Abstract
Background
Because granulocyte colony-stimulating factor (G-CSF) has anti-inflammatory and neuroprotective properties and is known to mobilize stem cells, it may be useful in the treatment of acute ischemic stroke. We sought to examine the feasibility, safety and efficacy of using G-CSF to treat acute stroke.
Methods
We conducted a randomized, blinded controlled trial involving 10 patients with acute cerebral infarction (middle cerebral artery territory as documented by the admission MRI) who presented within 7 days of onset and whose scores on the National Institutes of Health Stroke Scale (NIHSS) were between 9 and 20. Patients were assigned to either G-CSF therapy or usual care. The G-CSF group (n = 7) received subcutaneous G-CSF injections (15 μg/kg per day) for 5 days. The primary outcome was percentage changes between baseline and 12-month follow-up in mean group scores on 4 clinical scales: the NIHSS, European Stroke Scale (ESS), ESS Motor Subscale (EMS) and Barthel Index (BI). We also assessed neurologic functioning using PET to measure cerebral uptake of fluorodeoxyglucose in the cortical areas surrounding the ischemic core.
Results
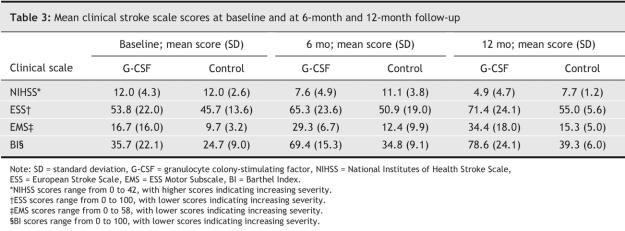
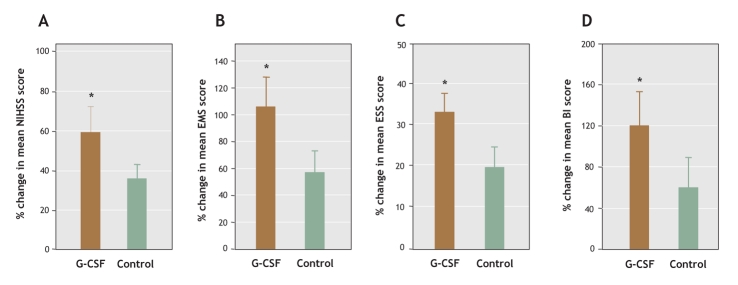
All of the patients completed the 5-day course of treatment, and none were lost to follow-up. No severe adverse effects were seen in patients receiving G-CSF. There was greater improvement in neurologic functioning between baseline and 12-month follow-up in the G-CSF group than in the control group (NIHSS: 59% change in the mean G-CSF group score v. 36% in the mean control group score, ESS: 33% v. 20%, EMS: 106% v. 58%, BI: 120% v. 60%). Although at 12 months there was no difference between the 2 groups in cerebral uptake of fluorodeoxyglucose in the ischemic core, uptake in the area surrounding the core was significantly improved in the G-CSF group compared with the control group. There was positive correlation between metabolic activity and EMS score following simple linear correlation analysis.
Interpretation
Our preliminary evidence suggests that using G-CSF as therapy for acute stroke is safe and feasible and leads to improved neurologic outcomes.
Cerebrovascular disease is a major cause of death and disability worldwide.1 Currently, some thrombolytic agents such as tissue plasminogen activator and urokinase are available to patients who have experienced acute stroke, but results may be complicated by hemorrhagic transformation, and the therapeutic window of only a few hours limits their wide use.2 However, recent studies have shown that neurons suitable for transplantation can be generated from stem cells in culture and that, in response to injury, the adult brain produces new neurons from endogenous stem cells.3,4 These findings indicate the possibility of developing stem cell therapies to treat human neurodegenerative disorders. For example, bone marrow cell transplantation following cerebral ischemia in an animal model has been shown to reduce infarction volume through the differentiation of bone marrow stem cells into both neurons and glial cells.5 In one clinical trial, 12 patients who had experienced chronic strokes affecting the basal ganglia received intracerebral transplantation of neurons generated from the human NT-2 teratocarcinoma cell line.6 Neurologic improvement was correlated by improved fluorodeoxyglucose uptake at the graft site in PET scans of 6 of 11 patients at 6 months after transplantation.7
Granulocyte colony-stimulating factor (G-CSF) is a member of the cytokine family of growth factors. Administration of G-CSF is known to mobilize hematopoietic stem cells from the bone marrow into the peripheral blood.8 Hematopoietic stem cells have been used in place of bone marrow cells in transplantation for the regeneration of nonhematopoietic tissues such as skeletal muscle and the heart.9 G-CSF has been used extensively for over 10 years in the treatment of neutropenia as well as for bone marrow reconstitution and stem cell mobilization.10 Furthermore, G-CSF has also been used as an anti-inflammatory agent in murine endotoxemia.11 This is particularly significant in view of findings that implicate inflammation in the pathophysiology of cerebral ischemic injury.12 For example, activated neutrophils have been shown to promote cerebral ischemic injury by microvascular plugging and the production of cytotoxic substances.13 Thus, a therapeutic approach that reduces inflammation may protect against cerebral ischemic injury. In a recent study, G-CSF exerted a potential neuroprotective effect in cell culture against excitotoxicity and in rats with cerebral ischemia.14 Because of its anti-inflammatory and neuroprotective properties and its capacity to mobilize stem cells, G-CSF has the potential to be used in clinical applications, including those following myocardial infarction and stroke.
In this study, we evaluate the clinical effects of G-CSF therapy for acute stroke by measuring as our primary end point the percentage change in group scores between baseline and 12-month follow-up on the National Institutes of Health Stroke Scale (NIHSS), European Stroke Scale (ESS), ESS Motor Subscale (EMS) and Barthel Index (BI). On the basis of results achieved in our previous animal study,15 we postulated that patients given G-CSF would show greater improvement in neurologic functioning than patients not given G-CSF. We also sought to determine the safety and adverse events of G-CSF therapy.
Methods
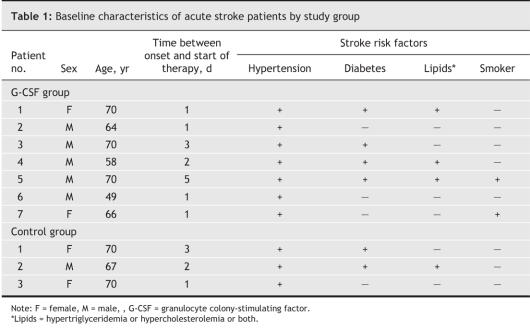
We recruited patients 35–75 years of age with a first episode of acute cerebral infarction localized in the middle cerebral artery territory as confirmed by MRI who presented to the emergency department within 7 days of onset. Patients were eligible if they were between 35 and 75 years of age and had a score of between 9 and 20 on the NIHSS. We excluded patients with other intracranial pathologies (e.g., tumours, infection); a history of major bleeding requiring blood transfusion or of leukopenia, thrombocytopenia, or hepatic or renal dysfunction; or evidence of malignant disease. We also excluded patients who were pregnant or nursing.
After giving informed consent to the study, patients were randomly assigned to receive G-CSF therapy or usual care. Randomization was carried out in the emergency department in the following manner: the clinician responsible for the patient selected an opaque envelope that contained the treatment assignment. The envelopes were prepared by one person who was responsible for treatment assignment and was unfamiliar with the patients.
Following enrollment, patients were assessed with a medical history, review of current medication and physical and neurologic examination. We determined stroke symptoms using an inhospital questionnaire that included questions about lifestyle before symptom onset and the acute or rapid progressive onset of symptoms such as limb weakness and slurred speech. To determine stroke severity we used the NIHSS administered at hospital admission (scores range from 0 to 42, with higher scores indicating increasing severity) as well as the ESS (scores range from 0 to 100, with lower scores indicating increasing severity), EMS (scores range from 0 to 58, with lower scores indicating increasing severity) and BI (scores range from 0 to 100, with lower scores indicating increasing severity).16,17 Blood samples were obtained for routine laboratory tests, including complete blood counts and serum cytokine and C-reactive protein levels. Neuroimaging studies consisting of the MRI scan taken at hospital admission and fluorodeoxyglucose PET (FDG-PET) were used to ascertain intracranial vascular condition.
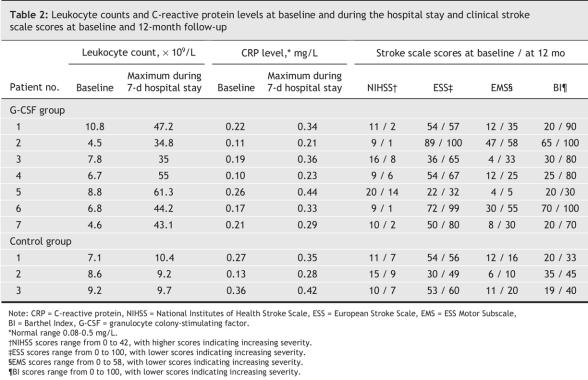
After all of the data had been recorded, patients assigned to receive G-CSF were given 15 μg/kg G-CSF (Filgrastim, Kirin, Japan) subcutaneously for 5 consecutive days. Other routine medications, such as fluid supplements and antiplatelet or anticoagulant agents, were given to all of the study participants unless contraindicated. Patients with hypertension were given antihypertensive drugs at the discretion of their physician. Leukocyte counts were measured daily from blood samples. One week after admission, neurologic function was assessed again in all of the participants using all 4 clinical stroke scales, and each patient was then discharged.
After discharge, all of the patients were followed up every 1–3 months in the out-patient department for one year. At each visit, any adverse events following G-CSF administration were recorded, and the results of NIHSS, ESS, EMS and BI assessments were examined to complete a formal chart. Standard laboratory tests were also conducted, and MRI and PET scans were taken at the 6-month visit. An independent safety committee from Tzu-Chi Hospital, Hualien, Taiwan, monitored the results of the trial. All clinical information and evaluation data from each patient were assessed by blinded data recorders and clinicians.
At 12 months, each patient also underwent FDG-PET to examine the metabolic activity of the brain neurons and MRI to investigate the abnormality or injury of the cerebral structures. FDG-PET was performed with a whole-body PET scanner in 3-dimensional mode (septa retracted) using a Discovery LS PET-CT System (GE Healthcare, Waukesha, Wis.). The overall examination procedure was the same as that described in a previous study.5 Patients received a single dose of FDG (7 mCi) intravenously, and emission scanning was performed for a total of 30 minutes beginning 40 minutes after injection. The data from PET were corrected for radioactive decay and attenuation by transmission, reconstructed with zoom at 2.5 and Hanning at 0.5, and then summed.
MRI was performed using an EXCITE 3T (GE Healthcare, Waukesha, Wis.) at 3.0 Tesla. The entire examination procedure was the same as that described in a previous study.18 The patient was supported on a wooden cradle, and his or her head was placed in a human head coil with an outer diameter of 5 cm. After the acquisition of scout images, 6–8 coronal plane images were taken between 3 mm behind the olfactory bulb and the caudal portion of the cerebellum. The slices were each 2 mm thick without any gaps. MRI to determine where the stroke was localized was performed when patients were first admitted to hospital; this scan included axial T1-weighted, T2-weighted and diffusion-weighted image and angiography. T2-weighted MRI data were spatially registered to the image generated by PET using automated image registration19 and were used to guide region-of-interest (ROI) analysis of the data from PET.
To semiquantitate FDG uptake, the data generated by PET and fused with MRI were assessed by a senior neuroradiologist (C.C.L.) using hand-drawn MRI-guided ROI analyses generated with software made available over the Internet (FSL, Oxford University; MRIcro and Image J, National Institutes of Health). The examination procedure described in an earlier study5 was used with some modification. Briefly, a region surrounding the infarcted core was calculated using a 1.5-cm-thick circumferential ROI analysis. For regional sampling of semiquantitative radioactivity, the ROI ipisilateral data were mirrored to the contralateral data to produce a radioactivity ratio. After correcting for the difference between the ipsilateral and contralateral hemispheres of the brain through computer calculation using applied software (FSL, Oxford University; MRIcro and Image J, NIH), the PET scans taken at 12 months were subtracted from the baseline PET scans to highlight those areas of the brain in which recovery had occurred.
The 12-month scores from the NIHSS, ESS, EMS and BI were used to assess treatment efficacy. Improvement was defined as the percentage change in mean group scores between baseline and 12 months. Statistical analyses were performed after all of the participants had completed the 12-month evaluation. The scale scores were analyzed in terms of percentage of recovery in neurologic deficits, which were calculated as the mean and standard deviation (SD) from baseline for each efficacy variable. Data showing normal distribution were analyzed using a Student t test (2-sided), whereas data not meeting this criterion were examined with nonparametric Wilcoxon 2-sample techniques. Comparison between groups was performed with Bonferroni-corrected ANOVA testing. Simple linear correlation analysis was used to investigate the relation between regional metabolic activity surrounding the infarction core under FDG-PET and the EMS score relative to baseline. Statistical significance was assumed at a value of p less than 0.05.
The Institutional Review Board of the Buddist Tzu-Chi Hospital, Hualien, Taiwan, approved the study protocol, and the study was conducted in accordance with the Declaration of Taiwan.
Results
A total of 10 patients were enrolled in the study, of whom 3 received usual care and 7 G-CSF therapy (Fig. 1). There were no losses to follow-up over the study period. There was no significant difference in baseline characteristics between the 2 groups (Table 1). Fig. 2 shows the baseline MRI scans of all of the study participants.
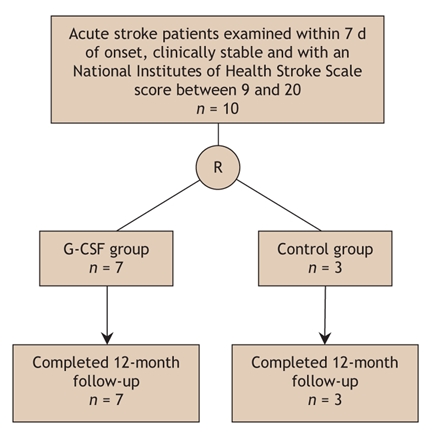
Fig. 1: Flow of participants through the study.
Table 1
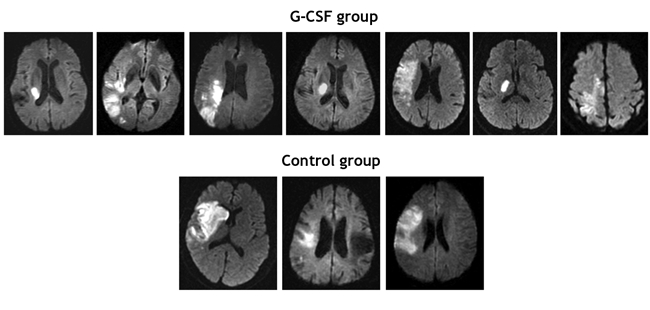
Fig. 2: Diffusion-weighted MRI scans at baseline of patients in the G-CSF (n = 7) and control (n = 3) groups.
Of the 7 patients receiving G-CSF, 3 reported headache, bone pain and transient impairment of liver function during the 3 days before and after the period of G-CSF administration. All of the patients completed the course of therapy, and the adverse reactions stopped after therapy ended. We observed no aggravations of stroke symptoms during the course of therapy or 7-day hospital stay. G-CSF successfully mobilized peripheral blood stem cells, as shown by elevated leukocyte counts, but there was no change in inflammation, as indicated by elevated C-reactive protein levels (Table 2). During the periprocedural and G-CSF injection periods, no patients receiving G-CSF experienced a thrombosis complication.
Table 2
We observed no aggravation of limb weakness, speech impediment or sensory impairment during the 12-month follow-up in either study group.
At 12-month follow-up, patients who had received G-CSF injections showed significant improvement in neurologic function according to the clinical scales (Table 2, Table 3, Fig. 3.) The mean EMS score in the G-CSF group showed a significant increase over that of the control group from the sixth month after treatment (Fig. 4).
Table 3
Fig. 3: Percent change in mean stroke scale scores between baseline and 12-month follow-up: (A) NIHSS, (B) EMS, (C) ESS, (D) BI. *p < 0.05.
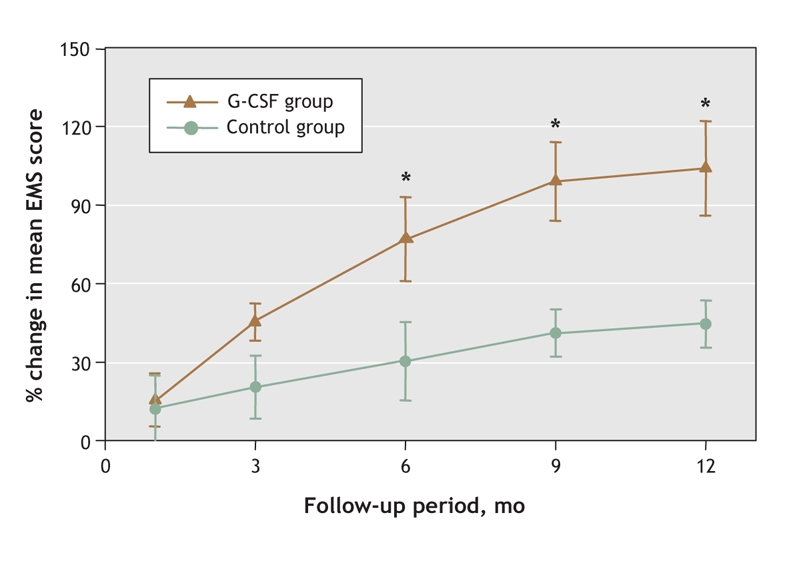
Fig. 4: Changes in EMS score in the G-CSF and control groups between baseline and 12 months. *p < 0.05.
MRI scans revealed no anatomic or structural changes, including cerebral hemorrhage, between baseline and 12-month follow-up in the brains of patients who received G-CSF therapy. There was no significant difference between the 2 groups (p ≥ 0.24) with regard to infarction size at baseline and at 12-month follow-up.
At baseline, FDG-PET revealed marked localized hypometabolism in both study groups that corresponded to the infarcted regions shown by MRI. At 12-month follow-up, there was no significant difference between the 2 study groups in the uptake of FDG in core stroke areas (Fig. 5). However, the lateral-to-contralateral ratio of uptake activity in the surrounding stroke area was significantly higher in the G-CSF group than in the control group (0.45% [SD 0.22%] v. 0.23% [SD 0.12%]) (p < 0.05). Fig. 6 shows significant correlation between the glucose metabolic activity surrounding the stroke area and the EMS score.
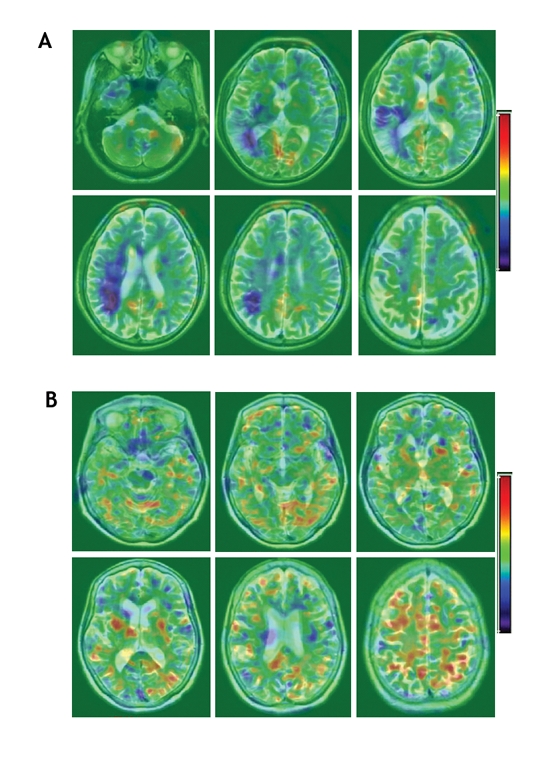
Fig. 5: Fusion images of MRI and PET at 12-month follow-up; (A) are of a G-CSF patient and (B) of a control patient. The images show successive views from the base of the skull (top left) to the vertex (bottom right). Areas of increasing metabolic activity, indicated by yellow and red colour, are distributed around the infarcted core, which is indicated by deep blue. Differences between the ipsilateral and contralateral hemispheres of the brain have been corrected.
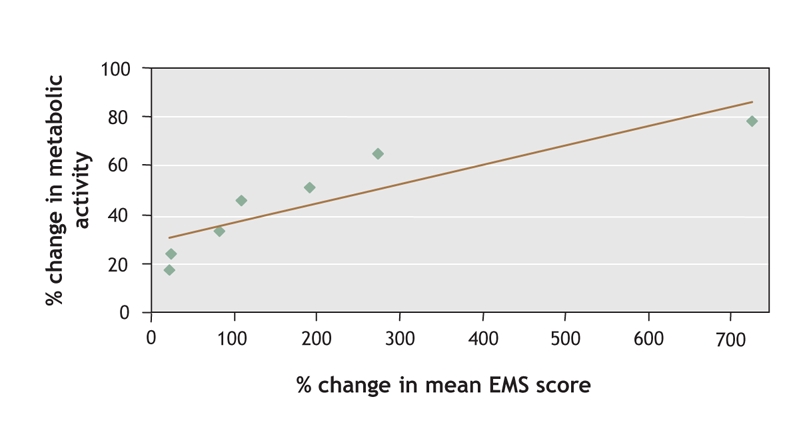
Fig. 6: Correlation between PET-generated data and clinical neurologic analysis. The relation between the ratio of fluorodeoxyglucose radioactivity in regions surrounding the infarction core and mean European Stroke Scale Motor Subscale (EMS) score was significant in the G-CSF group (r = 0.78; p = 0.01).
Interpretation
The results of our study show the feasibility of giving patients subcutaneous G-CSF therapy within 7 days of the onset of acute ischemic stroke symptoms. G-CSF therapy did not aggravate either inflammation or ischemia, and there were no consistent or clinically significant differences in blood or laboratory test results or urinalysis between the 2 study groups during the treatment period. Further, this study also provides preliminary data on the efficacy of G-CSF therapy in improving stroke-related neurologic deficits. At 12-month follow-up, the mean stroke severity scores of patients given G-CSF were significantly improved over those of patients who received usual care. FDG-PET neuroimaging studies verified greater recovery in the G-CSF group. All measurements were consistent in identifying a trend toward improved neurologic functional recovery in the G-CSF group.
Despite this success, it should be noted that this was a preliminary study and, because of the small number of participating patients, any inferences are tentative. In the future, we plan to conduct larger, double-blinded, placebo–control randomized studies to better evaluate the effect of G-CSF in patients with acute stroke.
In animal studies of cerebral ischemia, G-CSF exerts several functions, including neuroprotection, anti-inflammation, angiogenesis and mobilization of bone marrow-derived stem cells to the peripheral blood.14,15,20,21 Hence, G-CSF not only enables the penumbric neurons to survive, but also generates “new” neural tissue formation by mobilizing stem cells to reconstruct the neural circuits. According to this evidence, we can speculate that G-CSF, used either alone or in combination with another agent, should be an effective strategy in the treatment of cerebral infarction.
In this study, we have shown a relative increase in metabolic activity in the region surrounding infarction after G-CSF therapy. We postulate that this observed increase indicates enhanced cellular activity of the surviving neurons over the penumbric area “protected” by G-CSF. G-CSF may exert this neuroprotection through its neuronal receptor.14 On the basis of the results of our animal study,15 the increase in glucose metabolic activity over the peri-infarcted brain region of patients given G-CSF might be ascribed to new neural circuit formation generated by the homing of stem cells. In fact, this neuroplastic effect may have been induced by G-CSF-stimulated mobilization of stem cells from bone marrow, and their subsequent homing to the ischemic brain region. This theory of mobilization and homing prompted by G-CSF was suggested in our previous report.15 PET-generated measurements of cerebral metabolism suggest enhanced functional activity in the brain tissue surrounding the infarction region of patients receiving G-CSF.
Of the 7 patients given G-CSF therapy, 4 started treatment one day after the onset of cerebral ischemia. These 4 patients showed greater improvement as measured by clinical stroke scale scores than the other 3 patients in the intervention group, who received G-CSF later than one day after onset. Further clinical investigation is needed to see whether starting therapy within one day of infarction is associated with greater recovery in neurologic function.
In summary, the results of our study show an improvement in neurologic outcome among patients given G-CSF therapy according to both clinical stroke scale measurements and FDG-PET imaging studies. Further randomized, double-blinded and placebo-controlled trials are needed to confirm these preliminary findings.
@ See related article page 954
Acknowledgments
We thank Dr. K. Deen for his critical reading of this manuscript. This work was supported in part by research grants from the Chen-Han Foundation for Education, Academia Sinica (AS92IMB3) and the National Science Council (NSC93-2314-B-303-009).
Footnotes
Published at www.cmaj.ca on Mar. 3, 2006.
Editor's take
• Current treatment for ischemic stroke has a narrow therapeutic window for use. Therapies that mobilize endogenous stem cells have shown promise in animal research because of their anti-inflammatory, neuroprotective and angiogenic properties.
• In this pilot study, acute stroke patients who received G-CSF therapy showed greater improvement in neurologic functioning after 6 months than patients who received usual care, as shown by mean clinical stroke scale scores and metabolic activity measured by PET.
Implications for practice: G-CSF therapy shows promise as therapy for ischemic stroke; this needs to be confirmed with larger randomized studies.
This article has been peer reviewed.
Contributors: Woei-Cherng Shyu and Hung Li were responsible for the study concept and design and statistical analysis. Study supervision was undertaken by Shinn-Zong Lin and Hung Li. Woei-Cherng Shyu, Hung Li and Shinn-Zong Lin were responsible for the acquisition of data, and all of the authors were responsible for the analysis and interpretation of data. Shinn-Zong Lin, Chau-Chin Lee and Hung Li drafted the manuscript. All of the authors revised the manuscript for important intellectual content and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Hung Li, Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan; hungli@ccvax.sinica.edu.tw, and Dr. Woei-Cherng Shyu, Neuro-Medical Scientific Center, Tzu-Chi Buddhist General Hospital, Tzu-Chi University, Hualien, Taiwan
REFERENCES
- 1. Van Gijn J, Dennis MS. Issues and answers in stroke care. Lancet. 1998;352 Suppl 3:SIII23-7. [DOI] [PubMed]
- 2.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598-605. [DOI] [PubMed]
- 3.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 2003;92(6):692-9. [DOI] [PubMed]
- 4.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 2004;63:84-96. [DOI] [PubMed]
- 5.Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant 2001;10:31-40. [PubMed]
- 6.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000;55:565-9. [DOI] [PubMed]
- 7.Meltzer CC, Kondziolka D, Villemagne VL, et al. Serial [18F] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. [discussion 591-2]. Neurosurgery 2001;49:586-91. [DOI] [PubMed]
- 8.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood 1991;78:2791-808. [PubMed]
- 9.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 2001;98:10344-9. [DOI] [PMC free article] [PubMed]
- 10.Weaver CH, Buckner CD, Longin K, et al. Syngeneic transplantation with peripheral blood mononuclear cells collected after the administration of recombinant human granulocyte colony-stimulating factor. Blood 1993;82:1981-4. [PubMed]
- 11.Gorgen I, Hartung T, Leist M, et al. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol 1992;149:918-24. [PubMed]
- 12.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 1999;19:819-34. [DOI] [PubMed]
- 13.Wang PY, Kao CH, Mui MY, et al. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke 1993;24:236-40. [DOI] [PubMed]
- 14.Schabitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 2003;34:745-51. [DOI] [PubMed]
- 15.Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation 2004; 110:1847-54. [DOI] [PubMed]
- 16.D'Olhaberriague L, Litvan I, Mitsias P, et al. A reappraisal of reliability and validity studies in stroke. Stroke 1996;27:2331-6. [DOI] [PubMed]
- 17.Hantson L, De Weerdt W, De Keyser J, et al. The European Stroke Scale. Stroke 1994;25:2215-9. [DOI] [PubMed]
- 18.Lie C, Hirsch JG, Rossmanith C, et al. Clinicotopographical correlation of corticospinal tract stroke: a color-coded diffusion tensor imaging study. Stroke 2004;35: 86-92. [DOI] [PubMed]
- 19.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993;17:536-46. [DOI] [PubMed]
- 20.Willing AE, Vendrame M, Mallery J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant 2003;12:449-54. [DOI] [PubMed]
- 21.Six I, Gasan G, Mura E, et al. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol 2003;458:327-8. [DOI] [PubMed]