Abstract
Numerous studies show that the pathology of Alzheimer's disease is present decades before a clinical diagnosis of dementia can be made. Given the likelihood that agents will become available that reliably delay onset and/or slow progression of Alzheimer's disease, it will be important to detect preclinical Alzheimer's disease as early as possible for maximal treatment effect. Detection of individuals by sensitive cognitive measures provides one way to identify people who are at high risk of developing clinical Alzheimer's disease. However, it is likely that those with considerable brain or cognitive reserve will be able to mask cognitive deficits until very close to the onset of the dementia, rendering such cognitive measures insensitive. Optimum biomarkers for Alzheimer's disease therefore need to target the severity of underlying brain pathology independently of brain reserve. Findings are presented showing the importance of higher education and larger brain size in masking the underlying disease pathology.
Keywords: Alzheimer's disease, biomarkers, prevention
Autopsy,1 longitudinal cognitive,2-5 and other studies6 suggest that Alzheimer's disease (AD) begins relatively early in life and progresses “behind the scenes” for decades before it is clinically expressed. This raises the possibility that individuals who will eventually express the disease can be identified long before the initial symptoms appear and that interventions to prevent disease expression can be targeted to such people at high risk. Detection of individuals by sensitive cognitive measures provides one way to identify people who in the future are likely to develop AD. However, it is likely that those with considerable brain or cognitive reserve will be able to mask cognitive deficits until very close to the onset of the dementia, rendering such cognitive measures insensitive. Optimum biomarkers for AD therefore need to target the severity of underlying brain pathology independently of brain reserve.
ALZHEIMER NEUROPATHOLOGY AND COGNITIVE/LINGUISTIC MARKERS IN EARLY ADULT LIFE
Evidence supporting the presence of Alzheimer neuropathology decades before the age at which clinical expression usually occurs is provided by autopsy studies of individuals without dementia. Braak and Braak1 studied Alzheimer neuropathology in routine autopsies of 2661 individuals and found that by age 25 about one fifth of those autopsied were in Braak neurofibrillary stage I, the mildest stage of pathology. Because Braak stage can only be ascertained at autopsy, it is impossible to demonstrate that the disease progresses through an orderly sequence of stages from I to VI. However, the presence of individuals who have attained an advanced age and show little or no neurofibrillary degeneration at autopsy is consistent with this stage representing the earliest manifestation of the AD degenerative process.
Snowdon et al6 used autobiographies written by Catholic sisters at an average age of 22 to show that characteristics of these autobiographies strongly predicted who would develop both clinical and pathological AD 60 years later. The critical characteristic, idea density, measured the semantic content of the essays. Nuns with idea density in the lowest tertile were almost 60 times more likely to fulfill criteria for clinical and neuropathological AD in late life compared with those who scored in the upper two tertiles of this measure. Other studies based on cognitive assessments given as part of longitudinal studies of aging suggest that those who are destined to become demented in the future score differently on a variety of cognitive measures decades before the initial signs of the dementing illness become apparent.2-5
TWO SETS OF RISK FACTORS FOR AD
The fact that pathological lesions can be present long before clinical symptoms appear suggests that there may be 2 distinct sets of risk factors for this illness, one for the pathology and the other for the clinical expression. We have argued elsewhere7 that the pathology of the disease is primarily genetic in origin. The strongest data in support of this view come from studies of older twin pairs. Heritability estimates from existing studies (.74, .58) suggest that AD is likely to be strongly inherited.8,9 However, differences in the age of onset in concordant twin pairs imply a role for nongenetic factors in determining the age of onset.8 A second line of evidence comes from autopsy studies, which show that the presence of 1 or more ε4 alleles for apolipoprotein E is generally associated with an increase in the severity of the neuropathological findings.10-13 In the Nun Study, we found that the presence of APOE-ε4 genotypes was associated with a sixfold increase in the probability of meeting neuropathological criteria for AD at autopsy but only a twofold increase in dementia.13
RISK FACTORS THAT MODIFY THE CLINICAL EXPRESSION OF AD PATHOLOGY
One of the reasons for the difference in risk of pathologically and clinically defined AD associated with APOE-ε4 may be that a substantial proportion of individuals satisfying neuropathological criteria for AD at autopsy do not become demented before death. As shown in Table 1, this percentage can be quite large, suggesting that there are likely to be risk factors that modify the clinical expression of the pathology of AD. Two of these factors are attained education and the size of the fully developed brain. Numerous studies have found low educational attainment to be a risk factor for prevalent18-30 and incident31-41 dementia, although a few studies have failed to confirm this association.42-44 The effect of lower education on the expression and detection of AD is likely multifactorial, related to lower IQ, poorer test-taking ability, and less mental exercise.
Table 1.
Prospective Studies of Nondemented Elderly: Percent Meeting Neuropathological Criteria for Alzheimer's Disease (AD)
Smaller brain size attained in childhood,45 estimated from scans46-48 or from head circumference,49-51 has been associated with increased prevalence,48,51 increased incidence,52 earlier onset of symptoms,47 and increased severity of cognitive deficit46,49,51 in AD as well as with lower scores on cognitive screening tests in a community sample of nondemented older adults.50 However, negative studies have been published in which total intracranial volume, a measure of maximum attained brain size, was found not to be associated with the presence of AD in studies of clinically derived cases and cognitively normal community volunteers.53,54
Figure 1 shows findings from a large study of older persons who were screened with a mental status exam called the Cognitive Abilities Screening Instrument or CASI.49,55 Head circumferences above 55 cm had little effect, but smaller circumferences were associated with lower scores on this screening test. When individuals with probable AD were examined separately from those without this illness, it was found that the entire effect of head circumference on mental status was attributable to individuals with probable AD. Patients with probable AD who had smaller head circumferences had lower scores on the global mental status test, whereas head circumference had no effect on global cognition among those not meeting study criteria for probable AD.
Figure 1.
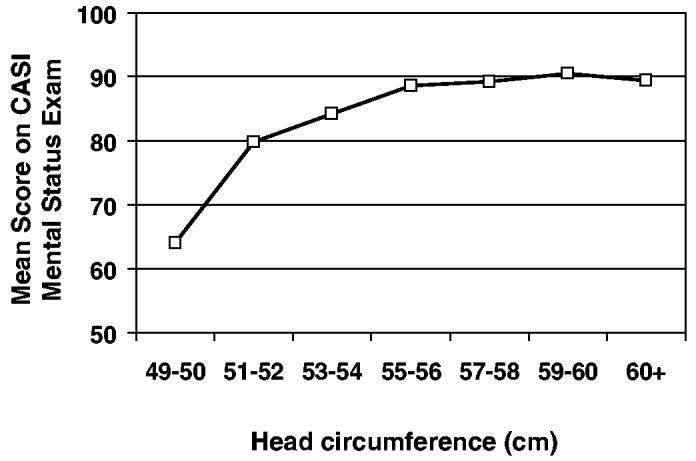
Mean scores on the Cognitive Abilities Screening Instrument (CASI) mental status exam by head circumference categories in a sample of 1985 Japanese Americans age 65 and over (the Kame Study). Copyright 1996 from “Head Circumference as a Measure of Cognitive Reserve: Association With Severity of Impairment in Alzheimer's Disease” by Graves et al.49 Adapted and reprinted with permission of the Royal College of Psychiatrists.
Borenstein-Graves et al52 later showed that head circumference was related to incidence of AD among initially nondemented older persons followed longitudinally who carried 1 or more e4 alleles for apolipoprotein E but not among people who did not carry this allele. The combination of a strong risk factor for Alzheimer neuropathology and a risk factor for clinical expression predicted who would become demented in the future.
Given the protective value of higher education and larger brain size, one can ask whether the combination of these two characteristics is more beneficial than either one in isolation. Recently, we examined this possibility using data from the Nun Study.56 The findings, summarized in Figure 2, show that larger head circumference is an important protective risk factor for the clinical expression of dementia, but only among those with lower education. Either high education or a larger brain is sufficient to lower the risk of dementia appreciably, and the additional benefit from having both is small. Autopsy data from patients in this study showed that neither head circumference nor attained education was related to satisfying neuropathological criteria for AD, consistent with findings of other investigators.57
Figure 2.
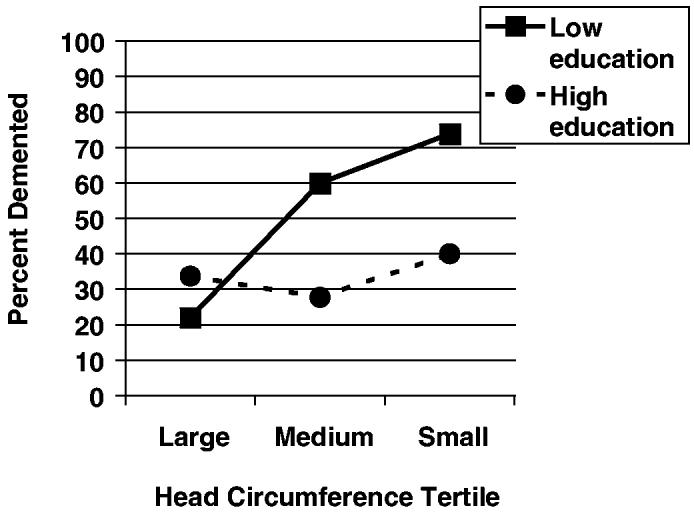
Percentage of Catholic sisters demented in six groups defined by educational attainment (high = bachelor's degree or more, low = less than a bachelor's degree) and head circumference tertile. Copyright 2003 from “Head Circumference, Education and Risk of Dementia: Findings From the Nun Study” by Mortimer et al.56 Reproduced by permission of Taylor & Francis Group, LLC., http://www.taylorandfrancis.com.
The result of further investigation of the modification of clinical status by education is shown in Figure 3. Among those Catholic sisters with high (vs low) educational attainment, the frequency of dementia before death was reduced by 26% for those in milder neuropathological states of the disease (Braak neurofibrillary stages I-III) and 13% for those with more severe pathology (Braak neurofibrillary states IV-VI). Education has a powerful effect in reducing the severity of cognitive impairment in individuals with moderate AD pathology. As the severity of the pathology increases, the protective role of education remains but is diminished.
Figure 3.
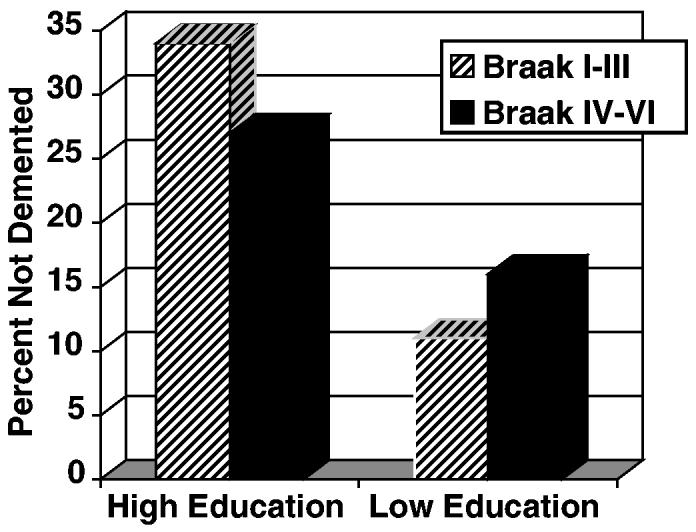
Percentages of non-demented Catholic sisters by educational attainment (high = bachelor's degree or more, low = less than a bachelor's degree) and Braak neurofibrillary stage at autopsy.
NEUROPATHOLOGY OF MILD COGNITIVE IMPAIRMENT
In an effort to intervene earlier in the disease course, considerable attention is being given to identifying individuals at the stage of mild cognitive impairment. Although patients at this clinical stage do have milder pathology (Figure 4), approximately 50% are already in Braak neurofibrillary stage III or higher, and 1 in 5 are likely to be in advanced stages of neuropathology.58 Clearly, earlier identification is needed before substantial and possibly irreversible degeneration has occurred. Ideally, persons at risk for AD should be identified 3 to 4 decades in advance of clinical expression when the opportunity to prevent substantial degeneration is likely greater.
Figure 4.
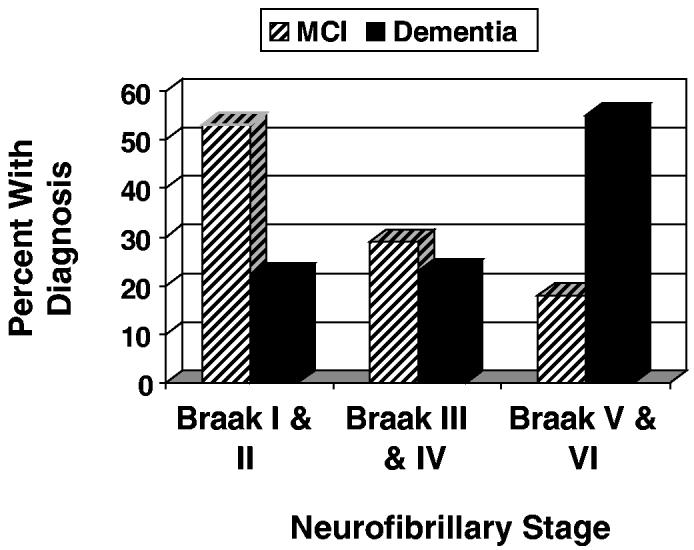
Percentages of Catholic sisters with dementia and mild cognitive impairment (MCI) by Braak neurofibrillary stage.
BIOMARKERS OF PATHOLOGY THAT ARE INDEPENDENT OF BRAIN RESERVE
Although it is clearly beneficial to have higher education or a larger brain or both, either of these characteristics is sufficient to mask the presence of substantial pathology. How does one go about identifying individuals who because of their reserve are likely to postpone cognitive impairment for years and even decades? To do this, one needs to focus on biomarkers that identify pathology independent of brain reserve. Validation ideally would involve correlation of such a marker against pathological findings at autopsy. To be useful for population screening, the biomarker also should be as noninvasive as possible and relatively inexpensive. Markers for in vivo brain β-amyloid, such as the Pittsburgh Compound B (PIB) that requires a positron-emission tomography (PET) scan, are unlikely to be useful for population screening. Less costly, but invasive, would be markers obtained from samples of cerebrospinal fluid. Ideally, we would like a simple blood test similar to prostate specific antigen that would identify people at high risk of AD. If such a blood test became available, those with positive tests could be evaluated with more specific tests, including magnetic resonance imaging (MRI) and PET scan measures closely correlated with pathology.
HIPPOCAMPAL ATROPHY AS A MARKER OF AD PATHOLOGY
The key to viable biomarkers is the demonstration that they identify individuals with considerable Alzheimer pathology while they are still nondemented and preferably free of memory impairment. Hippocampal atrophy is perhaps the best studied structural MRI marker of AD. Recently, we have shown using postmortem MRI scans from the Nun Study that hippocampal volume is strongly correlated with Braak neurofibrillary stage.59 Surprisingly, MRI appears to track even the earliest stages of the disease, offering hope that it can be used to identify individuals with the illness decades before clinical symptoms appear. Similar findings have been published by others60 based on correlations between antemortem MRI scans and autopsy findings.
Table 2 summarizes the findings of a recent study of 32 Catholic sisters who were nondemented at the times of their deaths.61 Forty-four percent of these sisters met neuropathological criteria for AD at autopsy. The sensitivity of hippocampal volume was 100% for all categories, and the specificity ranged between 83% and 100%. Overall classification accuracy ranged from 91% in cognitively normal sisters to 100% in those showing memory impairment short of dementia.
Table 2.
Prediction by Left Hippocampal Volume of Fulfillment of Alzheimer's Disease (AD) Neuropathological Criteria in Nondemented Groups of Catholic Sisters From the Nun Study
| Group/Subgroup | Sensitivity | Specificity | Classification Accuracy | Percent Meeting Neuropathological Criteria for AD |
|---|---|---|---|---|
| Nondemented (n = 32) | 1.00 | .83 | .92 | 43.8 |
| Memory impaired, not demented (n = 8) | 1.00 | 1.00 | 1.00 | 87.5 |
| Cognitively normal (n = 24) | 1.00 | .83 | .91 | 29.2 |
Source: Adapted from Gosche et al.61
COMBINATIONS OF BIOMARKERS
Because there are multiple causes of hippocampal atrophy, it is unlikely that this single measure alone will be sufficient to identify individuals harboring pathological AD. However, combinations of hippocampal volume with global atrophy measures and volumes of other regions thought to be involved early in AD may provide a reliable index of AD neuropathology, especially at more advanced Braak stages.
A variety of different biomarkers need to be investigated for their association with one another and with the severity of neuropathology assessed at autopsy. These biomarkers include but are not limited to structural and functional MRI markers, PET markers of amyloid β pathology, olfactory loss, plasma Aβ1-42, antibodies to Aβ, and levels of other serum proteins.
ULTIMATE BENEFICIARIES OF VERY EARLY DETECTION
Given the likelihood of development of agents capable of slowing the disease process, we need to be able to identify individuals who are at very high risk of developing AD, preferably decades before symptoms appear. The prospects for very early detection and prevention of AD are promising. Although initially, early detection procedures for this illness likely will be used in those in middle age and beyond, the children of Alzheimer patients, who are at heightened genetic risk, will be the ultimate beneficiaries of this technology when it becomes available.
Footnotes
This work was supported, in part, by grant R01AG09862 from the National Institute on Aging. This article was presented at the American Association of Geriatric Psychiatry Annual Meeting, March 6, 2005, in San Diego, California.
Contributor Information
Karen M. Gosche, From the NeuroImaging Research, Alachua, FL.
David A. Snowdon, From the Sanders-Brown Center on Aging and Department of Neurology, University of Kentucky, Lexington, KY..
References
- 1.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 2.LaRue A, Jarvik LF. Cognitive function and prediction of dementia in old age. Int J Aging Hum Dev. 1987;25:79–89. doi: 10.2190/DV3R-PBJQ-E0FT-7W2B. [DOI] [PubMed] [Google Scholar]
- 3.Linn R, Wolf P, Bachman D, Knoefel J. The “preclinical phase” of probable Alzheimer's disease: a 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 4.Elias MF, Beiser A, Wolf P, et al. The preclinical phase of Alzheimer's disease: a 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 5.Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon DA, Kemper SJ, Mortimer JA, et al. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life: Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 7.Ashford JW, Mortimer JA. Non-familial Alzheimer's disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4:169–177. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- 8.Gatz M, Pedersen NL, Berg S, et al. Heritability of Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52A:M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 9.Bergem AL, Engedal K, Kringlen E. The role of heredity in late-onset Alzheimer disease and vascular dementia. A twin study. Arch Gen Psychiatry. 1997;54:264–270. doi: 10.1001/archpsyc.1997.01830150090013. [DOI] [PubMed] [Google Scholar]
- 10.Polvikoski T, Sulkava R, Haltia M, et al. Apolipoprotein E, dementia and cortical deposition of β-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 11.Ghebremedhin E, Schultz C, Thal DR. Gender and age modify the association between APOE and AD-related neuropathology. Neurology. 2001;56:1696–1701. doi: 10.1212/wnl.56.12.1696. [DOI] [PubMed] [Google Scholar]
- 12.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Mortimer JA, Markesbery WR, Snowdon DA. Apolipoprotein E-e4 as a risk factor for Alzheimer and non-Alzheimer's dementias: findings from the Nun Study. Neurobiol Aging. 2000;21:S32–S33. [Google Scholar]
- 14.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: non-demented subjects with pathologically-confirmed Alzheimer's disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 15.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 16.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 17.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 18.Dartigues JF, Gagnon M, Michel P, et al. The Paquid research program on the epidemiology of dementia. Methods and initial results. Rev Neurol. 1991;147:225–230. [PubMed] [Google Scholar]
- 19.Bowirrat A, Friedland RP, Farrer L, et al. Genetic and environmental risk factors for Alzheimer's disease in Israeli Arabs. J Mol Neurosci. 2002;19:239–245. doi: 10.1007/s12031-002-0040-4. [DOI] [PubMed] [Google Scholar]
- 20.Callahan CM, Hall KS, Hui SL, et al. Relationship of age, education, and occupation with dementia among a community-based sample of African-Americans. Arch Neurol. 1996;53:134–140. doi: 10.1001/archneur.1996.00550020038013. [DOI] [PubMed] [Google Scholar]
- 21.Chibnall JT, Eastwood R. Postsecondary education and dementia risk in older Jesuit priests. Int Psychogeriatr. 1998;10:359–368. doi: 10.1017/s1041610298005456. [DOI] [PubMed] [Google Scholar]
- 22.De Ronchi D, Fratiglioni L, Rucci P, et al. The effect of education of dementia occurrence in an Italian population with middle to high socioeconomic status. Neurology. 1998;50:1231–1238. doi: 10.1212/wnl.50.5.1231. [DOI] [PubMed] [Google Scholar]
- 23.Haan MN, Mungas DM, Gonzalez HM, et al. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 24.Hall K, Gureje O, Gao S, et al. Risk factors and Alzheimer's disease: a comparative study of two communities. Aust N Z J Psychiatry. 1998;32:698–706. doi: 10.3109/00048679809113126. [DOI] [PubMed] [Google Scholar]
- 25.Kokmen E, Beard CM, O'Brien PC, Kurland LT. Educational attainment and Alzheimer's disease: reassessment of the Rochester, Minnesota data (1975-1984) Neurology. 1993;43:A317. [Google Scholar]
- 26.Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Precipe M, Casini AR, Ferretti C, et al. Prevalence of dementia in an elderly rural population: effects of age, sex, and education. J Neurol Neurosurg Psychiatry. 1996;60:628–633. doi: 10.1136/jnnp.60.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravaglia G, Forti P, Maioli F, et al. Education, occupation, and prevalence of dementia: findings from the Conselice study. Dement Geriatr Cogn Disord. 2002;14:90–100. doi: 10.1159/000064930. [DOI] [PubMed] [Google Scholar]
- 29.Schmand B, Smit J, Lindeboom J, et al. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997;50:1025–1033. doi: 10.1016/s0895-4356(97)00121-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 31.Di Carlo A, Baldereschi M, Amaducci L, et al. Incidence of dementia, Alzheimer's disease, and vascular dementia in Italy. The ILSA Study. J Am Geriatr Soc. 2002;50:41–48. doi: 10.1046/j.1532-5415.2002.50006.x. [DOI] [PubMed] [Google Scholar]
- 32.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 33.Fratiglioni L, Viitanen M, von Strauss E, et al. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungshomen Project, Stockholm. Neurology. 1997;48:132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 34.Karp A, Kareholt I, Qiu C, et al. Relation of education and occupation-based socioeconomic status to incident Alzheimer's disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- 35.Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 36.Letenneur L, Gilleron V, Commenges D, et al. Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 38.Ott A, van Rossum CTM, van Harskamp F, et al. Education and the incidence of dementia in a large population-based study: The Rotterdam Study. Neurology. 1999;52:663–666. doi: 10.1212/wnl.52.3.663. [DOI] [PubMed] [Google Scholar]
- 39.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 40.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Katzman R, Yu E, et al. A preliminary analysis of incidence of dementia in Shanghai, China. Psychiatry Clin Neurosci. 1998;52:S291–S294. doi: 10.1111/j.1440-1819.1998.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 42.Beard CM, Kokmen E, Offord KP, Kurland LT. Lack of association between Alzheimer's disease and education, occupation, marital status, or living arrangement. Neurology. 1992;42:2063–2068. doi: 10.1212/wnl.42.11.2063. [DOI] [PubMed] [Google Scholar]
- 43.Cobb JL, Wolf PA, Au R, et al. The effect of education on the incidence of dementia and Alzheimer's disease in the Framingham Study. Neurology. 1995;45:1707–1712. doi: 10.1212/wnl.45.9.1707. [DOI] [PubMed] [Google Scholar]
- 44.Graves AB, Larson EB, Edland SD, et al. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The Kame Project. Am J Epidemiol. 1996;144:760–771. doi: 10.1093/oxfordjournals.aje.a009000. [DOI] [PubMed] [Google Scholar]
- 45.Dobbing K, Sands J. Quantitative growth and development of the human brain. Arch Dis Child. 1973;49:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori E, Hirono N, Yamashita H, et al. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am J Psychiatry. 1997;154:18–24. doi: 10.1176/ajp.154.1.18. [DOI] [PubMed] [Google Scholar]
- 47.Schofield PW, Mosesson RE, Stern Y, Mayeux R. The age at onset of Alzheimer's disease and an intracranial area measurement. A relationship. Arch Neurol. 1995;52:95–98. doi: 10.1001/archneur.1995.00540250103019. [DOI] [PubMed] [Google Scholar]
- 48.Wolf H, Julin P, Gertz HJ, et al. Intracranial volume in mild cognitive impairment, Alzheimer's disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19:995–1007. doi: 10.1002/gps.1205. [DOI] [PubMed] [Google Scholar]
- 49.Graves AB, Mortimer JA, Larson EB, et al. Head circumference as a measure of cognitive reserve: association with severity of impairment in Alzheimer's disease. Br J Psychiatry. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds MD, Johnson JM, Dodge HH, et al. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurology. 1999;53:228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- 51.Schofield PW, Logroscino G, Andrews HF, et al. An association between head circumference and Alzheimer's disease in a population-based study of aging and dementia. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- 52.Borenstein-Graves A, Mortimer JA, Bowen JD, et al. Head circumference and incident Alzheimer's disease: modification by apolipoprotein E. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 53.Edland SD, Xu Y, Plevak M, et al. Total intracranial volume: normative values and lack of association with Alzheimer's disease. Neurology. 2002;59:272–274. doi: 10.1212/wnl.59.2.272. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins R, Fox NC, Rossor AM, et al. Intracranial volume and Alzheimer's disease: evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- 55.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiologic studies of dementia. Int Psychogeriatr. 1994;6:45–56. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 56.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;5:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 57.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 58.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 59.Gosche KM, Mortimer JA, Smith CD, et al. An automated technique for measuring hippocampal volumes from MR imaging studies. AJNR. 2001;22:1686–1689. [PMC free article] [PubMed] [Google Scholar]
- 60.Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gosche KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Alzheimer neuropathology. Findings from the Nun Study. Neurology. 2002;58:1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]


