Abstract
The molecular chaperone Hsp104 is not only a key component of the cellular machinery induced to disassemble aggregated proteins in stressed cells of Saccharomyces cerevisiae but also plays an essential role in the propagation of the [PSI+], [URE3], and [RNQ/PIN+] prions in this organism. Here we demonstrate that the fungal pathogen Candida albicans carries an 899-residue stress-inducible orthologue of Hsp104 (CaHsp104) that shows a high degree of amino acid identity to S. cerevisiae Hsp104 (ScHsp104). This identity is significantly lower in the N- and C-terminal regions implicated in substrate recognition and cofactor binding, respectively. CaHsp104 is able to provide all known functions of ScHsp104 in an S. cerevisiae hsp104 null mutant, i.e., tolerance to high-temperature stress, reactivation of heat-denatured proteins, and propagation of the [PSI+] prion. As also observed for ScHsp104, overexpression of CaHsp104 leads to a loss of the [PSI+] prion. However, unlike that of ScHsp104, CaHsp104 function is resistant to guanidine hydrochloride (GdnHCl), an inhibitor of the ATPase activity of this chaperone. These findings have implications both in terms of the mechanism of inhibition of Hsp104 by GdnHCl and in the evolution of the ability of fungal species to propagate prions.
The molecular chaperone Hsp104 was originally identified in the yeast Saccharomyces cerevisiae as a high-molecular-weight, stress-inducible protein that was not essential for survival at either normal or elevated growth temperatures but was essential for the induction of acquired thermotolerance (33, 34). Hsp104 is also one of a number of molecular chaperones induced by heat shock whose primary role is to disassemble and remodel cellular proteins that have misfolded and aggregated in heat-stressed cells (31). This activity requires at least two other cellular chaperones, namely, Hsp70 (Ssa1) and Hsp40 (Ydj1), to resolubilize protein aggregates and remodel proteins to their native biological conformations (13). In this respect, Hsp104 plays a similar role to its Escherichia coli orthologue, ClpB (26).
Hsp104 is classed as a member of the AAA (ATPases associated with a variety of cellular activities) family of proteins because it has both sequence and functional similarities to other members of this relatively diverse group of cellular proteins (15). A property common to all members of the AAA family is the ability to bind and hydrolyze ATP, which in turn drives the unfolding or disassembly of either protein-protein complexes or protein-DNA complexes. In this role, many of the AAA proteins form oligomers, and in most cases, including that of Hsp104, these are symmetrical ring-like hexamers whose formation is essential for the ATPase-dependent “unfoldase” activity. AAA+ proteins can contain either one (class II) or two (class I) nucleotide binding domains (NBD) of approximately 220 residues, with each NBD containing the characteristic Walker A and B motifs. Both Hsp104 and ClpB are class I AAA+ proteins (15).
In addition to its well-established role in protein remodeling, Hsp104 also plays an essential role in the propagation of all three known yeast prions, [PSI+], [URE3], and [RNQ/PIN+] (20). This intriguing additional dimension to the cellular role played by Hsp104 was first uncovered by the discovery that the [PSI+] prion form of the translation termination factor Sup35p could not be maintained in an hsp104 null mutant (7). Furthermore, overexpression of Hsp104 in a [PSI+] strain also resulted in the loss of the prion, even if Hsp104 was only transiently overexpressed (7, 30). Evidence for Hsp104's role in prion propagation has come from the finding that treating [PSI+] cells with the protein denaturant guanidine hydrochloride (GdnHCl) leads to a loss of the [PSI+] prion (42) as a consequence of inhibition of Hsp104 function (12, 14, 22, 23).
Several models have been proposed to explain the role of Hsp104 in yeast prion propagation, with the most plausible being that Hsp104 (presumably in conjunction with Hsp70 and Hsp40) acts on the highly aggregated dead-end form of the prion protein to generate low-molecular-weight oligomeric forms of the protein that can seed new rounds of protein polymerization (24). We now refer to the seeds as propagons (9). A lack of Hsp104 activity would therefore lead to a failure to generate new propagons, while overexpression of Hsp104 most likely results in complete disaggregation of the prion aggregates back to their soluble form without any seeding activity (24). Consistent with this model is the report that inactivation results in an increase in the size of Sup35p-green fluorescent protein aggregates (46). A recent in vitro polymerization study of the Sup35p NM region (39) is consistent with this model, although this study also suggested that Hsp104 may be required for the de novo formation of small Sup35p oligomers necessary for the nucleation of new Sup35p fibrils. It is clear, however, that Hsp104 is not required for newly synthesized Sup35p to enter polymers in cells (29).
In this paper, we report a functional analysis of an Hsp104 orthologue from the pathogenic yeast species Candida albicans. This species is closely related to S. cerevisiae and encodes orthologues of the S. cerevisiae prion proteins Sup35p (CaSup35p) (32, 35) and Ure2p (CaUre2p) (4, 11). The native CaSup35p protein is unable to establish a prion-like state in S. cerevisiae (32, 35). However, when the N-terminal region of ScSup35p is replaced with the equivalent region from CaSup35p, the chimeric protein is able to convert to an aggregated prion state but is not able to transmit this property to wild-type ScSup35p (35). A form of species barrier to prion transmission therefore exists in fungi (35). Nevertheless, as we show here, the CaHsp104 protein is able to propagate the prion form of ScSup35p in S. cerevisiae, indicating that C. albicans has the key cellular component necessary for prion propagation.
MATERIALS AND METHODS
Bacterial and yeast strains.
For plasmid amplification and construction, the Escherichia coli strain DH5α (14a) was used. Four different strains of S. cerevisiae were used for this study, as follows. The 74D-694 strain has the genotype MATα ade1-14 trp1-289 his3Δ-200 ura3-52 leu2-3,112 [PSI+] (7). Weak and strong [PSI+] variants of this strain were provided by Eric Fernandez-Bellot (University of Kent). The BSC783/4a strain has the genotype MATa SUQ5 ade2-1 his3-11,15 leu2-3,112 ura3-1 [PSI+]. The BSC783/4aΔhsp104 strain is strain BSC783/4a with an hsp104::HIS3 disruption and consequently is [psi−]. The YJW532 strain has the genotype MATa ade1-14 his3-11,15 leu2-3,112 ura3-1 trp1-1 can1-100 hsp104::HIS3 (p316HpHSP104) [PSI+] [PIN+] and was a kind gift from L. Osherovich and J. Weissman, University of California at San Francisco. E. coli was grown in standard Luria broth (LB). For growth of the various yeast strains, standard growth media were used, and cells were routinely cultured at 30°C as previously described (29). To ensure plasmid retention, transformed cells were grown on a glucose-based synthetic medium (YNBD) containing all necessary supplement combinations (ForMedium, Norwich, United Kingdom). When required, 3 mM GdnHCl was added to yeast extract-peptone-dextrose (YEPD) medium, but this was increased to 5 mM in YNBD. The [PSI+]-mediated suppression of the ade1-14 marker was routinely assessed by the color of colonies formed on 1/4YPD medium (YEPD but with 2.5 g/liter yeast extract rather than 10 g/liter) and confirmed on YNBD-adenine defined medium supplemented with 2.5% (vol/vol) YEPD. The growth conditions used to induce the GAL1 promoter were as previously described (29).
DNA transformation.
Plasmid DNAs were introduced into E. coli by using the standard CaCl2 transformation method (8). Yeast cells were transformed with plasmid DNA using the whole-cell lithium acetate transformation method, essentially as described by Ito et al. (18).
Cloning and sequencing of C. albicans HSP104 gene.
Using the S. cerevisiae HSP104 gene sequence (EMBL accession no. M67479), the C. albicans genome database was interrogated using the BLASTN (3) search engine. A single contig (4-2922) was identified that contained a gene whose open reading frame (ORF) encoded a protein showing significant sequence identity to the S. cerevisiae Hsp104 protein sequence. Two oligonucleotides (5′-ATAAAGAATGCGGCCGCCTACCGCATACAAGTGAC-3′ and 5′-CATTCTTACGCCGGCGCTTAACTCATTGGCGTCC-3′) were designed and used in a high-fidelity PCR to amplify a 3.71-kb DNA fragment from the genomic DNA of C. albicans strain 2005E. The Roche Expand High Fidelity PCR system was used with an assay volume of 50 μl containing the following final concentrations or amounts of reagents: deoxynucleoside triphosphates, 200 μM (each); primers, 300 nM (each); template DNA, 0.75 μg; Mg2+ buffer, 3 mM; and Taq/Pwo polymerase, 2.6 U. The primers produced unique HpaII (5′) and NheI (3′) restriction sites at either end of the amplified sequence. The HpaII/NheI-digested PCR product was ligated to ClaI/XbaI-digested pRS416 to generate plasmid pUKC1845. This cloning strategy was repeated to generate additional, independently derived clones that were designated pUKC1846, pUKC1847, and pUKC1849.
Using various oligonucleotide primers (synthesized by MWG, Eisberg, Germany), both strands of the cloned CaHSP104 gene were sequenced (by MWG) from two independent clones, namely, pUKC1847 and pUKC1849, using the dideoxy chain termination method.
Plasmid construction.
A single-copy LEU2-based plasmid carrying the CaHSP104 gene was generated by digesting pUKC1847 with XhoI/NotI and ligating the product to XhoI/NotI-digested pRS315. The resulting plasmid was designated pUKC1857. Plasmid pUKC1828, a single-copy URA3-based plasmid carrying the ScHSP104 gene, was previously described (12). To construct GAL1-regulated CaHSP104, the coding sequence of the gene was amplified in three independent reactions by high-fidelity PCR using the primers 5′-GTCGGGATCCATCATGGAAGATTTTACAG-3′ and 5′-GGATCTCGAGGTTTAGTCAAGTCCAGGTG-3′, with plasmid pUKC1847 as the template. The resulting fragments were digested with XhoI/BamHI and ligated into similarly digested plasmid pYES2 (Invitrogen), which is a URA3-based multicopy plasmid carrying the GAL1 promoter. The DNA sequences of the amplified products were confirmed for the three plasmids generated, which were designated pUKC1859, pUKC1860, and pUKC1861. Plasmid pUKC1860 was used in the studies reported here. As a control, the S. cerevisiae HSP104 gene was expressed under the control of the GAL1 promoter, using the plasmid pUKC1832 (12).
Antibody preparation.
The peptide CDEDDDEARFTSPGLD, corresponding to the C-terminal 15 amino acids of C. albicans Hsp104p, was synthesized on a Shimadzu PSSM-8 multiple peptide synthesizer using a 9-fluorenylmethoxy carbonyl-based strategy. A cysteine residue (underlined) was added to the N terminus of the peptide to enable coupling to activated keyhole limpet hemocyanin (Calbiochem) carrier protein. The covalently conjugated protein was desalted on a Pharmacia PD-10 column (Sephadex G25), and the concentration of the peptide was determined using the Coomassie Plus protein assay (Pierce). An aliquot (400 μg) of the peptide was used for each immunogen dose, and the immunization schedule was as follows: primary immunization was done with 400 μg conjugate in Freund's complete adjuvant and was followed by boosters at 2 weekly intervals with 400 μg conjugate in Freund's incomplete adjuvant. Serum was obtained after the sixth injection, and Western blot analysis indicated that a 1:2,000 dilution of the unpurified serum was sufficient to detect a protein with the predicted molecular weight with minimal background.
Subcellular fractionation analysis.
Protein extracts were prepared by glass bead lysis in the presence of a cocktail of protease inhibitors (Roche) and were separated into soluble and high-molecular-weight fractions as previously described (29), using centrifugation (100,000 × g for 15 min at 4°C). Protein samples were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Immobilon-PSQ transfer membrane (Millipore). Western blot analysis was carried out using the following primary antibodies: rabbit anti-S. cerevisiae Hsp104 (a gift of S. Lindquist), used at a 1:5,000 dilution, and rabbit anti-C. albicans Hsp104 (obtained as described above), used at a 1:2,000 dilution. Antibody-antigen interactions were revealed using standard chemiluminescence techniques (ECL; Amersham-Pharmacia), utilizing a horseradish peroxidase-conjugated swine anti-rabbit antibody (DAKO P0217) at a 1:5,000 dilution.
Thermotolerance assay.
To assay cells' ability to survive at 50°C, a culture of the test strain was first grown in the appropriate defined medium to maintain plasmid selection to an optical density at 600 nm (OD600) of 0.4 to 0.6 at 30°C. The culture was then transferred to 37°C for 1 h, after which the cells were pelleted by centrifugation at 1,500 × g for 5 min at room temperature and then resuspended in an equal volume of fresh medium prewarmed to 50°C. The culture was maintained at 50°C in a water bath for the duration of the experiment. Cell aliquots were then removed every 5 min, diluted as required, and plated onto YEPD plates to determine the numbers of CFU.
Luciferase refolding assay.
The relevant strains were transformed with the plasmid pGPDLuxAB(HIS), which expresses a temperature-sensitive Vibrio harveyi luciferase (31). The transformed cells were grown in appropriate selective medium to an OD600 of approximately 0.2, and the cells were then pelleted and transferred to fresh YEPD, after which the culture was incubated until an OD600 of ∼0.4 was reached. The culture was then transferred to 46°C, and after 10 min of incubation at this temperature, cycloheximide was added to a final concentration of 10 μg/ml. The culture was then incubated for a further 10 min, after which the cell culture was transferred back to 25°C to allow the cells to recover. Cell samples were taken immediately to determine the level of luciferase activity and then collected every 30 to 45 min for up to 4 h. The luciferase activity was determined by using 300 μl cells plus 5 μl decylaldehyde (Sigma), and the resulting luminescence was immediately quantified using a Bio Orbit 1253 luminometer. Three independent samples were taken per time point.
Nucleotide sequence accession numbers.
The CaHSP104 gene sequences in pUKC1847 and pUKC1849 were deposited in the EMBL database under accession numbers AF362390 and AF362391, respectively.
RESULTS
Candida albicans carries an orthologue of the Saccharomyces cerevisiae HSP104 gene.
The complete DNA sequence of the coding region of the S. cerevisiae HSP104 gene (accession no. M67479) was used to search the C. albicans genome database (19). A match was found to a partial sequence on the C. albicans 17.7-kb genomic contig 4-2922, and the ORF Finder program (NCBI) was used to locate the ORF in the putative HSP104 orthologue. The sequence was amplified from C. albicans strain 2005E by PCR, and a 3,120-bp region corresponding to the coding region and 178 bp upstream and 242 bp downstream of the ORF was sequenced. Two different DNA sequences (CaHSP104-1 [EMBL accession no AF362390] and CaHSP104-2 [EMBL accession no. AF362391]) were obtained that differed at five positions within the ORF and gave rise to two polymorphisms at the protein sequence level, namely, Lys96/Arg96 and Asn427/Ser427. Independent PCRs and sequencing were used to confirm that these sequence differences represented natural polymorphisms at the CaHSP104 locus. C. albicans is a diploid organism and shows heterozygosity at many loci (21).
The predicted 899-residue sequences of CaHsp104-1 and CaHsp104-2 showed 64% overall identity to the 908-residue S. cerevisiae Hsp104 protein (ScHsp104), although the N- and C-terminal domains showed significantly lower identities (49.3% and 47.6%, respectively) (Fig. 1A). The two nucleotide binding domains, NBD1 and NBD2, and the mobile linker region between these two domains all showed between 70 and 72% identity. Apart from complete conservation of the Walker A (GxxxxGKT) and Walker B (hhhhDE) motifs, other regions implicated in ATP binding and hydrolysis were also conserved in CaHsp104, particularly the sensor-1 and sensor-2 regions and the associated sensor and substrate discrimination motif originally described by Smith et al. (40) (Fig. 1B). These regions are highly conserved in evolution and are found across a wide evolutionary range of members of the Hsp104/ClpB family, including the Arabidopsis Hsp101 protein (Fig. 1B).
FIG. 1.
The putative Candida albicans HSP104 gene encodes an 899-residue protein that contains all of the key sequence features of the S. cerevisiae molecular chaperone Hsp104. (A) Domain organization of S. cerevisiae Hsp104 (ScHsp104) and C. albicans Hsp104 (CaHsp104) proteins and % amino acid identity at the domain level between the two proteins. The domains were identified by sequence comparison with the Escherichia coli ClpB protein sequence and structure (25). The residues that define the approximate positions of these domains are indicated for ScHsp104 (above) and CaHsp104 (below). (B) Alignment of amino acid sequences of putative Hsp104 orthologues from fungi and Arabidopsis thaliana. Three regions implicated in ATP binding or hydrolysis are shown, with invariant residues boxed. The sequences were obtained from GenBank, and the species are as follows: Sc, Saccharomyces cerevisiae; Sb, Saccharomyces bayanus; Spx, Saccharomyces paradoxus; Sm, Saccharomyces mikatae; Sct, Saccharomyces castellii; Sk, Saccharomyces kudriavzevii; Ca, Candida albicans; Spm, Schizosaccharomyces pombe; Nc, Neurospora crassa; and At, Arabidopsis thaliana. (C) The C-terminal 20 residues of members of the Hsp104 family, including CaHsp104, contain large proportions of the acidic residues D and E (in bold) and two highly conserved residues (LD; boxed) at the extreme C terminus.
The extreme C terminus of CaHsp104 had only 12/41 residues identical to those of ScHsp104, but the last 20 residues, like all members of the Hsp104/ClpB family, showed a strong bias towards acidic residues (D/E) (Fig. 1C). The two C-terminal residues (LD) of CaHsp104 were identical to those in Hsp104 proteins from a number of closely related yeast species (Fig. 1C). The putative Hsp104 orthologue in C. albicans therefore has all the sequence features consistent with it being the functional orthologue of ScHsp104.
The CaHSP104 gene is induced by heat shock in C. albicans.
In Saccharomyces cerevisiae, HSP104 expression is strongly induced by a variety of stresses, including heat shock and entry into stationary phase (33). This transcriptional response requires two specific cis-acting DNA sequence elements, the AGGG stress element (STRE) and a canonical heat shock element (27, 38). Both elements are present within the first 500 bp of DNA upstream of the CaHSP104 coding sequence, with a single STRE element (AGGGG) present at positions 142 to 146 and two heat shock elements (GAATCATCC [positions 382 to 387] and GAAGGTTCC [positions 263 to 271]).
To assess whether CaHsp104 protein levels increased in heat-stressed cells, a polyclonal antibody was raised against the C-terminal 15 residues of CaHsp104, one of the most highly divergent regions between CaHsp104 and ScHsp104 (Fig. 1). This antibody recognized a protein of the expected molecular weight in total C. albicans extracts which was not recognized by an anti-ScHsp104 polyclonal antibody raised against a C-terminal peptide of ScHsp104 (Fig. 2). The level of the CaHsp104 protein increased in cells in exponential-phase growth when they were switched from 30°C to 37°C for 1 hour, with the level of induction being similar to that seen for ScHsp104 in similarly heat-shocked S. cerevisiae cells (Fig. 2). Furthermore, introduction of the CaHSP104-1 gene into S. cerevisiae on the single-copy plasmid pUKC1847 resulted in the synthesis of a protein with the same electrophoretic mobility as the protein detected by the anti-CaHsp104 antibody in C. albicans cells. Although the intensity of the signal obtained would suggest that there was a higher level of CaHsp104 than the endogenous ScHsp104 levels, since different antibodies were used to detect the two proteins it cannot be concluded that this difference reflects real differences in the numbers of the respective Hsp104 molecules.
FIG. 2.
CaHsp104 is encoded by a heat shock-inducible gene in Candida albicans. (A) Locations of key motifs important for stress-inducible gene transcription in the CaHSP104 promoter. The positions of motifs shown are relative to the A of the ATG codon. (B) Analysis of Hsp104 levels in C. albicans and S. cerevisiae before and after a 1-h heat shock at 37°C. Total protein extracts were separated by SDS-PAGE. Left, C. albicans strain 2005E, probed with anti-CaHsp104 antibody; middle, S. cerevisiae strain BSC783/4a, probed with anti-ScHsp104 antibody (top panel) or with anti-CaHsp104 antibody (bottom panel; the same filter shown in the top panel was stripped and reprobed); right, S. cerevisiae strain BSC783/4a transformed with plasmid pUKC1847, probed with anti-CαHsp104 antibody.
Functional complementation analysis of CaHsp104 in Saccharomyces cerevisiae.
In S. cerevisiae, Hsp104 is important for cell survival of heat-stressed cells (34) and for refolding and reactivating heat-damaged and aggregated proteins (31). Using well-established assays, we examined whether CaHsp104 was able to replace ScHsp104 with respect to these two major functional properties in S. cerevisiae.
The CaHSP104-1 gene was ligated into the plasmid pRS416 to generate the plasmid pUKC1847, and this plasmid was then introduced into strain BSC783/4a carrying an hsp104::LEU2 disruption. The ability of the transformed cells to survive severe heat stress was compared, in a number of independent experiments, to those of the nontransformed hsp104::LEU2 parent strain (Fig. 3A) and cells transformed with the plasmid pUKC1828, which carries the wild-type ScHSP104 gene (12). Both the pUKC1847 and pUKC1828 transformants had the ability to survive heat stress. Likewise, a comparative analysis of the abilities of these three strains to reactivate heat-inactivated luciferase [expressed from plasmid pGPD LuxAB(His)] (31) confirmed that CaHsp104 and plasmid-encoded ScHsp104 could restore the ability of the hsp104::LEU2 parent strain to reactivate the heat-denatured luciferase. This analysis therefore confirmed that CaHsp104 was also able to functionally replace endogenous Hsp104 in S. cerevisiae with respect to its role in thermotolerance (Fig. 3B).
FIG. 3.
CaHsp104 complements both the thermotolerance and protein reactivation properties of ScHsp104. (A) Strain BSC783/4aΔhsp104 (squares) was transformed with either the plasmid pUKC1847 (diamonds) that carries the CaHSP104 gene or pUKC1828 (triangles), a pRS316-derived plasmid carrying the ScHSP104 gene with its native promoter (12). Plating of cells onto YEPD was used to measure cell survival after exposure to 37°C for 60 min and then 50°C for 0 to 20 min. The resulting numbers of CFU were used to estimate % survival. (B) Reactivation of heat-denatured firefly luciferase was determined in BSC783/4aΔhsp104 cells (squares) and BSC783/4aΔhsp104 cells transformed with either pUKC1828 (triangles) or pUKC1847 (diamonds), as described in Materials and Methods. Three independent samples were assayed per time point, and the averages are shown.
Maintenance of [PSI+] prion by CaHsp104.
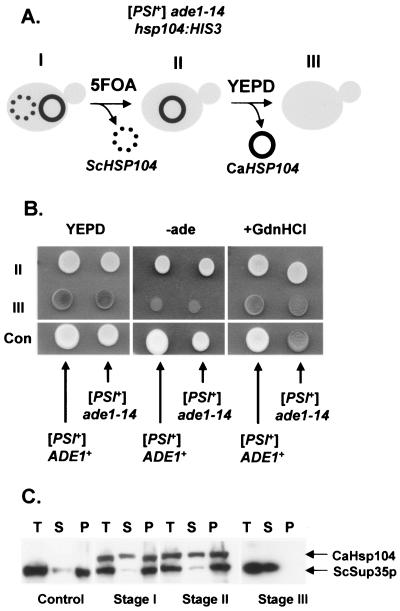
The continued propagation of the three yeast prions described so far, i.e., [PSI+], [URE3], and [PIN+/RNQ+], is absolutely dependent on ScHsp104. The propagation of the [PSI+] prion, but not the other two prions, is also perturbed when the levels of ScHsp104 are elevated in nonstressed cells (20). To determine whether this chaperone-specific function was also conserved between ScHsp104 and CaHsp104, we replaced the ScHSP104 gene with the CaHSP104-1 gene and assessed the consequences on [PSI+] propagation by using a plasmid shuffling strategy (Fig. 4A). For this assay, we used the ade1-14 hsp104::HIS3 strain YJW532, which is able to propagate the [PSI+] prion because it carries the URA3-based plasmid p316TpHSP104 that encodes wild-type ScHsp104. In addition, this strain contains the ade1-14 allele, which is suppressed in [PSI+] cells but not in [psi−] cells (7). The nonsuppressed [psi−] phenotype is readily detected because the cells form red colonies and are adenine auxotrophs, while [PSI+] suppressed cells form white prototrophic colonies (Fig. 4B). Transformation of YJW532(pRS316TpHSP104) with the plasmid pUKC1857 resulted in a mitotically stable [PSI+] strain expressing both CaHsp104 and ScHsp104. Ura− cells that only expressed CaHsp104 were selected using 5-fluoroorotic acid and gave mitotically stable [PSI+] Ade+ cells (Fig. 4B). As expected, these strains did not produce any ScHsp104, as defined by Western blot analysis (data not shown). The stage II cells all gave rise to colonies with pale pink coloration, indicative of a relatively strong [PSI+] phenotype similar to that of the parent strain. Subcellular fractionation analysis confirmed that >90% of the Sup35p protein was present in the high-molecular-weight fractions of cells from different stages of the shuffling experiment (Fig. 4C), consistent with the relative strength of the [PSI+] phenotype, i.e., strong [PSI+] variants have very low levels of Sup35p in the soluble fraction (43). CaHsp104 is therefore able to replace ScHsp104 to allow continued propagation of the S. cerevisiae [PSI+] prion. CaHsp104 was detected equally in both the soluble and high-molecular-weight fractions of these strains (Fig. 4C), a distribution also seen for ScHsp104 in normal [PSI+] strains using this fractionation protocol (data not shown).
FIG. 4.
CaHsp104 can mediate propagation of the [PSI+] prion in the absence of ScHsp104. (A) Plasmid shuffling strategy used to assess the ability of CaHsp104 (encoded by the plasmid pUKC1857) to mediate propagation of the [PSI+] prion. Stage I, double transformant expressing both ScHsp104 and CaHsp104; stage II, transformants which had lost the URA3-based plasmid expressing ScHsp104 (pRS316HpHSP104) were selected by growth on 5-fluoroorotic acid-containing medium; stage III, loss of the CaHsp104-expressing plasmid (pUKC1857) by growth on a rich medium (YEPD). (B) Phenotypes of transformants at stages II and III. Colony colors after growth on rich growth medium, in the absence of adenine (−ade), and on YEPD containing 3 mM GdnHCl (+GdnHCl) were determined. Two control strains (Con) are also shown for each assay. (C) Subcellular distribution of CaHsp104 and ScSup35p in [PSI+] cells expressing different combinations of CaHsp104 and/or ScHsp104, as indicated. Total protein extracts (T) were fractionated by ultracentrifugation, as described in Materials and Methods, into soluble (S) and pellet (P) fractions. After fractionation of the different samples by SDS-PAGE and transfer to a nitrocellulose filter, the filter was simultaneously probed with both anti-CaHsp104 and anti-ScSup35p, with relative protein positions indicated by arrows.
CaHsp104 function is not inhibited by guanidine hydrochloride in vivo.
The [PSI+] prion is readily eliminated from cells grown in 3 to 5 mM GdnHCl (42) as a consequence of inhibition of the ATPase activity of Hsp104 (12, 14, 22, 23). However, when [PSI+] prion- and pUKC1857-carrying cells were plated on rich medium containing 3 mM GdnHCl, there was no detectable loss of the [PSI+] prion observed other than through segregational loss of the pUKC1857 plasmid, i.e., no [psi−] Leu+ cells were detected. This level of GdnHCl normally results in a rapid loss of the [PSI+] prion from cells that only express ScHsp104 (Fig. 4B). Subsequent replating of the [PSI+] prion- and pUKC1857-carrying cells on 3 mM GdnHCl plates failed to generate any [psi−] Leu+ cells, suggesting that CaHsp104 function is not inhibited in vivo by GdnHCl.
Subcellular fractionation analysis of GdnHCl-treated cells revealed that there was a redistribution of Sup35p from the high-molecular-weight pellet fraction to the soluble fraction in cells expressing ScHsp104. However, such a redistribution was not seen in cells expressing CaHsp104 (Fig. 5B), consistent with the failure of GdnHCl to eliminate the [PSI+] prion in these cells. In both strains, Hsp104 was equally distributed between the soluble and pellet fractions in either the presence or absence of GdnHCl (Fig. 5B).
FIG. 5.
CaHsp104 activity is not inhibited in vivo by growth in the presence of GdnHCl. (A) Effects of GdnHCl on the ability of strain BSC783/4aΔhsp104 to withstand lethal heat stress at 50°C. For these experiments, the strain was transformed with either the plasmid pUKC1828 carrying the ScHSP104 gene (upper graph) or pUKC1847 carrying the CaHSP104 gene (lower graph). Cells were grown in the absence (•) or presence (▪) of 3 mM GdnHCl. (B) Subcellular distributions of CaHsp104 and ScSup35p in YJW532Δhsp104 [PSI+] cells expressing either ScHsp104 (left panels) or CaHsp104 (right panels). Cells were grown overnight in defined (selective) medium in the absence (upper panels) or presence (lower panels) of 5 mM GdnHCl. Total protein extracts (T) were fractionated by ultracentrifugation, as described in Materials and Methods, into soluble (S) and pellet (P) fractions. After fractionation of the different samples by SDS-PAGE and transfer to a nitrocellulose filter, the filter was simultaneously probed with both anti-CaHsp104 and anti-ScSup35p, with relative protein positions indicated by arrows.
To determine whether the [PSI+] prion maintained in the YJW532(pUKC1857) strain was eliminated by growth in the presence of 3 mM GdnHCl when the CaHSP104 gene was replaced with the ScHSP104 gene, we mated the YJW532(pUKC1857) strain to YJW509, a [psi−] ade1-14 strain of the opposite mating type. The resulting diploid showed the expected [PSI+] Ade+ white phenotype. Eight complete tetrads were obtained from this cross, and analysis of these spores confirmed that the [PSI+] prion was faithfully transmitted at meiosis to spores containing the endogenous ScHSP104 gene and to hsp104::HIS3 spores also carrying the plasmid pUKC1857. In hsp104::HIS3 spores lacking the plasmid pUKC1857, the [PSI+] prion was no longer maintained. Furthermore, the [PSI+] spores containing the ScHSP104 gene were cured of the [PSI+] prion by growth on 3 mM GdnHCl, consistent with the proposal that it was the CaHsp104 protein that was insensitive to GdnHCl and not the generation of an unusual form of [PSI+] prion that did not require Hsp104 for continued propagation. These data also confirm that ScSup35p propagons generated by CaHsp104 in the original YJW532(pUKC1857) strain could be transmitted through meiosis and continue to seed prion propagation in the absence of CaHsp104.
The ability of GdnHCl to inhibit thermotolerance in cells expressing just CaHsp104 was also examined. As previously reported (12, 23), the presence of 3 mM GdnHCl significantly inhibited survival after heat shock of [PSI+] cells expressing ScHsp104 (Fig. 5A), but for cells expressing only CaHsp104 (Fig. 3), no inhibition by GdnHCl was observed. Both the prion propagation and thermotolerance functions of CaHsp104 are therefore resistant to the inhibitory effects of GdnHCl, suggesting that these two functions work via a similar molecular mechanism.
Overexpression of CaHsp104 eliminates the [PSI+] prion.
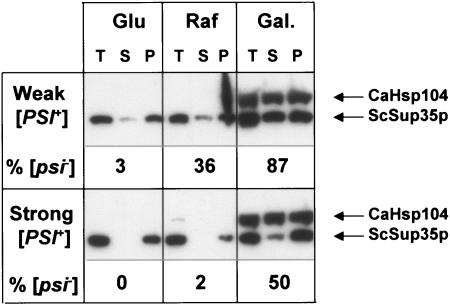
The [PSI+] prion is readily eliminated from cells by the overexpression of ScHsp104 (7), and this elimination is more rapid in cells with a weak variant of the [PSI+] prion (10). The CaHSP104-1 gene was therefore placed under the transcriptional control of the GAL1 promoter (plasmid pUKC1860) and introduced into both a weak and a strong variant of the [PSI+] strain 74D-694. For both strains, a significant loss of the [PSI+] prion was observed after growing cells for 10 to 12 generations following the transfer to galactose-based medium, with the greatest loss (87%) being observed with the weak [PSI+] variant (Fig. 6). In a parallel experiment in which ScHSP104 was overexpressed from the GAL1 promoter using the plasmid pUKC1832 (12), after eight or nine generations of growth, 97% of the weak variant cells were [psi−], while 22.7% of the strong variant cells were [psi−]. In the postinduction cells, there was also a significant increase in the amount of Sup35p found in the soluble fraction for both the weak and strong [PSI+] variants. Consequently, high levels of CaHsp104 in nonstressed cells also result in a failure to propagate the [PSI+] prion in S. cerevisiae, as observed for ScHsp104.
FIG. 6.
Overexpression of CaHSP104 leads to loss of the [PSI+] prion from growing cells. Weak (upper) and strong (lower) [PSI+] variants of strain 74D-694 were transformed with the plasmid pUKC1860 that expresses the CaHSP104 gene under the control of the galactose-inducible GAL1 promoter. Cells were initially grown overnight in glucose-based medium (Glu) before being switched to a preinduction medium containing raffinose (Raf) for a further one to three generations. Galactose (Gal) was then added, and the cells were incubated for a further eight to nine generations. Total extracts (T) were prepared from cells at each of the three stages, and the subcellular distributions of CaHsp104 and ScSup35p in the cells were analyzed as described in the legend to Fig. 5, looking at both soluble (S) and pellet (P) fractions. In addition, cells were taken from each stage and plated onto 1/4YPD to determine the % of cells that were prion-free (i.e., [psi−]), as indicated.
DISCUSSION
Although much has already been learned about the structure and function of Hsp104 in Saccharomyces cerevisiae and of its bacterial orthologue ClpB (15, 26), there have been few reports of functional orthologues of Hsp104 in higher eukaryotes. The exception is plants, as Hsp104 orthologues have been described for Arabidopsis thaliana (36) and rice (2). Both plant proteins show significant (i.e., >40%) amino acid identity to S. cerevisiae Hsp104 and restore thermotolerance to an S. cerevisiae hsp104 null mutant (2, 36), indicating that there is considerable evolutionary conservation of this key property of Hsp104. However, in spite of considerable scrutiny of databases, no orthologue of Hsp104 has been uncovered in flies, frogs, worms, or mammals, although there are at least two mammalian AAA+ proteins that share both structural and functional features with Hsp104, namely, p97/CDC48 (45) and torsin A (5). Intriguingly, when ScHsp104 was expressed in cultured human cells, it enhanced their survivability at high temperatures, implying that the yeast chaperone was able to deal with stress-induced protein aggregates within the context of a heterologous protein chaperone network (28), but which endogenous protein(s) is assigned this function remains to be verified.
All fungi for which annotated genome sequences are available carry Hsp104 orthologues, and these orthologues show high degrees of amino acid identity within the two nucleotide binding domains, NBD1 and NBD2 (Fig. 1A), but diverge to a much greater extent at both the N-terminal and C-terminal regions (data not shown). It is notable, however, that the extreme C-terminal region encompassing the last 20 residues contains a large proportion of charged (Asp/Glu) residues, including the Arabidopsis orthologue, where 10 of the last 20 residues are Asp or Glu. With the exception of Schizosaccharomyces pombe Hsp104, the C-terminal residue in plant and fungal Hsp104 proteins (including CaHsp104) is an Asp residue.
The interactions between AAA+ proteins and their substrate(s) are mediated largely through their N- and/or C-terminal regions, which either recognize the substrate directly or may bind adaptor proteins which in turn mediate substrate binding (47). Three Hsp90-binding cochaperones, Sti1p, Cpr7p, and Cnr1p, interact with Hsp104 in respiring cells via the acidic C terminus (1), but although both Sti1p and Cpr7p play a minor, nonessential role in [PSI+] propagation, this interaction appears to be mediated via their regulation of the substrate binding properties of Ssa1p (Hsp70) rather than Hsp104 (19). The extreme C terminus of CaHsp104 (FTSPGLD) contains a C-terminal Asp (underlined), but since CaHsp104 carries out all the known functions of ScHsp104, it is possible that Hsp104-cochaperone interactions may just require a single C-terminal acidic residue.
Comparison of the CaHsp104 and ScHsp104 sequences shows that the N and C termini show <50% amino acid identity, in contrast to the >70% amino acid identity for the NBD1-linker-NBD2 regions of the molecules (Fig. 1A). Yet since both the Ca- and ScHsp104 molecules are able to propagate the [PSI+] prion, the data suggest that heterologous Hsp104 is able to recognize the substrate—ScSup35p—to facilitate disaggregation of the ScSup35p polymer to generate Sup35p-based propagons. Previous structure-function studies have identified the importance of the conserved sequences, important for ATP binding and/or hydrolysis, in the prion propagation properties of Hsp104, including the sensor-1 and sensor-2 motifs (7, 16, 17). These sequences, including the Walker A and B boxes and the sensor-1 and sensor-2 motifs, are conserved between CaHsp104 and ScHsp104 (Fig. 1A and B). In addition, Schirmer et al. (37) have demonstrated that a region within the linker region of Hsp104 (TATAADLRYFA [residues 499 to 509 of the ScHsp104 sequence]) is crucial to the regulation of Hsp104 function, possibly facilitating interdomain communication (6). This sequence is in the CaHsp104 protein (residues 497 to 507) and is part of a stretch of 22 residues (residues 490 to 511) that shows 100% identity between CaHsp104 and ScHsp104. Whether this region is crucial for yeast prion propagation remains to be established.
The continued propagation of the [PSI+] prion depends on a critical level of ScHsp104, since either depletion or overexpression of ScHsp104 can lead to a loss of the prion from dividing cells (7, 46). In our analysis, a direct comparison of the levels of endogenous ScHsp104 versus CaHsp104 in the transformed strains was not possible because different antibodies were used to detect the two proteins. It is therefore not clear whether the continued propagation of the [PSI+] prion requires the same amount of CaHsp104 as ScHsp104, although overexpression of the CaHSP104 gene clearly perturbed the propagation mechanism (Fig. 6).
While three of the assayable functions of Hsp104 seem to be conserved, namely, maintenance of the [PSI+] prion, reactivation of denatured luciferase, and heat shock protection, between CaHsp104 and ScHsp104, there is one important difference: the function of CaHsp104 in vivo is not inhibited by millimolar concentrations of the protein denaturant GdnHCl (Fig. 4 and 5). Jung et al. (23) reported the isolation of an hsp104 mutant that was resistant to GdnHCl-induced elimination of the [PSI+] prion. This mutation was an Asp184Asn substitution, but the corresponding CaHsp104 residue (Asp182) is the same as that in wild-type GdnHCl-sensitive ScHsp104. GdnHCl inhibits the ATPase activity of Hsp104 in vitro by enhancing the binding of nucleotides to the chaperone in its hexameric form, and this in turn may lead to reduced ATP turnover (14). Such binding presumably occurs via a pocket within the hexameric form of the Hsp104 molecule that encompasses residue 184 in ScHsp104. One possibility is that the presence of an Asp residue at this position is not essential for such binding and inhibition, and given the high degree of amino acid conservation in this region, which is located 27 residues from the Walker A box in NBD1, this would suggest that other regions of the Hsp104 molecule play an important role in GdnHCl-induced inhibition.
To date, prions have only been reported for two fungal species, S. cerevisiae and the filamentous fungus Podospora anserina (44), and it remains to be established whether or not other fungal species have endogenous prions that can be effectively propagated during mitosis. Previous studies have shown that although CaSup35p can form self-seeding fibrils in vitro (35), there is no evidence that full-length CaSup35p can be propagated in this form in S. cerevisiae (32). This is most likely because it is unable to interact with the endogenous Sup35p prion, with the reason for this cross-species barrier being the difference in the extreme N-terminal aggregation domains of these two proteins (35). However, this barrier can be overcome by certain conformational prion variants generated using chimeric Ca/ScSup35pNM lacking the functional C-terminal region of Sup35p (41). No natural variants of either full-length CaSup35p or ScSup35p that can cross transmit have yet been described. The presence of the key chaperone for the propagation of S. cerevisiae prions in C. albicans and its ability to facilitate the propagation of ScSup35p as a prion suggest that the cellular machinery necessary for prion propagation exists in C. albicans. The development of a sensitive assay, such as that based on readthrough of a stop codon in either the ADE1 or ADE2 gene, is now required in order to search for CaSup35p-based prions in C. albicans.
Acknowledgments
The research described in this paper was supported by a project grant awarded to M.F.T. by the Biotechnology and Biological Sciences Research Council.
We thank Eric Fernandez-Bellot, Susan Lindquist, and Lev Osherovich for various reagents used in this study and Justin O'Sullivan, Mikayala King, and Martin Carden for help in the early phases of this project.
REFERENCES
- 1.Abbas-Terki, T., O. Donze, P. A. Briand, and D. Picard. 2001. Hsp104 interacts with Hsp90 cochaperones in respiring yeast. Mol. Cell. Biol. 21:7569-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal, M., C. Sahi, S. Katiyar-Agarwal, S. Agarwal, T. Young, D. R. Gallie, V. M. Sharma, K. Ganesan, and A. Grover. 2003. A molecular characterization of rice hsp101: complementation of yeast hsp104 mutation by disaggregation of protein granules and differential expression in indica and japonica rice types. Plant Mol. Biol. 51:543-553. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Baudin-Baillieu, A., E. Fernandez-Bellot, F. Reine, E. Coissac, and C. Cullin. 2003. Conservation of the prion properties of Ure2p through evolution. Mol. Biol. Cell 14:3449-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, G. A., S. Cao, C. C. Gelwix, E. G. Sexton, and K. A. Caldwell. 2004. An animal model to discern torsin function: suppression of protein aggregation in C. elegans. Adv. Neurol. 94:79-85. [PubMed] [Google Scholar]
- 6.Cashikar, A. G., E. C. Schirmer, D. A. Hattendorf, J. R. Glover, M. S. Ramakrishnan, D. M. Ware, and S. L. Lindquist. 2002. Defining a pathway of communication from the C-terminal peptide binding domain to the N-terminal ATPase domain in a AAA protein. Mol. Cell 9:751-760. [DOI] [PubMed] [Google Scholar]
- 7.Chernoff, Y. O., S. L. Lindquist, B. Ono, S. G. Inge-Vechtomov, and S. W. Liebman. 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor Psi+. Science 268:880-884. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, S. N., A. C. Chang, and L. Hsu. 1972. Non-chromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, B. S., F. Ness, and M. F. Tuite. 2003. Analysis of the generation and segregation of propagons: entities that propagate the [PSI+] prion in yeast. Genetics 165:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derkatch, I. L., Y. O. Chernoff, V. V. Kushnirov, S. G. Inge-Vechtomov, and S. W. Liebman. 1996. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edskes, H. K., and R. B. Wickner. 2002. Conservation of a portion of the S. cerevisiae Ure2p prion domain that interacts with the full-length protein. Proc. Natl. Acad. Sci. USA 99(Suppl. 4):16384-16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira, P. C., F. Ness, S. R. Edwards, B. S. Cox, and M. F. Tuite. 2001. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol. Microbiol. 40:1357-1369. [DOI] [PubMed] [Google Scholar]
- 13.Glover, J. R., and S. L. Lindquist. 1998. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73-82. [DOI] [PubMed] [Google Scholar]
- 14.Grimminger, V., K. Richter, A. Imhof, J. Buchner, and S. Walter. 2004. The prion curing agent guanidinium chloride specifically inhibits ATP hydrolysis by Hsp104. J. Biol. Chem. 279:7378-7383. [DOI] [PubMed] [Google Scholar]
- 14a.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Hanson, P. I., and S. W. Whiteheart. 2005. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 6:519-529. [DOI] [PubMed] [Google Scholar]
- 16.Hattendorf, D. A., and S. L. Lindquist. 2002. Analysis of the AAA sensor-2 motif in the C-terminal ATPase domain of Hsp104 with a site-specific fluorescent probe of nucleotide binding. Proc. Natl. Acad. Sci. USA 99:2732-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattendorf, D. A., and S. L. Lindquist. 2002. Cooperative kinetics of both Hsp104 ATPase domains and interdomain communication revealed by AAA sensor-1 mutants. EMBO J. 21:12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, G., Y. Song, S. Chung, and D. C. Masison. 2004. Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol. Cell. Biol. 24:3928-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, G. W. and M. F. Tuite. 2005. Chaperoning prions: the cellular machinery for propagating an infectious protein? Bioessays 27:823-832. [DOI] [PubMed] [Google Scholar]
- 21.Jones, T., N. A. Federspiel, H. Chibana, J. Dungan, S. Kalman, B. B. Magee, G. Newport, Y. R. Thorstenson, N. Agabian, P. T. Magee, R. W. Davis, and S. Scherer. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 101:7329-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung, G. M., and D. C. Masison. 2001. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr. Microbiol. 43:7-10. [DOI] [PubMed] [Google Scholar]
- 23.Jung, G. M., G. Jones, and D. C. Masison. 2002. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc. Natl. Acad. Sci. USA 99:9936-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushnirov, V. V., and M. D. Ter-Avanesyan. 1998. Structure and replication of yeast prions. Cell 94:13-16. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S., M. E. Sowa, Y. H. Watanabe, P. B. Sigler, W. Chiu, M. Yoshida, and F. T. Tsai. 2003. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell 115:229-240. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S., M. E. Sowa, J. M. Choi, and F. T. Tsai. 2004. The ClpB/Hsp104 molecular chaperone—a protein disaggregating machine. J. Struct. Biol. 146:99-105. [DOI] [PubMed] [Google Scholar]
- 27.Marchler, G., C. Schuller, G. Adam, and H. A. Ruis. 1993. Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 12:1997-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosser, D. D., S. Ho, and J. R. Glover. 2004. Saccharomyces cerevisiae Hsp104 enhances the chaperone capacity of human cells and inhibits heat stress-induced proapoptotic signalling. Biochemistry 43:8107-8115. [DOI] [PubMed] [Google Scholar]
- 29.Ness, F., P. Ferreira, B. S. Cox, and M. F. Tuite. 2002. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol. Cell. Biol. 22:5593-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newnam, G. P., R. D. Wegrzyn, S. L. Lindquist, and Y. O. Chernoff. 1999. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19:1325-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsell, D. A., A. S. Kowal, M. A. Singer, and S. Lindquist. 1994. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372:475-478. [DOI] [PubMed] [Google Scholar]
- 32.Resende, C. G., S. N. Parham, C. Tinsley, P. Ferreira, J. A. B. Duarte, and M. F. Tuite. 2002. The Candida albicans Sup35p protein: function, prion-like behaviour and an associated polyglutamine length polymorphism. Microbiology 148:1049-1060. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez, Y., and S. L. Lindquist. 1990. HSP104 is required for induced thermotolerance. Science 248:1112-1115. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez, Y., J. Taulien, K. A. Borkovich, and S. L. Lindquist. 1992. Hsp104 is required for tolerance to many forms of stress. EMBO J. 11:2357-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoso, A., P. Chien, L. Z. Osherovich, and J. S. Weissman. 2000. Molecular basis of a yeast prion species barrier. Cell 100:277-288. [DOI] [PubMed] [Google Scholar]
- 36.Schirmer, E. C., S. Lindquist, and E. Vierling. 1994. An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell 6:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirmer, E. C., O. R. Homann, A. S. Kowal, and S. L. Lindquist. 2004. Dominant gain-of-function mutations in Hsp104p reveal crucial roles for the middle region. Mol. Biol. Cell 15:2061-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seppa, L., A. L. Hanninen, and M. Makarow. 2004. Up-regulation of the Hsp104 chaperone at physiological temperature during recovery from thermal insult. Mol. Microbiol. 52:217-225. [DOI] [PubMed] [Google Scholar]
- 39.Shorter, J., and S. Lindquist. 2004. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304:1793-1797. [DOI] [PubMed] [Google Scholar]
- 40.Smith, C. K., T. A. Baker, and R. T. Sauer. 1999. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc. Natl. Acad. Sci. USA 96:6678-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka, M., P. Chien, K. Yonekura, and J. S. Weissman. 2005. Mechanism of cross-species prion transmission: an infectious conformation compatible with two highly divergent yeast prion proteins. Cell 121:49-62. [DOI] [PubMed] [Google Scholar]
- 42.Tuite, M. F., C. R. Mundy, and B. S. Cox. 1981. Agents that cause a high frequency of genetic change from [psi+] to [psi−] in Saccharomyces cerevisiae. Genetics 98:691-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uptain, S. M., G. J. Sawicki, B. Caughey, and S. Lindquist. 2001. Strains of [PSI+] are distinguished by their efficiencies of prion-mediated conformational conversion. EMBO J. 20:6236-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uptain, S. M., and S. Lindquist. 2002. Prions as protein-based genetic elements. Annu. Rev. Microbiol. 56:703-741. [DOI] [PubMed] [Google Scholar]
- 45.Wang, Q., C. Song, and C. C. Li. 2004. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 146:44-57. [DOI] [PubMed] [Google Scholar]
- 46.Wegrzyn, R. D., K. Bapat, G. P. Newnam, A. D. Zink, and Y. O. Chernoff. 2001. Mechanism of prion loss after Hsp104 inactivation in yeast. Mol. Cell. Biol. 21:4656-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weibezahn, J., B. Bukau, and A. Mogk. 2004. Unscrambling an egg: protein disaggregation by AAA+ proteins. Microb. Cell Fact. 3:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]