × 105/well of a 24-well plate and subjected to various amounts of freshly resuspended doxycycline (Sigma) for 48 h. Lysates were prepared in standard RIPA buffer supplemented with 1 μg each of aprotinin, leupeptin, and pepstatin per ml, 0.5 mM phenylmethylsulfonyl fluoride, and 1 mM each NaVO4 and NaF (Sigma). The protein was quantitated by the Bradford method and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10 to 12% polyacrylamide gels. Semidry transfer to a polyvinylidene difluoride membrane (Millipore) was performed using transfer buffer (48 mM Tris, 39 mM glycine, 0.037% SDS, 20% methanol). Blots were probed with primary antibody (Ab) overnight at 4°C. Horseradish peroxidase-conjugated secondary Ab was added after washing and was detected by an enhanced chemiluminescence system (ECL) (Amersham). For anti-phosphokinase blots, the blots were stripped after detection of the phosphorylated form and reprobed with Ab to total enzyme. The following primary Abs were used: anti-phospho-p38, anti-phospho-ERK1/2, anti-phospho-AKT, anti-phospho-IκBα, and anti-total-AKT (Cell Signaling Technology); anti-total ERK1/2-ct (Upstate); and anti-total p38 and anti-total IκBα (C-21) (Santa Cruz). Polyclonal vIL-6 antibody was derived in our laboratory by immunizing rabbits with peptide PDVTPDVHDK, as reported by Moore et al. (46), and used at 1:1,000. Anti-β-actin was from Sigma. Band intensity was quantified with NIH Image (http://rsb.info.nih.gov/nih-image/). Bands were normalized to the respective controls bands and then reported as fold induction over the values for baseline non-vGPCR-expressing conditions.
ELISA for VEGF.
BC-3.14 cells growing exponentially were exposed to doxycycline and/or GROα for 36 h to cause the expression of vGPCR and then serum starved overnight in RPMI 1640. Then 6 × 105 cells were plated for each sample in triplicate in 48-well plates, again in RPMI 1640 without serum. The cells were centrifuged, and 24 h later the supernatants were analyzed for VEGF expression by enzyme-linked immunosorbent assay (ELISA) (Cytokine Analysis Laboratory at the Fred Hutchinson Cancer Research Center, Seattle, WA, George B. McDonald, MD, Director).
RESULTS
Establishment of a vGPCR-expressing PEL cell line using a novel single-plasmid construct for tetracycline-mediated gene expression.
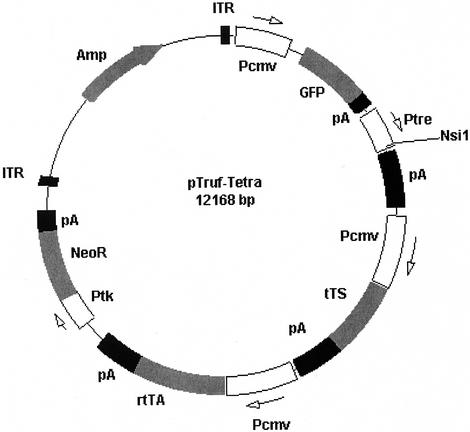
Our aim in this study was to assess vGPCR signaling pathways and downstream effects in KSHV-infected hematopoietic cells. Given the important role of hematopoietic cells as a reservoir for KSHV and a potential source of influential cytokines, a study of such cells is relevant not only to PEL but also to all KSHV-mediated disease (51). Initially, standard transient and stable expression transfection studies were attempted, but unlike the transformative properties displayed in other cell types, standard overexpression of vGPCR via an unregulatable promoter appeared to be toxic in all lymphoma cell lines tested, including PEL lines. It became clear that an inducible gene expression system was needed. Although tetracycline-inducible expression is not new, we sought to derive a plasmid vector that required just one round of transfection and clonal selection (29). Furthermore, since hematopoietic cells do not tend to integrate foreign DNA as readily as many commonly used cell lines, we utilized the ITR sequences derived from AAV-2: sequences thought to promote viral DNA integration into the host cell genome (25). Figure 1 shows a map of the resulting plasmid pTRUF2-Tet. In addition to the AAV-2 ITRs, the commercially available tetracycline-responsive promoter TRE, along with a unique NsiI cloning site and SV40 poly(A) tail are present. The rtTA sequence shown encodes the fusion protein that binds tetracycline derivatives to become a transcriptional activator. tTS is a more recently developed transcriptional silencer that minimizes background expression from the TRE promoter in the absence of tetracycline, an attribute vital to the successful regulation of a potentially lethal gene product (Clontech) (26).
FIG. 1.
Map of pTruf-Tet, a single plasmid designed to allow tetracycline-mediated induction of transgenes cloned into the unique NsiI site. ITR, inverted terminal repeat from AAV-2; Pcmv, CMV-derived promoter region; GFP, green fluorescent protein; pA, polyadenylation site from SV40; Ptre, tetracycline-responsive promoter; tTS, transcriptional silencer; rtTA, reverse tetracycline-responsive transcriptional activator; Neor, conveys neomycin resistance.
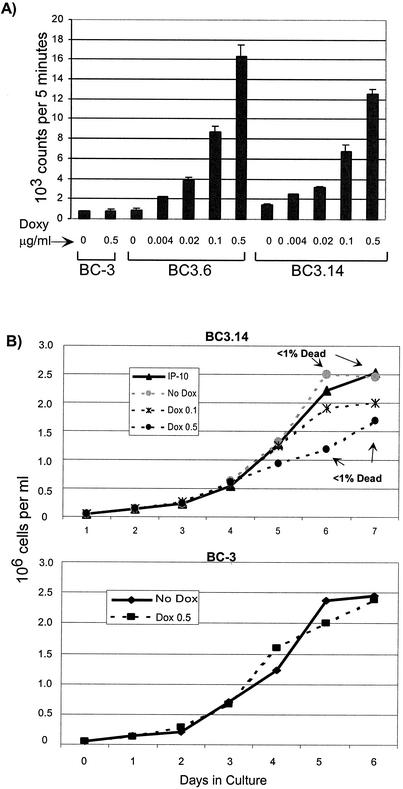
To create PEL lines that inducibly express KSHV vGPCR, we cloned vGPCR into pTRUF2-Tet and transfected BC-3, an EBV-negative, KSHV-positive PEL line. Clonal cell lines were derived by limiting dilution onto irradiated fibroblast feeder layers. The resulting lines were screened first for green fluorescent protein expression and then for the expression of rtTA and tTS by transient transfection with pTRE-Luc (Clontech). By transfecting each line with luciferase under the control of the tetracycline-responsive promoter pTRE, we identified lines displaying low uninduced luciferase expression but high levels on induction with doxycycline. The clones considered to have promising inducibility underwent the final screening step for vGPCR expression by binding assays with radiolabeled GROα, a known agonist. Figure 2A shows the results of a dose-response binding assay using various doses of doxycycline in two BC-3 derivative cell lines, BC-3.6 and BC-3.14. The data show exquisite doxycycline sensitivity, with no detectable baseline increase of vGPCR expression compared to that in the parent BC-3 line. As an initial evaluation of functional vGPCR expression, Fig. 2B shows dose-response growth curves in vGPCR-expressing cells. At any given time point, the number of viable cells is inversely proportional to vGPCR expression. Interestingly, the smaller numbers of viable cells are not accounted for by increased cell death, as determined by daily trypan blue staining; instead, they reflect slower proliferation. These data are in line with our initial observation in transfection studies with standard nonregulatable CMV-derived promoters: namely, that vGPCR expression adversely affects cell growth. These initial growth curves confirmed that these cell lines express vGPCR to a level high enough to affect cell physiology, thus making them suitable lines for further experiments.
FIG. 2.
Establishment of doxycycline-inducible vGPCR-expressing PEL lymphoma cell lines. (A) Binding assay shows increased vGPCR protein expression with increasing doses of doxycycline. Cells were incubated with125I-GROα, and pellets were counted in a γ counter. The first two lanes verify that doxycycline has no independent effect on control BC-3 cells. (B) vGPCR expression slows the growth of PEL cells in culture. Cells were plated at 5 × 104 cells/ml with the doxycycline doses shown (in micrograms per milliliter) and counted daily by trypan blue exclusion. (Top) BC3.14 with increasing doxycycline doses and thereby increasing vGPCR expression. The percentage of dead cells does not vary between vGPCR-expressing and -nonexpressing cells. (Bottom) Control BC-3 cells incubated with 2 μg/of doxycycline per ml shows that doxycycline has no independent effect on cell growth. Shown is average of three independent experiments; error bars are omitted for clarity.
KSHV vGPCR activates mitogen- as well as stress-induced kinases.
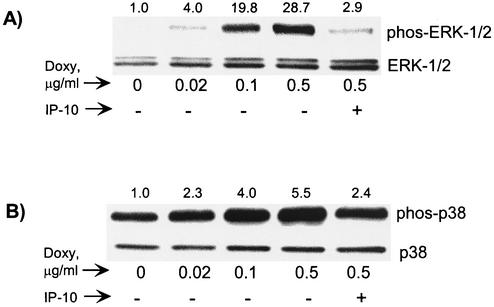
With a functional vGPCR-expressing PEL line, we next examined vGPCR-mediated effects on MAPK and SAPK. Constitutive activity of the MAPK ERK-1/2 is associated with oncogenesis in both GPCR-dependent and -independent models (20). Published studies show that in HEK 293 cells, vGPCR did not activate ERK-1/2, but constitutive activation was later shown in COS-7 cells (8, 69). Such variation points to the importance of considering cellular context when interpreting signaling studies. In Fig. 3A we show that vGPCR does indeed induce ERK-2 phosphorylation and thus activation in PEL cells. BC-3.6 cells were grown in the presence of doxycycline for 48 h as shown, and Western blot analyses were performed by probing first for phospho-ERK-1/2. After stripping of the blot, total ERK-1/2 was detected to show that overall levels of this kinase had not changed. IP-10 is an inverse agonist of vGPCR and was included to verify that kinase activation was specifically due to vGPCR. Interestingly, vGPCR seems to have a preferential effect on ERK-2. It is unclear why ERK-1 is unaffected.
FIG. 3.
vGPCR signaling activates ERK-2 and p38. (A) Western blots for total and phosphorylated (active) ERK-1/2 show increasing enzyme activity with increasing doxycycline doses. BC-3.14 cells were grown at 37°C for 48 h in the presence of doxycycline doses as shown. Protein extracts (30 μg/well) were subjected to SDS-PAGE and blotted first with α-phospho-ERK1/2 and then stripped and reprobed with α-total ERK1/2. (B) Western blots for phosphorylated and total p38. Protein extracts (40 μg) from cells stimulated as above were first probed with α-phospho-p38 and then stripped and reprobed with α-total p38. Numbers above the bands represent the average fold induction in band intensity relative to baseline (three independent experiments).
c-Jun amino-terminal kinase (JNK) and p38 are often discussed together as the SAPK family of serine/threonine kinases. Like ERK-1/2, they are ubiquitous in nature, but they are generally activated by physiologic stresses and inflammatory cytokines instead of by growth factors. Their physiologic role varies considerably among cell type and other coexisting signaling cascades. p38 is known to activate the transcription factors ATF2, CHOP, and CREB among others (57, 75). Although KSHV vGPCR can activate p38 in various cell lines, the most exciting recent finding is that p38, along with ERK, is involved in the vGPCR-induced expression of VEGF from endothelial cells (69). VEGF is a vital growth factor in all KSHV-mediated disease. Figure 3B shows that in PEL cells, vGPCR can cause the phosphorylation and activation of p38. Depending on the available upstream isoforms of MAPK kinase (MKK), JNK and p38 can be activated together or as independent SAPK modules (58). JNK activity is often associated with apoptosis, but in some models it plays a mitogenic role. In human B cells for example, JNK is activated by CD40 and has an antiapoptotic effect (61). Despite using multiple methods, we have not detected significant vGPCR-mediated JNK activation in PEL cells (data not shown).
vGPCR activates AP-1, CREB, and NF-κB-mediated transcription.
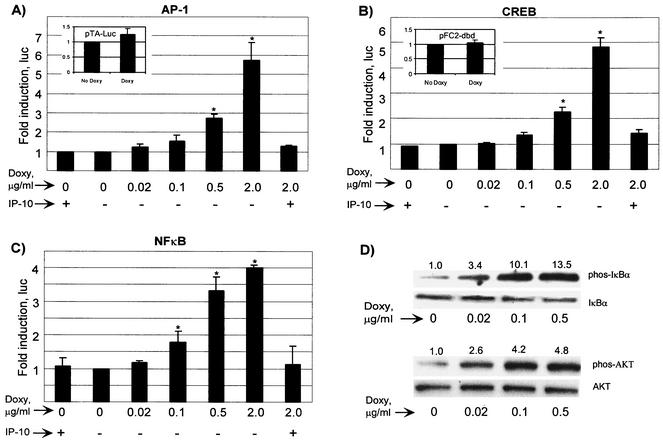
AP-1 is an important transcription factor involved in cellular growth control. vGPCR can activate AP-1-mediated transcription in HEK 293 and COS-1 cells, but it was unknown if this is the case in PEL cells. To address this possibility, a reporter construct with an AP-1/phorbol ester-responsive element upstream of luciferase was utilized. BC3.14 cells in exponential growth were transfected with pAP1-(PMA)-TA-Luc by electroporation and then exposed to increasing doses of doxycycline to induce vGPCR expression. After 48 h, lysates were assayed for luciferase activity. A direct relationship between vGPCR and AP-1 activity was shown (Fig. 4A). Again, AP-1-mediated transcription was sensitive to IP-10, thereby confirming the role of vGPCR. The very dramatic inhibitory effect of IP-10 is partly a reflection of assay sensitivity. Unlike experiments based on transient expression from multiple plasmid copies of vGPCR, our model cells transcribe vGPCR from as few as one integrated copy. In addition, only a small proportion of PEL cells are transfected with the reporter vectors. With fewer receptors expressed than in other overexpression systems, inhibition with IP-10 leaves very few actively signaling receptors, the full effects of which may not be accurately detected.
FIG. 4.
vGPCR activates AP-1, CREB, and NF-κB in PEL cells. BC-3.14 cells were transfected with the appropriate luciferase reporter and plated with the doses of doxycycline shown with or without IP-10 (100 nM), an inverse agonist of vGPCR. At 48 h, lysates were assayed for luciferase activity. (A) Dose-response curve of AP-1 activation. The inset shows the response to doxycycline of the negative control plasmid pTA-luc for the AP-1 and NF-κB constructs. (B) CREB activation. The inset shows the negative control plasmid, pFC2-dbd. (C) NF-κB activation. The results shown represent at least three independent transfections. Values are normalized to the no-doxycycline point. (D) vGPCR causes the phosphorylation of IκBα and AKT. Cells were subjected to the doses of doxycycline shown for 48 h, after which lysates (40 μg) were transferred to PVDF, probed for phosphorylated enzyme, stripped, and probed for total enzyme. Numbers above the bands represent the average fold induction (two independent experiments) in band intensity relative to baseline. ∗, In panels A to C, P ≤ 0.05 relative to the baseline uninduced value.
The CREB family of transcription factors is generally defined by their ability to bind cyclic AMP (cAMP)-responsive elements after phosphorylation by cAMP-activated protein kinase A (PKA). It is now clear, however, that CREB activity can also be regulated by MAPK and SAPK family members like p38 (75). Furthermore, several viruses produce proteins to attenuate the function of CREB family members, including adenovirus, human T-cell leukemia virus (HTLV), and, KSHV (27, 37, 39). Given the effect of vGPCR on MAPK and SAPK members, we studied its effect on CREB by using a trans-activation luciferase reporter system (PathDetect; Stratagene). Transfections and luciferase assays were performed as described above. We found that vGPCR also up-regulates CREB-mediated transcription (Fig. 4B). Our novel finding that vGPCR up-regulates this important transcription factor has important implications for the ability of KSHV to regulate both viral and cellular gene products.
NF-κB is a vital transcription factor for B-cell maturation and survival. Its dysregulation has been associated with oncogenesis in several models, and its inhibition of leads to the death of some lymphoma lines including PEL (35). vGPCR activates NF-κB in primary endothelial cells, the monocytic line THP-1, and Jurkat T cells (17, 51, 65). Furthermore, vGPCR activates NF-κB via coupling to Gα13 in HeLa cells (67). Using an NF-κB-luciferase reporter, we show that NF-κB transcriptional activity is enhanced by vGPCR expression in PEL cells (Fig. 4C). Consistent with this response, vGPCR can clearly cause phosphorylation of IκBα, a reaction upstream of NF-κB nuclear translocation (Fig. 4D). Treatment with IP-10 abrogated the activation of NF-κB by the induced expression of vGPCR.
vGPCR activation of NF-κB has been linked to its signaling via the kinase AKT/PKB, the main effector of PI3K (17, 45). GPCRs of both the pertussis-sensitive and -insensitive families, Gαi and Gαq, respectively, have recently been shown to activate the PI3K-AKT axis (47). Figure 4D shows that vGPCR induces the phosphorylation and thereby the activation of AKT in PEL cells.
KSHV vGPCR activates NFAT and transcription via ORF 50 and ORF 57 promoters.
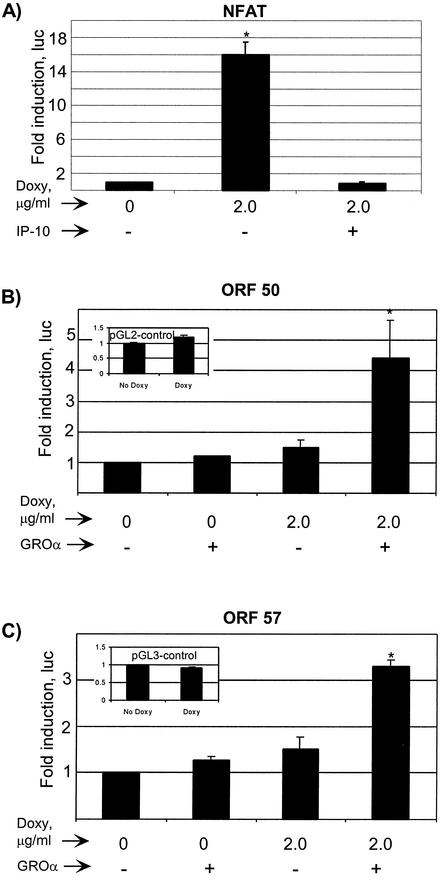
In immune cells, NFAT, a Ca2+-dependent transcription factor, can act in conjunction with AP-1 to enhance the expression of numerous proteins involved in the productive immune response, some of which are crucial to the biology of KSHV-mediated disease (23, 41, 68). Furthermore, in B cells, NFAT and NFκB are common downstream targets of PI3K and phospholipase Cγ (for a review, see reference 43). Since we knew that AP-1 and NF-κB were activated in PEL cells by vGPCR, we studied the effect of vGPCR on NFAT-mediated transcription (Fig. 5A). The implications of increased NFAT activity on the role of vGPCR in the transactivation of viral genes are interesting because NFAT is absolutely required for the Ca2+-induced latent-to-lytic program switch in KSHV in vitro (81). With the knowledge of such broad activation of important transcription factors, we went on to assess the effects of vGPCR on certain KSHV lytic genes including ORF 50 and ORF 57; the former gene product is a homologue of EBV RTA, has broad transactivation potential, and is necessary and sufficient to induce lytic replication of KSHV. ORF 57 is a lytic gene product up-regulated by ORF 50 and is thought to have transactivation potential of its own (21, 40). Luciferase reporter assays show clear transcriptional activation via both the ORF 57 and ORF 50 promoters by vGPCR (Fig. 5B and C). Although constitutive vGPCR signaling had minimal effect, the presence of the agonist GROα caused three- and fourfold induction, respectively, in vGPCR-expressing cells. This requirement for an agonist differs from the results of the transcription factor assays in Fig. 4 and 5A and is discussed below.
FIG. 5.
vGPCR activates transcription by NFAT as well as the KSHV ORF 50 and ORF 57 promoters. BC-3.14 cells in exponential growth were transfected with the respective luciferase reporter construct and exposed to does of doxycycline and/or GROα as shown, to induce vGPCR expression and further increase signaling, respectively. After 48 h, lysates were assayed for luciferase activity as described in Materials and Methods. (A) NFAT activity. The negative control was as in the inset of Fig. 4A. (B) ORF 50 promoter. The inset shows the response to doxycycline of the negative control plasmid pGL2-control. (C) ORF 57 promoter. The inset shows the response to doxycycline of the negative control plasmid pG32-control. Results shown represent at least three independent transfections. ∗, P ≤ 0.05 relative to the baseline uninduced value.
KSHV vGPCR up-regulates vIL-6 and VEGF expression from PEL cells.
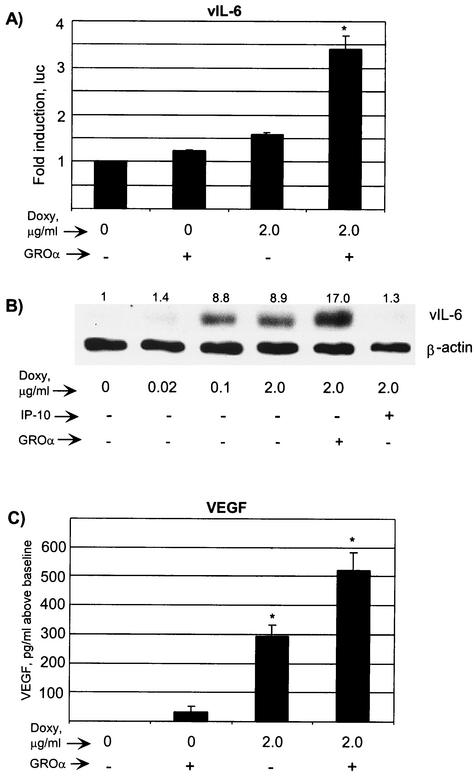
vGPCR induces VEGF expression from both fibroblasts and endothelial cells, in the latter case via p38 (8, 69). KS depends on VEGF for its highly vascular morphology, and PEL depends on VEGF for tumor growth and acsites production in mice (2, 3, 44, 62). Furthermore, VEGF clearly plays a role in the clinical behavior of MCD (49). Should vGPCR cause VEGF expression from PEL cells, it would have potential to mediate paracrine effects on infected or uninfected endothelial cells. Using an ELISA, we showed that vGPCR can indeed increase VEGF expression from PEL cells (Fig. 6A). The expression of vGPCR in the presence of the agonist GROα led to a 500-pg/ml increase in VEGF expression, which represented an approximately twofold change from the baseline serum-starved state. In murine fibroblasts, VEGF expression is enhanced by the KSHV homologue of IL-6 (vIL-6) (1). Furthermore, vIL-6 is functional and plays an important role in the growth of PEL and MCD (1, 32, 38). The vIL-6 promoter contains both AP-1 and NFAT sites and is known to be upregulated by ORF 50 (18). We sought, therefore, to determine if vGPCR affects vIL-6 expression. Using both a luciferase reporter assay (K2P-luc was a kind gift from Ren Sun) and Western blotting, we showed that vGPCR signaling positively regulated vIL-6 expression (Fig. 6B and C). The induction of both vIL-6 and VEGF confirms the growth and angiogenic potential of vGPCR, particularly as regards a paracrine role in PEL.
FIG. 6.
vGPCR increases the expression of KSHV vIL-6 and VEGF. (A) BC-3.14 cells were transfected with the vIL-6-luciferase construct, incubated with or without doxycycline and with or without GROα (100 nM) for 48 h, and assayed for luciferase activity. Negative control is as in the inset in Fig. 5C. Results of three independent transfections are shown. (B) BC-3.14 cells were plated at 5 × 105 cells/ml in the doses of doxycycline shown. The cells were lysed in RIPA buffer after 48 h, and 40 μg of protein per lane was loaded onto the SDS-PAGE gel. Western blot analysis was performed using anti-vIL6 Ab, stripped, and then the blot was reprobed with anti-β-actin Ab to control for loading. Numbers above the bands represent average fold induction in band intensity relative to baseline (three independent experiments). (C) BC-3.14 cells growing exponentially were preincubated in full medium with or without doxycycline and with or without GROα (100 nM) for 36 h. They were then washed and replated in serum free medium with or without doxycycline and with or without GROα for another 12 h. Then 6 × 105 cells per point were plated in serum-free medium, and at 24 h, the supernatant was assayed for VEGF by ELISA. Results represent three independent experiments and are expressed as picograms per milliliter over baseline production. Uninduced cells expressed 535 pg of VEGF per ml. vGPCR-expressing cells stimulated with GROα expressed 1,054 pg/ml. ∗, P ≤ 0.001 relative to the baseline uninduced value.
DISCUSSION
KSHV vGPCR is a bona fide constitutively signaling molecule that can couple to both Gαi and Gαq G proteins, thereby giving it broad signaling capabilities. It activates the MAPK and SAPK cascades and enhances transcription via NF-κB and AP-1. Important growth and angiogenic factors like VEGF are influenced by vGPCR, and in NIH 3T3 cells, vGPCR is directly transforming. However, little is known about the signaling cascades utilized and the downstream phenotypic effects of vGPCR in hematopoietic cells such as monocytes and B lymphocytes, cells that serve as reservoirs of KSHV in vivo and are the source of important inflammatory cytokines. We sought, therefore, to study vGPCR in such a highly biologically relevant cellular context. As described above, however, our repeated attempts to express vGPCR constitutively in B-cell lines failed, in that none of the many lymphoma lines used recovered after transfection of vGPCR under a constitutive promoter; this finding was surprising, given that vGPCR is considered a transforming viral oncogene (8). To establish a workable model, we decided to use an inducible gene transcription system to express vGPCR in PEL cells. Inducibility offers several advantages over constitutive transient or stable expressions (i) As in our case, gene products that are toxic to cells or cause a slowing of cell growth can be studied more effectively. (ii) Graded inducible expression can avoid the “forced” protein-protein and protein-DNA interactions that may result from enormous overexpression of an exogenous product under a nonregulatable promoter. (iii) Transfection of some cell types is very inefficient, results in much cell death, and thus makes certain experiments exceedingly difficult when transient-expression assays are used. For our experiments, we chose to use the tetracycline-inducible system (Tet-On; Clontech). The standard approach requires the establishment of a clonal line that efficiently expresses the tetracycline-binding protein (rtTA), followed by a second round of transfection and clonal screening for the expression of the gene of interest that is downstream of the tetracycline-responsive promoter (pTRE). Given the general difficulty in establishing stable hematopoietic cell lines, we wanted to derive a vector that would allow a single transfection and selection effort. Although single-plasmid systems containing both the rtTA and transgene under the control of pTRE have been reported (33, 64), we added two more elements to our construct: (i) the tTS gene product, which has been recently developed to minimize leaky expression from the tetracycline-responsive promoter (26), and (ii) the ITR sequences from AAV-2. AAV-2 is a promising candidate as a gene therapy vector, given its ability to integrate into the host cell genome. It has generally been thought that the ITR regions at the distal ends of the AAV-2 genome are highly recombinogenic (although very recent data suggest that other AAV-2 sequences are required for maximal integration efficiency) (50, 53, 63). Our resulting plasmid, pTRUF2-Tet, was used to successfully establish vGPCR-expressing PEL lines that have proven to be a promising and unique model for studying vGPCR signaling as well as the interaction between vGPCR and other KSHV genes.
Our first goal with these inducible vGPCR-expressing lines was to establish the effect of vGPCR on the major limbs of the MAPK and SAPK pathways. MAPK and SAPK are ubiquitous signaling molecules with an array of downstream effects in response to mitogens, inflammatory cytokines, and physiologic stresses. Dysregulation of both MAPK and SAPK has been associated with dysregulated growth and tumorigenesis. GPCRs are known to signal via the MAPK and SAPK pathways, and KSHV vGPCR is no exception. Cellular context is, however, crucial to any signaling molecule's choice of effectors, their potential cross talk, and the ultimate effect on gene transcription and cellular physiology. This is highlighted in a recent study by Polson et al., where significant differences were identified by using transient transfection of vGPCR in the BJAB and SLK cell lines, representing B and endothelial cells, respectively, followed by gene expression profiling (54). Here we have shown that, unlike for some other cell types studied, vGPCR can activate both ERK-2 and p38 in PEL cells. This gives vGPCR a great potential to affect a huge array of genes involved in cell cycling, death, and cytokine secretion.
Indeed, this potential to affect gene transcription in PEL cells was extended by our finding that even in the absence of ligand, vGPCR has an activating effect on AP-1, NF-κB, and CREB; all three transcription factors have been thoroughly studied and are known to influence lymphocytic cells and malignant phenotypes. It is known that AP-1 is involved in cellular growth control, since it was found to be stimulated by phorbol ester tumor promoters and to be composed of c-Jun and c-Fos, the cellular homologues of v-Fos and v-Jun. Its overall effects are largely cell type specific, however, leading to apoptosis in some and to increased survival in others (for a review, see reference 66). Our studies show for the first time that vGPCR can also activate CREB. The CREB/ATF transcription factor family is involved in regulating various cellular genes, and CRE sites are found in the regulatory elements of many human viruses including members of Herpesviradae and HTLV-1 (4). Thus, CREB activation (or mimicry in the case of HTLV-1 Tax) may provide a link between the regulation of gene expression by cellular signaling pathways and their subversion by viral transactivating proteins (31). In some cases, this subversion may serve as a primary method by which these viruses manipulate cellular machinery toward the goal of viral replication (for a review, see reference 28). Therefore, the exact function of vGPCR-mediated CREB activation in KSHV warrants further study.
NF-κB is also proving to be an important downstream effector of vGPCR. Not only does vGPCR directly activate NF-κB-mediated transcription in endothelial cells, but also conditioned medium from vGPCR-expressing endothelial cells activates NF-κB and several of its dependent cytokines in non-vGPCR-expressing cells (51). Until now, however, a link between the two has not been shown in a naturally KSHV-infected hematopoietic cell. Our results confirm and extend the importance of vGPCR-mediated NF-κB activation in that we now know that in such cells, vGPCR has the potential to affect cytokine production, host cell antiviral responses, and even cell survival via NF-κB. Although the pathway from vGPCR to NF-κB involves the PI3K/AKT axis in COS-7 cells, it is not certain that the same holds true in hematopoietic cell types. We have shown that vGPCR activates AKT in PEL cells, and we know that this reaction is wortmannin sensitive, i.e., PI3K mediated (data not shown), but we are currently assessing whether PI3K/AKT contributes substantially to vGPCR-mediated NF-κB activation. Regardless of a direct effect on NF-κB, however, the ability to signal via PI3K/AKT greatly extends the signaling repertoire of vGPCR in PEL cells. AKT has many potential downstream effectors, is associated with an antiapoptotic function, and is necessary to vGPCR-mediated endothelial cell survival (45, 56).
For several reasons, we also wanted to assess the effects of vGPCR on the Ca2+-calcineurin-dependent transcription factor NFAT. First, although the major pathways activating NFAT and AP-1 are distinct, cross talk does occur and a large range of NFAT/AP-1 composite binding sites occur upstream of genes in immune cells (e.g., genes for IL-2 and granulocyte-macrophage colony-stimulating factor) (41). Second, both NFAT and NF-κB are downstream effectors of PI3K and protein lipase Cγ (PLCγ) in B-cell activation (for a review, see reference 43). Third, NFAT is required for the Ca2+-induced KSHV lytic cycle (81). Because of its broad signaling capacity including Ca2+ mobilization and AP-1 activation (like phorbol esters used to induce the KSHV lytic phase), we postulated that vGPCR was a candidate molecule for affecting KSHV transcription patterns. Our novel results showing vGPCR-mediated NFAT activation mean that vGPCR warrants further investigation with regard to its transactivation potential.
Recently, Chiou et al. have elegantly investigated the transactivation potential of vGPCR and shown that it can up-regulate transcription via the KSHV T1.1/PAN lytic promoter, the LLP latency leader promoter, and the K1 promoter (15). We extend these results by showing that vGPCR can activate transcription via both the ORF 50 and ORF 57 promoters. It is noteworthy that unlike vGPCR-mediated activation of the various transcription factors tested, detection of transcription via the ORF 50 and ORF 57 (and vIL-6) promoters required the addition of GROα, a vGPCR agonist (Fig. 5B and C and 6A). This agonist requirement probably reflects the low copy number of vGPCR expressed. In addition, although transcription factor luciferase reporter constructs are purposely designed for maximum response to a specific transcription factor (often with multiple copies of the binding element in tandem), the viral promoters used here are much more complex and include both positive and negative regulatory elements. Therefore, the effect of vGPCR on ORFs 50 and 57, shown in Fig. 5B and C, is the net result of the integration of many events caused by this broadly signaling receptor. It is unclear whether vGPCR-mediated transcription from the ORF 57 promoter is direct, indirect (via ORF 50), or a combination of the two mechanisms. Moreover, although the ORF 50 promoter contains NFAT-, AP-1-, and NF-κB-binding sites (and the ORF 57 promoter contains both AP-1 and NF-κB sites), determining which of these are involved vGPCR-mediated transcription requires further deletion mutant studies.
Although transcriptional activation by vGPCR may be “nonspecific”, Chiou et al. astutely point out that an important physiologic role for vGPCR transactivation in the lytic phase of KSHV infection cannot be ruled out. The vGPCR gene is an early lytic gene as defined by transcript sensitivity to the viral DNA polymerase inhibitor foscarnet (48, 73). In a later study with a single cell line, the vGPCR transcript was detected temporally after ORF 59 and called a late lytic product, but no inhibitor studies were included (52). Interestingly, a very recent study by Dezube et al. argues that vGPCR is vigorously expressed on initial infection of endothelial cells as part of an immediate and synchronous lytic infection (19). Therefore, although it would be difficult to postulate that, as a lytic product, vGPCR can induce the KSHV lytic switch de novo from latently infected cells, it is clear that vGPCR has the potential to affect both viral and cellular gene transcription during at least part of the lytic cycle. It is conceivable that the slower proliferation of vGPCR-expressing cells (Fig. 2) reflects a role for a vGPCR-mediated subversion of the cell cycle during the lytic phase. In fact, inhibition of cellular DNA replication is a common effect mediated by several herpesvirus proteins and is thought to be important during lytic herpesvirus replication, perhaps to prevent competition for nucleotide pools. This may be induced directly or indirectly, for example by induction of other lytic genes such as the replication-associated protein, which was recently shown by Wu et al. to induce a G1 cell cycle arrest (79). Furthermore, through its ability to respond both negatively and positively to various CC- and CXC-type chemokines (including KSHV-derived chemokine homologues), vGPCR could help dictate the KSHV response to changes in the extracellular environment (59).
In addition to possible roles in the KSHV life cycle or host cell cycle, it is very likely that by augmenting both vIL-6 and VEGF expression from KSHV-infected lymphoma cells, vGPCR has an important role with respect to angiogenesis and cellular proliferation in a paracrine manner. Such mechanisms fit nicely with the expression patterns of vGPCR and the repeated finding of small subsets of lytically infected hematopoietic cells in PEL, MCD, and KS lines and tissues, including within the KS-like lesions of transgenic vGPCR-expressing mice (15, 24, 34, 36, 72, 80). These few lytic cells may encourage growth or angiogenic properties among neighboring cells, KSHV infected or otherwise. Thus, it may be that vGPCR exerts its most important effects via a very small subset of cells.
Having established a novel KSHV-positive hematopoietic cell line inducible for vGPCR expression, we now have a model suited for further study of cell-type-specific vGPCR signaling and its effects on viral and cellular gene regulation. It will also facilitate further study of vGPCR-mediated changes in cellular phenotype including growth, proliferation, response to apoptotic stimuli, and even chemotaxis.
Acknowledgments
M.C. was a Fellow of the Lymphoma Foundation of America while much of this work was performed. This work was also funded by NIH grant CA73531 to E.C.
REFERENCES
- 1.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 2.Aoki, Y., and G. Tosato. 1999. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 94:4247-4254. [PubMed] [Google Scholar]
- 3.Aoki, Y., and G. Tosato. 2001. Vascular endothelial growth factor/vascular permeability factor in the pathogenesis of primary effusion lymphomas. Leuk. Lymphoma 41:229-237. [DOI] [PubMed] [Google Scholar]
- 4.Arima, N., and C. Tei. 2001. HTLV-I Tax related dysfunction of cell cycle regulators and oncogenesis of adult T cell leukemia. Leuk. Lymphoma 40:267-278. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 7.Asou, H., J. W. Said, R. Yang, R. Munker, D. J. Park, N. Kamada, and H. P. Koeffler. 1998. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91:2475-2481. [PubMed] [Google Scholar]
- 8.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 9.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 10.Burger, M., J. A. Burger, R. C. Hoch, Z. Oades, H. Takamori, and I. U. Schraufstatter. 1999. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 163:2017-2022. [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, E. H., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94:690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiou, C. J., L. J. Poole, P. S. Kim, D. M. Ciufo, J. S. Cannon, C. M. ap Rhys, D. J. Alcendor, J. C. Zong, R. F. Ambinder, and G. S. Hayward. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coughlin, S. R. 1994. Expanding horizons for receptors coupled to G proteins: diversity and disease. Curr. Opin. Cell Biol. 6:191-197. [DOI] [PubMed] [Google Scholar]
- 17.Couty, J. P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 18.Deng, H., M. J. Song, J. T. Chu, and R. Sun. 2002. Transcriptional regulation of the interleukin-6 gene of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 76:8252-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dezube, B. J., M. Zambela, D. R. Sage, J. F. Wang, and J. D. Fingeroth. 2002. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: early events. Blood 100:888-896. [DOI] [PubMed] [Google Scholar]
- 20.Dhanasekaran, N., L. E. Heasley, and G. L. Johnson. 1995. G protein-coupled receptor systems involved in cell growth and oncogenesis. Endocr. Rev. 16:259-270. [DOI] [PubMed] [Google Scholar]
- 21.Duan, W., S. Wang, S. Liu, and C. Wood. 2001. Characterization of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 ORF57 promoter. Arch. Virol. 146:403-413. [DOI] [PubMed] [Google Scholar]
- 22.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 23.Fiorelli, V., R. Gendelman, M. C. Sirianni, H. K. Chang, S. Colombini, P. D. Markham, P. Monini, J. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Gamma-interferon produced by CD8+ T cells infiltrating Kaposi's sarcoma induces spindle cells with angiogenic phenotype and synergy with human immunodeficiency virus-1 Tat protein: an immune response to human herpesvirus-8 infection? Blood 91:956-967. [PubMed] [Google Scholar]
- 24.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 25.Flotte, T. R., and B. J. Carter. 1995. Adeno-associated virus vectors for gene therapy. Gene Ther. 2:357-362. [PubMed] [Google Scholar]
- 26.Freundlieb, S., C. Schirra-Muller, and H. Bujard. 1999. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene Med. 1:4-12. [DOI] [PubMed] [Google Scholar]
- 27.Gachon, F., A. Peleraux, S. Thebault, J. Dick, I. Lemasson, C. Devaux, and J. M. Mesnard. 1998. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J. Virol. 72:8332-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilchrist, C. A., D. J. Orten, and S. H. Hinrichs. 1996. Evidence for the role of cyclic AMP-responsive elements in human virus replication and disease. J. Biomed. Sci. 3:293-306. [DOI] [PubMed] [Google Scholar]
- 29.Gossen, M., A. L. Bonin, S. Freundlieb, and H. Bujard. 1994. Inducible gene expression systems for higher eukaryotic cells. Curr. Opin. Biotechnol. 5:516-520. [DOI] [PubMed] [Google Scholar]
- 30.Gossen, M., S. Freundlieb, G. Bender, G. Muller, W. Hillen, and H. Bujard. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science 268:1766-1769. [DOI] [PubMed] [Google Scholar]
- 31.Hoeffler, J. P. 1992. Structure/function relationships of CREB/ATF proteins. J. Investig. Dermatol 98:21S-28S. [DOI] [PubMed] [Google Scholar]
- 32.Jones, K. D., Y. Aoki, Y. Chang, P. S. Moore, R. Yarchoan, and G. Tosato. 1999. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 94:2871-2879. [PubMed] [Google Scholar]
- 33.Jost, M., C. Kari, and U. Rodeck. 1997. An episomal vector for stable tetracycline-regulated gene expression. Nucleic Acids Res. 25:3131-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269:335-344. [DOI] [PubMed] [Google Scholar]
- 35.Keller, S. A., E. J. Schattner, and E. Cesarman. 2000. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537-2542. [PubMed] [Google Scholar]
- 36.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016-31022. [DOI] [PubMed] [Google Scholar]
- 38.Liu, C., Y. Okruzhnov, H. Li, and J. Nicholas. 2001. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 75:10933-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, F., and M. R. Green. 1994. Promoter targeting by adenovirus E1a through interaction with different cellular DNA-binding domains. Nature 368:520-525. [DOI] [PubMed] [Google Scholar]
- 40.Lukac, D. M., L. Garibyan, J. R. Kirshner, D. Palmeri, and D. Ganem. 2001. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J. Virol. 75:6786-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene 20:2476-2489. [DOI] [PubMed] [Google Scholar]
- 42.Maeda, Y., U. Ikeda, M. Shimpo, S. Ueno, Y. Ogasawara, M. Urabe, A. Kume, T. Takizawa, T. Saito, P. Colosi, G. Kurtzman, K. Shimada, and K. Ozawa. 1998. Efficient gene transfer into cardiac myocytes using adeno-associated virus (AAV) vectors. J. Mol. Cell. Cardiol. 30:1341-1348. [DOI] [PubMed] [Google Scholar]
- 43.Marshall, A. J., H. Niiro, T. J. Yun, and E. A. Clark. 2000. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cγ pathway. Immunol. Rev. 176:30-46. [DOI] [PubMed] [Google Scholar]
- 44.Masood, R., J. Cai, T. Zheng, D. L. Smith, Y. Naidu, and P. S. Gill. 1997. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 94:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 46.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 47.Murga, C., L. Laguinge, R. Wetzker, A. Cuadrado, and J. S. Gutkind. 1998. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase gamma. J. Biol. Chem. 273:19080-19085. [DOI] [PubMed] [Google Scholar]
- 48.Nador, R. G., L. L. Milligan, O. Flore, X. Wang, L. Arvanitakis, D. M. Knowles, and E. Cesarman. 2001. Expression of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor monocistronic and bicistronic transcripts in primary effusion lymphomas. Virology 287:62-70. [DOI] [PubMed] [Google Scholar]
- 49.Nishi, J., and I. Maruyama. 2000. Increased expression of vascular endothelial growth factor (VEGF) in Castleman's disease: proposed pathomechanism of vascular proliferation in the affected lymph node. Leuk. Lymphoma 38:387-394. [DOI] [PubMed] [Google Scholar]
- 50.Palombo, F., A. Monciotti, A. Recchia, R. Cortese, G. Ciliberto, and N. La Monica. 1998. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector. J. Virol. 72:5025-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Philpott, N. J., C. Giraud-Wali, C. Dupuis, J. Gomos, H. Hamilton, K. I. Berns, and E. Falck-Pedersen. 2002. Efficient integration of recombinant adeno-associated virus DNA vectors requires a p5-rep sequence in cis. J. Virol. 76:5411-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polson, A. G., D. Wang, J. DeRisi, and D. Ganem. 2002. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus. Cancer Res 62:4525-4530. [PubMed] [Google Scholar]
- 55.Ponnazhagan, S., M. C. Yoder, and A. Srivastava. 1997. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J. Virol. 71:3098-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pugazhenthi, S., A. Nesterova, C. Sable, K. A. Heidenreich, L. M. Boxer, L. E. Heasley, and J. E. Reusch. 2000. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 275:10761-10766. [DOI] [PubMed] [Google Scholar]
- 57.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 58.Robinson, M. J., M. Cheng, A. Khokhlatchev, D. Ebert, N. Ahn, K. L. Guan, B. Stein, E. Goldsmith, and M. H. Cobb. 1996. Contributions of the mitogen-activated protein (MAP) kinase backbone and phosphorylation loop to MEK specificity. J. Biol. Chem. 271:29734-29739. [DOI] [PubMed] [Google Scholar]
- 59.Rosenkilde, M. M., T. N. Kledal, H. Brauner-Osborne, and T. W. Schwartz. 1999. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 274:956-961. [DOI] [PubMed] [Google Scholar]
- 60.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakata, N., H. R. Patel, N. Terada, A. Aruffo, G. L. Johnson, and E. W. Gelfand. 1995. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J. Biol. Chem. 270:30823-30828. [DOI] [PubMed] [Google Scholar]
- 62.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152:1433-1443. [PMC free article] [PubMed] [Google Scholar]
- 63.Samulski, R. J., A. Srivastava, K. I. Berns, and N. Muzyczka. 1983. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell 33:135-143. [DOI] [PubMed] [Google Scholar]
- 64.Schultze, N., Y. Burki, Y. Lang, U. Certa, and H. Bluethmann. 1996. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat. Biotechnol. 14:499-503. [DOI] [PubMed] [Google Scholar]
- 65.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 66.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 67.Shepard, L. W., M. Yang, P. Xie, D. D. Browning, T. Voyno-Yasenetskaya, T. Kozasa, and R. D. Ye. 2001. Constitutive activation of NF-κB and secretion of IL-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involves Gα (13) and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 68.Sirianni, M. C., L. Vincenzi, V. Fiorelli, S. Topino, E. Scala, S. Uccini, A. Angeloni, A. Faggioni, D. Cerimele, F. Cottoni, F. Aiuti, and B. Ensoli. 1998. Gamma-interferon production in peripheral blood mononuclear cells and tumor infiltrating lymphocytes from Kaposi's sarcoma patients: correlation with the presence of human herpesvirus-8 in peripheral blood mononuclear cells and lesional macrophages. Blood 91:968-976. [PubMed] [Google Scholar]
- 69.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 70.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, and et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 71.Spiegel, A. M. 1996. Defects in G protein-coupled signal transduction in human disease. Annu. Rev. Physiol. 58:143-170. [DOI] [PubMed] [Google Scholar]
- 72.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swanton, C., D. J. Mann, B. Fleckenstein, F. Neipel, G. Peters, and N. Jones. 1997. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390:184-187. [DOI] [PubMed] [Google Scholar]
- 75.Tan, Y., J. Rouse, A. Zhang, S. Cariati, P. Cohen, and M. J. Comb. 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15:4629-4642. [PMC free article] [PubMed] [Google Scholar]
- 76.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, and A. S. Denton. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]
- 77.Wingender, E., X. Chen, R. Hehl, H. Karas, I. Liebich, V. Matys, T. Meinhardt, M. Pruss, I. Reuter, and F. Schacherer. 2000. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 28:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong, W. W. 1998. ICE family proteases inflammation and apoptosis. Agents Actions Suppl. 49:5-13. [DOI] [PubMed] [Google Scholar]
- 79.Wu, F. Y., Q. Q. Tang, H. Chen, C. ApRhys, C. Farrell, J. Chen, M. Fujimuro, M. D. Lane, and G. S. Hayward. 2002. Lytic replication-associated protein (RAP) encoded by Kaposi’s sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein alpha. Proc. Natl. Acad. Sci. USA 99:10683-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang, T., S. Chen, M. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. Jenh, S. Narula, S. Chensue, and S. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zoeteweij, J. P., A. V. Moses, A. S. Rinderknecht, D. A. Davis, W. W. Overwijk, R. Yarchoan, J. M. Orenstein, and A. Blauvelt. 2001. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood 97:2374-2380. [DOI] [PubMed] [Google Scholar]
- 82.Zolotukhin, S., M. Potter, W. W. Hauswirth, J. Guy, and N. Muzyczka. 1996. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 70:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]