Abstract
Nucleoporins are components of the nuclear pore, which is required for nucleo-cytoplasmic transport. We report a role for a subclass of nucleoporins in orienting the mitotic spindle in C. elegans embryos. RNAi-mediated depletion of any of five putative nucleoporins npp-1, npp-3, npp-4, npp-11, and npp-13 leads to indistinguishable spindle orientation defects. Transgenic worms expressing NPP-1::GFP or NPP-11::GFP show GFP localization at the nuclear envelope, consistent with their predicted function. NPP-1 interacts with the other nucleoporins in yeast two-hybrid assays suggesting that the proteins affect spindle orientation by a common process. The failed orientation phenotype of npp-1(RNAi) is at least partially epistatic to the ectopic spindle rotation in the AB blastomere of par-3 mutant embryos. This suggests that NPP-1 contributes to the mechanics of spindle orientation. However, NPP-1 is also required for PAR-6 asymmetry at the two-cell stage, indicating that nucleoporins may be required to define cortical domains in the germ line blasotmere P1. Nuclear envelope structure is abnormal in npp-1(RNAi) embryos but the envelope maintains its integrity and most nuclear proteins we assayed accumulate normally. These findings raise the possibility that these nucleoporins may have direct roles in orienting the mitotic spindle and the maintenance of cell polarity.
Keywords: nucleoporins, spindle orientation, Caenorhabditis elegans, embryo
INTRODUCTION
Faithful orientation and placement of the mitotic spindle is essential for the proper development of multi-cellular organisms. Proper spindle orientation is important in two major ways. First, proper spatial organization often requires that cells divide in a reproducible orientation and second, proper segregation of cytoplasmic determinants requires that spindles orient appropriately with respect to the axis of cellular asymmetry. In the Caenorhabditis elegans embryo, a series of asymmetric divisions beginning with the zygote (P0) leads to the production of the six founder cells AB, C, D, E, MS and P4 (Sulston et al., 1983). Each asymmetric division requires proper rotation and placement of the mitotic spindle. The zygote divides asymmetrically to form a large anterior cell, AB, and a smaller posterior cell, P1. This division requires that the centrosome/nuclear complex rotate to position the spindle longitudinally (Albertson, 1984). During the second round of division, the P1 centrosome/nuclear complex rotates to align the spindle along the long axis of the embryo and P1 divides asymmetrically to form P2 and EMS (Hyman and White, 1987).
Several proteins are known to be required for proper spindle positioning in C. elegans embryos. Cell polarity genes are required for differential regulation of spindle rotation in AB and P1. For example, PAR-2 activity is required for the centrosome/nuclear rotation in P1 (Cheng et al., 1995) and PAR-3, PAR-6 and PKC-3 are required to prevent the rotation of the spindle in AB (Cheng et al., 1995; Tabuse et al., 1998; Watts et al., 1996). Double mutant combinations of par-2 with par-3 or par-6 result in centrosome/nuclear complex rotation in both the AB and P1 indicating that the polarity proteins determine in which cell the centrosome nuclear complex rotates, but have no role in the rotation mechanism itself.
Microtubule motors are required for proper spindle positioning. Dynein (dhc-1) and members of the dynactin complex (dnc-1 and dnc-2) are required for this orientation (Gonczy et al., 1999; Skop and White, 1998). Dynein is a minus-end directed microtubule motor and the dynactin complex has been shown to bind cargoes to dynein and regulate the dynein motor function (Karcher et al., 2002). Studies in yeast suggest that dynein and the dynactin complex are required for proper interactions between microtubules and the cell cortex that are required for proper spindle positioning (Adames and Cooper, 2000). Members of the dynactin complex interact with specific nucleoporins in both yeast and vertebrates (Ito et al., 2001; Payne et al., 2003).
OOC-3 and the Torsin-related OOC-5 proteins are also required for proper spindle orientation (Basham and Rose, 1999). Mutations in ooc-3 or ooc-5 cause multiple embryonic defects including reduced size, loss of cell polarity, and failure of mitotic spindle orientation at the two-cell stage. OOC-3 and OOC-5 are localized to both the endoplasmic reticulum and the nuclear envelope (Basham and Rose, 2001). Similarly, the ATP-bound form of TorsinA has been shown to localize to the nuclear envelope (Naismith et al., 2004) suggesting its interaction partners may reside on the nuclear envelope.
Here we report that several nucleoporins are required for proper spindle positioning in C. elegans embryos. Nucleoporins are the individual protein components that make up the nuclear pore complex. Nuclear pore complexes (NPCs) are large multiprotein complexes that provide the only means known for transporting large macromolecules between the nucleus and the cytoplasm (Suntharalingam and Wente, 2003). The vertebrate NPC has a total mass of approximately 125 MDa, is 120 nm in diameter, and is composed of multiple copies of approximately 30 proteins that assemble into a cylindrical structure with an eight-fold rotational symmetry. The yeast nuclear pore contains a similar number of proteins and has a similar structure but is considerably smaller at 66 MDa, and 100 nm diameter.
The C. elegans NPC has a similar structure to that of yeast and vertebrates, but is closer in size to vertebrate NPC (Cohen et al., 2002). Similarly, the predicted C. elegans nucleoporins are more similar to nucleoporins from vertebrate than from yeast (Galy et al., 2003). Of the 30 identified vertebrate nucleoporins, 20 were found to have clear homologues in C. elegans; these have been named npp-1 through npp-20.
Recently, many nucleoporins have become implicated in cellular roles beyond their function in nuclear transport. Eleven vertebrate nucleoporins have been found to become associated with the kinetochore during mitosis (Belgareh et al., 2001; Harel et al., 2003; Joseph et al., 2002; Loiodice et al., 2004; Wang et al., 2001). The yeast nucleoporin Ndc1p localizes to spindle pole bodies and is required for their duplication (Chial et al., 1998). Yeast Nup53p interacts with the spindle checkpoint genes Mad1p and Mad2p and sequesters them to the NPC throughout most of the cell cycle until the spindle checkpoint is activated (Iouk et al., 2002).
In this report we demonstrate a new role for five nucleoporins. We show that C. elegans nucleoporins NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 (homologues of vertebrate Nup54, Nup205, Nup45/58, Nup62, and Nup93 respectively) are required for proper spindle rotation. While the exact molecular nature of this requirement is unknown, we propose that they could act in concert with the dynactin complex or OOC-3/OOC-5.
MATERIALS AND METHODS
Strains used
Nematodes were cultured under normal conditions (Brenner, 1974). The Bristol N2 strain was used as wild type. Strains used for this analysis were PD4251, ccIs4251[myo-3p::gfp] (Fire et al., 1998); SU93, jcIs1 [ajm-1p::ajm-1::gpf] IV (Mohler et al., 1998), PD4790 (myo-2p::gfp; pes-10p::gfp; F22B7.9p::gfp) (M. Edgley, J. Liu, D. Riddle, A. Fire, personal communication); DP132, edIs6[unc119::GFP] IV;) (D. Pilgrim personal communication); AZ244, unc-119(ed3) III; ruIs57[unc-119(+) pie-1p::gfp::tubulin] (Praitis et al., 2001); PGL-1::GFP-expressing strain (S. Strome, personal communication); XA3541, unc-119(ed3); qaIs3502[unc-119(+) + pie-1p::yfp::lmn-1]; ojIs1[unc-119(+) pie-1p::gfp::tbb-2] (Franz et al., 2005); XA3543, unc-119(ed3); qaIs3507[unc-119(+) + pie-1p::gfp::lem-2] III; ojIs1[unc-119(+) pie-1p::gfp::tbb-2]; XA3507 unc-119(ed3) qaIs3507[unc-119(+) + pie-1p::gfp::lem-2] III (Galy et al., 2003), PCNA::GFP, unc-119(ed3) isIs17 [pGZ295:pie-1p::GFP::PCNA (Brauchle et al., 2003); KK051, lon-1(e185) par-3(it71) III/qC1; JH227, axEx73[pJH3.92(pie-1::gfp), pRF4]; AZ212, unc-119(ed3) ruIs32[unc-119(+) pie-1p::gfp::h2b] III (Praitis et al., 2001); XA3501, unc-119(ed3) ruIs32[unc-119(+) pie-1p::gfp::h2b] III; ojIs1[unc-119(+) pie-1p::gfp::tbb-2] (Askjaer et al., 2002).
RNA interference
RNAi was carried out either by microinjection or by RNAi feeding. For microinjection, gene-specific primers containing T7 polymerase promoter sequences were used to PCR amplify a portion of each gene from genomic DNA. The PCR product was used as a template to make dsRNA using the T7 Ribomax kit (Promega). Injections were then performed as described in (Fire et al., 1998). Worms were analyzed for defects after at least 48 hours post-injection. RNAi feeding used protocols described in (Galy et al., 2003). Primer sequences and information about the sequences used for RNAi is available at http://www.embl-heidelberg.de/ExternalInfo/mattaj/elegans/primers.html or by request.
Generation of NPP-1 GFP expression
An NPP-1::GFP expression vector was constructed using pBluescript (Stratagene). Full length npp-1 genomic DNA, 556 base pairs upstream of the start codon, 524 base pairs directly downstream of the stop codon, and GFP were PCR amplified and ligated together (sequence available upon request). The 5’ and 3’ regions included all sequences up to the predicted transcripts of the flanking genes. The GFP was fused with the C-terminal end of full length npp-1 genomic DNA directly preceding the stop codon. An NPP-11::GFP construct was also created by amplifying the full length npp-11 cDNA from the ProQUEST cDNA library (Gibco) and cloning it into the npp-1 expression vector in place of the npp-1 genomic DNA.
Transgenic animals expressing extrachromosomal arrays were created using injection of each of these constructs independently with linearized genomic DNA and pRF4 (rol-6 dominant marker) using previously described methods (Kelly et al., 1997; Mello et al., 1991). A typical injection mix contained 1 ng/ul linearized pRF4, 1 ng/ul linearized npp-1 or npp-11::gfp construct, and 50 ng/ul PvuII cut genomic DNA.
Immunofluorescence
Immunostaining for CSN-5 and mAb414 was performed using a standard methanol fixation (Miller and Shakes, 1995). Worms were dissected on poly-lysine slides in water. Embryos were fixed by freeze-cracking them and immersing them in -20° C methanol. They were washed in phosphate-buffered saline (PBS) 0.5% Tween followed by two washes in PBS. Either anti-CSN-5 (1:50 in PBS) (Pintard et al., 2003) or mAb414 (1:200 in PBS) was added and they were incubated 8-12 hours at 4° C. After three washes in PBS, anti-rabbit (Jackson ImmunoResearch Lab #111-095-0030) or anti-mouse (Jackson ImmunoReseach Lab #115-096-062) antibodies conjugated to FITC (1:100 in PBS) were added and incubated 1-2 hours at 37° C. Slides were washed in PBS and mounted in Vectashield (Vector Labs Inc. #H-1000).
Microscopy and image collection
Leica DMRA2 and DMIRE2 microscopes with 63X HCX PL APO objectives were used to collect data. Wide-field images were captured with an ORCA-ER (Hamamatsu C4742-95) digital camera while confocal images were obtained with a Leica AOBS system. Images were collected and processed with ImageJ, Openlab 3.5 (Improvision) and Photoshop 7.0 (Adobe).
Yeast Two-hybrid analysis
Two hybrid analysis was performed essentially as previously described (James et al., 1996). Full length cDNAs for each gene to be tested were amplified from the ProQuest C. elegans cDNA library (Gibco-BRL #11288-026) using gene-specific primers with appropriate restriction sites (sequences available upon request). cDNAs were sequenced and found to be error free and in agreement with WORMBASE predictions. cDNAs were subsequently cloned into pGAD and pGBD (James et al., 1996). Pair wise combinations of all the nucleoporins were transformed into yeast strain PJ69-4A. Combinations of empty pGAD and pGBD vectors were also transformed into PJ69-4A with each construct to assess self-activation. They were then tested for their ability to grow on plates lacking adenine and plates lacking histidine.
RESULTS
Rotation of the centrosome/nuclear complex in P0 and P1 requires NPP-1
In an analysis of RNAi phenotypes of ovary-enriched genes in C. elegans we discovered that npp-1(RNAi) causes spindle orientation defects in P0 and P1 (Fig. 1) (Piano et al., 2002). We analyzed npp-1(RNAi) embryos from injected mothers 48 hours after dsRNA injection. The npp-1(RNAi) embryos displayed normal meiosis, pseudocleavage and pronuclear migration, but both the P0 spindle (55%, n=11) and the P1 (86%, n=35) spindle failed to rotate in npp-1(RNAi) embryos. Although embryos fertilized 24-48 hours after dsRNA injection failed to hatch, they did not display early spindle orientation defects and were not analyzed further. In addition to the spindle orientation defects, we noted that eggs from NPP-1 depleted worms were 33% smaller than controls, development was slowed by about 40% relative to controls and the injected worms ceased producing eggs by about 72 hours after injection.
Figure 1.
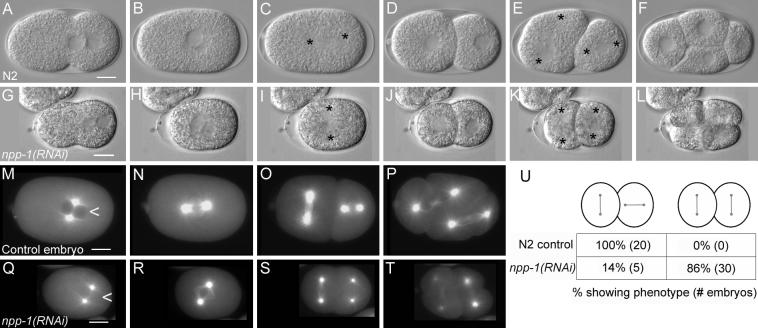
npp-1(RNAi) causes a failed rotation of the mitotic spindle. Panels A-F are a time series of DIC photomicrographs of control embryos (N2). Panels G-L show corresponding time points for npp-1(RNAi) embryos. Anterior is to the left in all embryos. Asterisks mark centrosome positions. No defects in pseudocleavage and pronuclear migration are apparent in npp-1(RNAi) embryos (A versus G). The mitotic spindle fails to rotate properly in P0 and in P1 in npp-1(RNAi) embryos (C, E versus I, K). Panels M-T are photomicrographs of embryos expressing β-Tubulin::GFP at time points comparable to those shown in B,C, E & F. Note the absence of obvious defects in spindle structure. Also note the smaller size of npp-1(RNAi) embryos. Nuclei of npp-1(RNAi) embryos also fail to exclude β-tubulin::GFP (arrowhead in M compared to Q) indicating a failure in nuclear envelope function. A summary table of the P1 spindle orientation defects observed in npp-1(RNAi) embryos is included (U). Bar represents 10 microns.
To determine whether npp-1(RNAi) affected microtubule organization we examined npp-1(RNAi) embryos expressing β-Tubulin::GFP (Fig. 1 M-T). As expected, npp-1(RNAi); β-Tubulin::GFP embryos displayed the same spindle orientation defects previously observed by differential interference microscopy. However, microtubule organization did not appear to be visibly different from controls at this level of resolution, indicating that NPP-1 controls spindle orientation by some mechanism other than by affecting microtubule organization. However, our analysis cannot rule out subtle effects such as changes in microtubule interactions with the cortex or changes in microtubule dynamics.
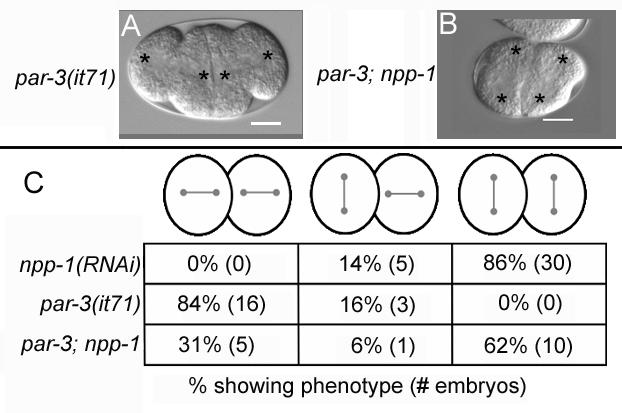
NPP-1 is required for the ectopic AB spindle rotation in par-3(it71) embryos
The spindle orientation defects of npp-1(RNAi) embryos are similar to defects that affect general polarity in the embryo (Cheng et al., 1995; Kemphues et al., 1988a). In wild type embryos, the AB centrosome/nuclear complex does not rotate and the spindle orients transversely. The polarity proteins PAR-3, PAR-6 and PKC-3 (anterior PAR proteins) are required to prevent the rotation of the AB spindle. In embryos mutant for these genes or depleted of protein by RNAi both the AB and P1 spindles are oriented longitudinally (Fig. 2A) (Kemphues et al., 1988b; Tabuse et al., 1998). To examine the functional relationship between NPP-1 and the anterior PAR proteins we carried out two epistasis experiments. In the first we carried out npp-1 RNAi in a par-3 mutant background and in the second we assessed the distribution of PAR-6::GFP in npp-1(RNAi) embryos. In par-3(it71) embryos, both the AB and P1 spindle were found to be oriented longitudinally in 84% of embryos and no embryos had both spindles oriented transversely (n=19) (Fig. 2B, C). In par-3(it71); npp-1(RNAi) embryos, only 31% of the embryos exhibited Par-3-like longitudinal orientations in both the AB and P1 spindles and 62% showed Npp-1-like transverse spindle orientations. Since a failed rotation of both AB and P1 is never seen in par-3(it71) embryos, we take this partial epistasis to indicate that rotation of the AB spindle in par-3embryos requires NPP-1.
Figure 2.
NPP-1 is required for ectopic spindle rotation of the AB cell in par-3 mutant embryos. A,B: Differential interference constrast photomicrographs of par-3 (it71) (A) and npp-1(RNAi); par-3(it71) (B) embryos. Anterior is to the left. Asterisks mark centrosome positions. C: Table summarizing spindle orientations observed in npp-1(RNAi), par-3(it71) and npp-1(RNAi);par-3(it71) embryos. Bar represents 10 microns.
In the second experiment we determined the effect of NPP-1 depletion on the distribution of one of the anterior PAR proteins, PAR-6. The anterior PAR proteins co-localize to the anterior pole of one-cell embryos. In the two-cell embryo, the proteins cover the cortex of the AB cell but are restricted to the anterior periphery of P1. We depleted NPP-1 in PAR-6::GFP-expressing embryos. Worms with this genetic background were hypersensitive to the npp-1(RNAi) sterility phenotype, so we sampled embryos at an earlier time point after injection when the embryonic phenotypes are less penetrant. We found that PAR-6::GFP (Cuenca et al., 2003) was properly localized in all one-cell npp-1(RNAi) embryos, but became uniformly distributed along the cortex in both AB and P1 in six of nineteen two-cell embryos examined (Fig. 3A-O). The mislocalization of PAR-6::GFP in npp-1(RNAi) embryos correlated with the failure of the P1 spindle to rotate; npp-1(RNAi) embryos that failed to orient the P1 spindle properly mislocalized PAR-6::GFP (n=6) while the npp-1(RNAi) embryos that showed proper spindle orientation displayed proper localization of PAR-6::GFP at the two-cell stage (n=13).
Figure 3.
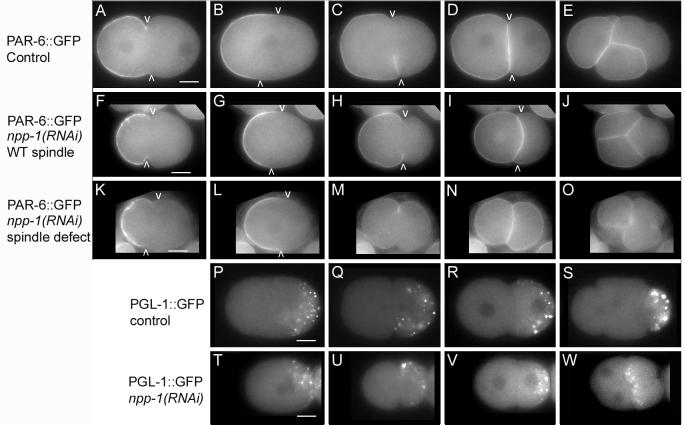
npp-1(RNAi) causes localization defects of PAR-6::GFP and segregation defects of PGL-1::GFP. A-O: Photomicrographs of wild-type embryos (A-E) and npp-1(RNAi) (F-O) embryos expressing PAR-6::GFP. Arrowheads mark the posterior boundary of cortical PAR-6::GFP. F-J: PAR-6::GFP is similar to wild type in an npp-1(RNAi) embryo with normal spindle orientation. K-O PAR-6::GFP is mislocalized at cytokinesis and in the two cell stage in an npp-1(RNAi) embryo with abnormal spindle orientation. P-W: Micrographs of PGL-1::GFP distribution in control embryos (P-S) and npp-1(RNAi) embryos (T-W) at similar time points. PGL-1::GFP initially localizes properly in P0 and P1 in npp-1 (RNAi) embryos (T-V). Subsequently, PGL-1::GFP is improperly segregated to both EMS and P2 in npp-1(RNAi) embryos (W). Bar represents 10 microns.
npp-1(RNAi) embryos improperly segregate P granules to P2 and EMS
Spindle orientation defects often correlate with general defects in polarity (Cheng et al., 1995; Kemphues et al., 1988b; Watts et al., 1996). To examine how cell polarity was affected, we examined PGL-1::GFP (kindly provided by S. Strome) localization in npp-1(RNAi) embryos. PGL-1 is a component of P granules (Kawasaki et al., 1998). P granules are asymmetrically distributed to the posterior of P0 and are segregated to P1 and P2 in subsequent divisions (Strome and Wood, 1982) (Fig. 3P-S). Transgenic animals expressing PGL-1::GFP were subjected to npp-1(RNAi). The distribution of PGL-1::GFP is unaffected in P0 or P1 (Fig. 3T-V) indicating that the initial cues required for establishing cellular polarity are not affected in npp-1(RNAi) embryos. Although PGL-1::GFP is properly localized in the two-cell embryo, the spindle orientation defect in P1 causes P granules to become mis-segregated to both EMS and P2 (Fig. 3W).
npp-1(RNAi) embryos fail to differentiate pharyx or gut cell types
An improperly aligned mitotic spindle is likely to cause the improper segregation of cell fate determinants and hence loss of specific cell types. A less specific affect on overall metabolism might be expected to affect all cell types equally. Because of this we were interested in which tissues are present in terminally differentiated npp-1(RNAi) embryos. Different strains expressing either AJM-1::GFP, MYO-3::GFP, UNC-119::GFP translational fusions or MYO-2::GFP and F22B7.9::GFP transcriptional fusion were subjected to npp-1(RNAi). As shown in supplemental figure 1, terminally differentiated embryos express AJM-1::GFP (an epidermal marker) (Mohler et al., 1998), MYO-3::GFP (a body wall muscle marker) (Fire et al., 1998), and UNC-119::GFP (a neuronal marker) (Maduro and Pilgrim, 1995), but fail to express MYO-2::GFP (pharyngeal muscle) (Ardizzi and Epstein, 1987) or F22B7.9::GFP (intestinal marker; data not shown). The differentiation of these tissues indicates that npp-1(RNAi) does not block differentiation in general, but only blocks the differentiation of cell types known to be dependent upon proper early segregation or activity of cytoplasmic determinants in the P1 lineage. (Bowerman et al., 1992; Kemphues et al., 1988b; Mello et al., 1992).
npp-1(RNAi) causes pleitropic defects
Besides the spindle orientation defect, npp-1(RNAi) embryos exhibit other defects including a reduced size and a slower cell cycle. Defects in cell size were observed in the oocytes produced by hermaphrodites 48 hours after injection. The width of the second through fourth oocyte proximal to the spermtheca was measured. The overall organization of the ovary was normal, but oocytes produced by npp-1(RNAi) animals were approximately 20% smaller than wild type oocytes and embryos were 33% shorter than wild type. Wild type embryos are 52.4 (+/- 1.6)μ in length (n=30): npp-1(RNAi) embryos are 35.0 (+\- 5.6)μ (n=32).
The cell cycle progressed 42% slower in npp-1 embryos as compared to wild type. In wild type embryos, 14.4 (+/- 0.6) minutes elapsed (n=13) from nuclear envelope breakdown of P0 to the nuclear envelope breakdown of AB while npp-1(RNAi) embryos took 20.4 (+/- 1.4) minutes (n=9).
npp-1(RNAi) results in reduced fecundity of the injected animals. In our experiments, injected hermaphrodites were moved to new plates every 24 hours and the number of embryos laid was counted. Similar numbers of embryos were laid from 24-48 hours post-injection with control animals laying an average of 136 embryos (n=10) while injected animals laid an average of 142 embryos (n=8) in that time period. Injected animals produced fewer embryos from 48-72 hours post injection. Uninjected animals laid an average of 100 embryos (n=10) while injected animals laid 65% fewer embryos with an average of 36 (n=8). After this time, injected animals were sterile (n=7) while uninjected animals laid an additional 24 embryos per animal (n=10) from 72-96 hours post-injection. The reduction in fecundity was not recovered by mating the injected animals to males. These defects imply that NPP-1 also has a role in oogenesis.
Other rare defects (< 10%) included a symmetric placement of the P0 mitotic spindle such that AB and P1 were of roughly equivalent sizes, the AB and P1 cells undergoing a synchronous second division, and the formation of multiple nuclei in AB and P1. These defects were not characterized further.
npp-1a and b splice variants are functionally redundant
NPP-1 corresponds to the predicted WORMPEP protein K07F5.13 (Galy et al., 2003; Harris et al., 2003). There are 3 predicted splice variants of this protein; all share the final 446 amino acids. The three forms, NPP-1a, NPP-1b, and NPP-1c, are predicted to encode proteins of 613, 550, and 640 amino acids respectively. Sequences of ESTs yk136e9, yk508f2, yk427d3 (provided by Y. Kohara) were in complete agreement with WORMBASE predictions, indicating that all three variants are produced. Protein domain searches with SMART (Letunic et al., 2002; Schultz et al., 1998) revealed no predictable domain structure in any splice variant. NPP-1c, the longest of the variants is 28% identical (88/312) and 50% similar (156/312) to human nucleoporin Nup54 at the amino acid level. NPP-1 is also related in sequence to the S. cerevisiae nucleoporin Nup57. In yeast, Nup57p is found on both the cytoplasmic and nucleoplasmic faces of the central channel of the NPC (Fahrenkrog et al., 1998). Nup57p also has an active role in protein import and mRNA export. Nup57p is the organizing center of the nucleoporin complex containing Nsp1p, Nup57p, Nup49p (Schlaich et al., 1997). Similar analysis in the vertebrate systems have shown that these interactions and functions are conserved (Finlay et al., 1991; Pante et al., 1994).
To determine which variants are required for proper spindle orientation, we targeted each splice variant by RNAi. We found that at least NPP-1a and NPP-1b are functionally redundant. Double stranded RNA that targeted both NPP-1a and NPP-1c forms but not the b form gave 99-100% viable progeny indicating that NPP-1b is sufficient for NPP-1 activity. Double stranded RNA targeting NPP-1b and NPP-1c but not NPP-1a was also injected. In this case only NPP-1a is expressed and 99-100% of the embryos hatch from each injected animal indicating NPP-1a is sufficient for NPP-1 function as well. Double standed RNA of similar size that targets all three forms of NPP-1 results in only 0-5% of embryos hatching. Unfortunately it is not possible to design a dsRNA that will specifically deplete NPP-1a and NPP-1b. We take these results to indicate that the different splice variants are functionally redundant and that at least NPP-1a and NPP-1b are each sufficient for NPP-1 function in the embryo.
NPP-1::GFP localizes to the nuclear envelope
In order to determine where and when NPP-1 is expressed, we made transgenic animals expressing NPP-1::GFP under the control of the npp-1 promoter and 3’UTR. We tagged the C-terminus of npp-1 with GFP and used 556 base pairs of the promoter sequence and 524 base pairs of the 3’ UTR to control the expression. This construct should express tagged forms of all three splice variants of npp-1.
NPP-1::GFP is localized to the nuclear envelope of most, if not all cells of transgenic animals (Fig. 4A-G and data not shown). This is consistent with the expected localization of a nucleoporin. We see no evidence for developmental regulation of npp-1 because all cells in all stages of development express NPP-1::GFP. Five stable lines were produced, all of which show identical expression patterns. Signal is detected at pronuclear formation and is found on the nuclear envelope of both the maternal and paternal pronucleus. Signal disappears at pronuclear breakdown, but returns to the nuclear envelope as the envelope reforms during telophase. There is also some punctate cytoplasmic GFP signal that can be observed moving in the cytoplasm. These punctuate cytoplasmic signals may be an artifact of expressing GFP because similar patterns are seen with negative control constructs (data not shown). There are also frequently punctate GFP signals inside the nucleus which are not apparent in the GFP-only control lines (Fig. 4G).
Figure 4.
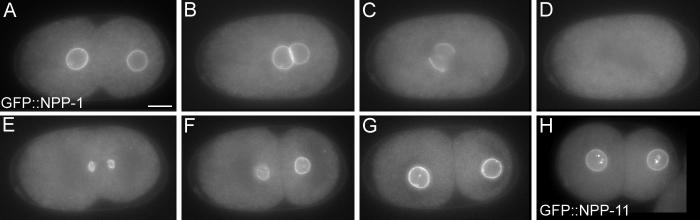
Putative nucleoporins NPP-1 and NPP-11 localize to the nuclear envelope. A-G: Developmental time course showing GFP::NPP-1 distribution. H: GFP::NPP-11 at the two-cell stage. Bar represents 10 microns.
npp-1(RNAi) affects nuclear function and structure
To determine the effect of depleting NPP-1 on the nuclear pore distribution we performed indirect immunofluorescence with the monoclonal antibody mAb414. This antibody was made against rat Nup62 but recognizes a subset of nucleoporins and has been shown to recognize the nuclear envelope in C. elegans (Browning and Strome, 1996; Davis and Blobel, 1987). In wild type embryos, mAb414 localizes in a smooth pattern around the entire nuclear envelope (Fig. 5B). In contrast, npp-1(RNAi) embryos show a punctate distribution along the nuclear envelope (Fig. 5F). Either a reduced number of nuclear pore complexes or a clustering of the nuclear pore complexes could cause this abnormal distribution. Similar defects in mAb414 localization are also seen after depletion of two other nucleoporins NPP-3 and NPP-13 (Galy et al., 2003).
Figure 5.
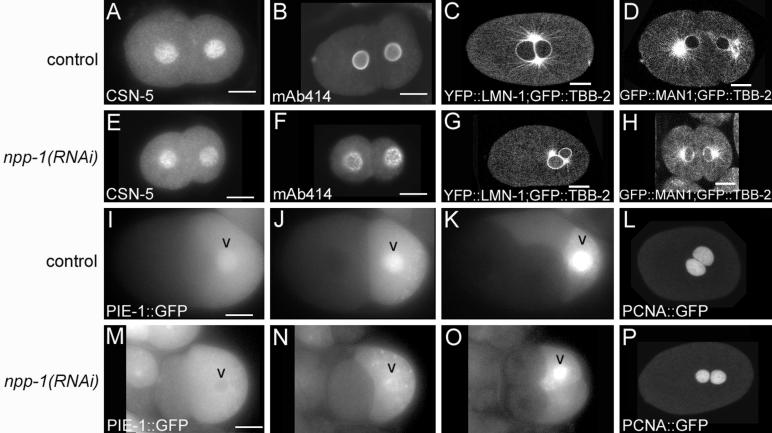
npp-1(RNAi) affects some aspects of nuclear function and structure. Comparision of protein distributions in control and npp-1(RNAi) embryos for the indicated proteins. A,B,E,F: Immunofluourescence images. C,D,G,H,L,P: Confocal images. I-K and M-O are time courses showing the delayed nuclear entry of PIE-1::GFP in npp-1(RNAi) versus control embryos. Bar represents 10 microns.
We also examined the integrity of the nuclear envelope by comparing YFP::LMN-1 and GFP::MAN1 distribution in npp-1(RNAi) and wild type embryos. lmn-1 encodes lamin, an important structural component of the nuclear envelope (Liu et al., 2000). MAN1 (encoded by lem-2) is an integral membrane protein of the nuclear envelope that interacts with lamin and localizes to the nuclear envelope in C. elegans (Lee et al., 2000; Liu et al., 2003). Neither YFP::LMN-1 (n=7) localization nor GFP::MAN1 (n=6) localization is affected in npp-1(RNAi) embryos (Fig. 5C-D, G-H).
To examine nuclear pore function, we examined the ability of NPP-1 depleted nuclei to exclude cytoplasmic proteins and to import and retain proteins normally localized to the nucleus. A nucleus with a properly formed nuclear envelope and NPCs will exclude cytoplasmic macromolecules from the nucleus (Shulga et al., 2000). If the nuclear pore complex structure is compromised, macromolecules can passively travel through the nuclear pore. In wild type embryos, β-Tubulin::GFP is excluded from the nucleus (Fig. 1M; Fig.5 C,D). In npp-1(RNAi) embryos, soluble β-Tubulin::GFP passively enters the nucleus indicating that there is a defect in the integrity of the nuclear envelope or nuclear pore complexes (Fig. 1Q; Fig 5G, H). Depletion of nucleoporins NPP-3 and NPP-13 cause a similar defect in nuclear exclusion; however, the abnormal chromatin behavior that accompanies this defect in npp-3(RNAi) and npp-13(RNAi) embryos (Galy et al., 2003) is not observed upon depletion of NPP-1 (n=6, data not shown). To assess the effect of NPP-1 depletion on nuclear protein localization, we assayed nuclear accumulation of three proteins known to become localized to the nucleus in early embryos: CSN-5, PCNA::GFP and PIE-1::GFP. CSN-5 encodes the homologue of subunit 5 of the COP9 signalsome complex which regulates ubiquitin ligase activity (Smith et al., 2002). Indirect immuno-fluorescence with anti-CSN-5 antibodies shows a strong nuclear localization in all cells of the C. elegans embryo (Pintard et al., 2003). PCNA (proliferating cell nuclear antigen) is an essential component of DNA replication and repair machinery. PCNA::GFP is imported into the interphase nucleus and persists until nuclear envelope breakdown (Brauchle et al., 2003). PIE-1 is a maternally expressed zinc finger-containing protein that becomes enriched in the paternal pronucleus of P0, and the nuclei of P1 and P2 (Mello et al., 1996). We assayed the nuclear accumulation of CSN-5 in both early and later stage embryos but only assayed the accumulation of PCNA::GFP in one-and two-cell embryos. We found that nuclear localization of CSN-5 (88%, n=17) and PCNA::GFP (100%, n=3) is normal in npp-1(RNAi) embryos (Fig. 5A, E, L, P), but that import of PIE-1::GFP is modestly affected. In contrast to normal development, after depletion of NPP-1, in 20 of 21 embryos examined, PIE-1::GFP fails to become enriched in either the paternal pronucleus or the P1 nucleus (Fig. 5M, N). However in 18 of 21 embryos, PIE-1::GFP is imported into the nuclei of P2, P3 and P4 blastomeres as in control embryos (Fig. 5O). While some aspects of nuclear function are clearly affected, most aspects that were analyzed were not severely compromised indicating that much of the nuclear function remains intact in npp-1(RNAi) embryos.
NPP-3, NPP-4, NPP11, and NPP-13 are also required for spindle rotation
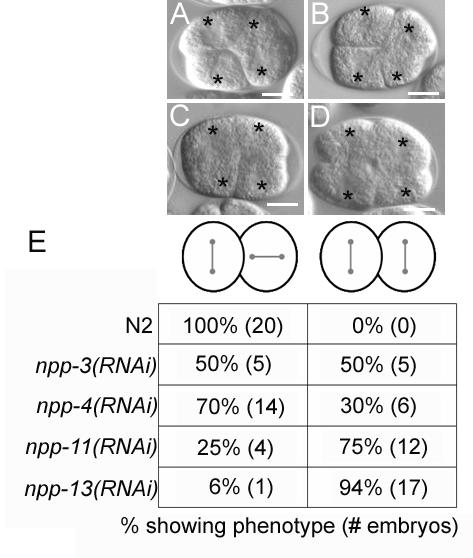
Recently C. elegans homologues of 20 vertebrate nucleoporins were identified (Galy et al., 2003). In that study each of the nucleoporins was depleted by RNAi and the effect on nuclear function examined. Depletion of seven nucleoporins gave viable embryos and depletion of one other, NPP-2, resulted in a low level of embryonic lethality; we did not analyze these eight. Eight other nucleoporins are required for embryonic viability and also required for nuclear envelope formation. Depletion of NPP-8 or NPP-9 via RNAi results in a failure to form a nuclear envelope and uncouples the centrosomes from the chromatin (Askjaer et al., 2002; Franz et al., 2005). In spite of these defects, centrosomes align along the long axis in the P0, and can orient properly in P1 (data not shown). In addition to npp-1, four nucleoporins are required for embryonic viability but not required for nuclear envelope formation, npp-3, npp-4, npp-11, and npp-13. These genes are homologues of vertebrate nucleoporins Nup205, Nup45/Nup58, Nup62, and Nup93 respectively. We tested this class of nucleoporins to see if any were required for proper spindle orientation via RNAi. Indeed, we found after more prolonged RNAi treatment than used in the previous study that all four nucleoporins were required for the proper spindle rotation of P1 (Fig. 6). In npp-3(RNAi) embryos, npp-4(RNAi) embryos, npp-11(RNAi) embryos, and npp-13(RNAi) embryos, 48 hours after RNAi, the P1 spindle failed to rotate 50% (n=10), 30% (n=20), 75% (n=16), and 94% (n=18) of the time, respectively. We also expressed NPP-11::GFP under the control of the npp-1 promoter and it also localized to the nuclear envelope supporting the prediction that it is a nucleoporin (Fig. 4H).
Figure 6.
The nucleoporins NPP-3, NPP-4, NPP-11, and NPP-13 are required for P1 spindle orientation. Differential interference contrast photomicrographs of npp-3(RNAi) (A), npp-4(RNAi) (B), npp-11(RNAi) (C), and npp-13(RNAi) (D) embryos. Asterisks mark centrosome positions. (E) Summary of results. Bar represents 10 microns.
NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 interact in yeast
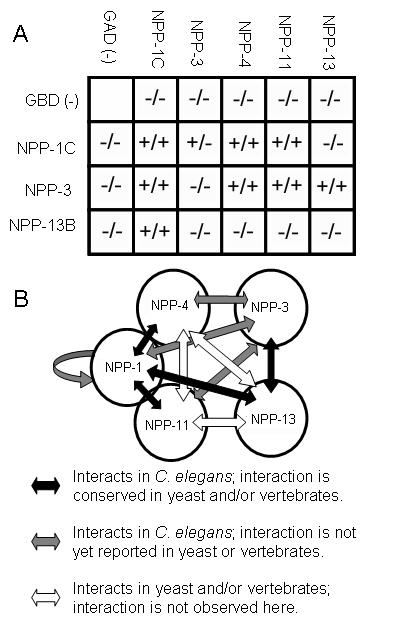
Homologues of NPP-1, NPP-4, NPP-11, and NPP-13 have been shown to form a protein complex in both yeast and vertebrate systems (Grandi et al., 1995; Hu et al., 1996; Ito et al., 2001). Similarly, human homologues of NPP-3 and NPP-13 have been shown to physically interact as well (Kosova et al., 1999). In order to test whether this subclass of nucleoporins also interact in C. elegans, we cloned full-length cDNAs of each into the pGAD and pGBD two-hybrid vectors (James et al., 1996). NPP-1 and NPP-13 have multiple splice variants. NPP-1c is the largest splice variant and includes all exons found in NPP-1a and NPP-1b. NPP-13b is a smaller splice variant and was used because of difficulties in cloning the a variant. Pairwise combinations among the five nucleoporins required for spindle orientation were transformed into the yeast strain PJ69-4A and tested for growth on two sets of plates, one lacking adenine and the other lacking histidine. We found that, as reported for yeast and vertebrates (Finlay et al., 1991; Grandi et al., 1993), most of these C. elegans nucleoporins interact with one another (Fig. 7). Many of the interactions found in the yeast and vertebrate nucleoporin homologs were also found here (black arrows Fig. 7) but a few were not (white arrows, Fig.7). We also identified interactions that have not been previously reported by homologues in other systems (gray arrows, Fig. 7). NPP-4 and NPP-11 are predicted to interact based on evidence from vertebrate and yeast (Finlay et al., 1991; Grandi et al., 1993), but we were unable to assess this interaction because both NPP-4 and NPP-11 activated reporters by themselves when fused to the DNA binding domain (pGBD).
Figure 7.
NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 physically interact via 2-hybrid. A summary of results from yeast two-hybrid analysis. The table indicates growth (+) or lack of growth (-) on either adenine drop out or histidine drop out plates. All of the tested nucleoporins interact with one another or are predicted to interact with one another based on what is known in vertebrates and yeast (B). Black arrows indicate interactions which have been demonstrated in yeast and/or vertebrates that have been reproduced in this study. Grey arrows indicate interactions observed in this study which have not yet been reported in other systems. White arrows indicate interactions demonstrated in vertebrates and/or yeast that were not reproduced here.
DISCUSSION
We have shown that the C. elegans nucleoporins NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 interact and are required for the proper orientation of the mitotic spindle in one- and two-cell stage C. elegans embryos. Although several proteins previously have been identified as determinants for spindle positioning, our work is the first to implicate nucleoporins in this process. The yeast two-hybrid interactions and the similar RNAi phenotypes of this subset of nucleoporins suggest that they act as a complex in a common cellular process that is required for proper spindle orientation. Because the interactions between the nucleoporins are conserved, it is possible that their involvement in spindle orientation is also conserved.
The mechanism by which NPP-1,-3,-4,-11, and -13 affect spindle orientation is not clear. The nucleoporins could be affecting spindle orientation either directly by participating in the process of centrosome/nuclear complex rotation or indirectly by affecting the polarity system. Mutation of par-2 results in a spindle orientation phenotype similar to that of npp-1(RNAi)—failure of the P1 spindle to rotate (Cheng et al., 1995), but the par-2 effect is indirect. Absence of PAR-2 leads to the abnormal accumulation of anterior complex proteins at the P1 cell cortex (Cuenca et al., 2003; Etemad-Moghadam et al., 1995). Because par-3 is epistatic to par-2 for the spindle orientation defect it appears that mislocalized PAR-3 blocks the spindle orientation in P1 in the par-2 mutant (Cheng et al., 1995). npp-1(RNAi), like par-2 mutants, results in abnormal accumulation of cortical PAR-6::GFP in the P1 cell, consistent with a role in localizing the polarity proteins. However, in contrast to the par-2 par-3 double mutant result, the npp-1(RNAi) spindle phenotype is at least partially epistatic to par-3. We interpret this partial epsistas to mean that NPP-1 and presumably NPP-3,-4,-11 and -13 are contributing to the mechanics of spindle rotation independently of their effect on distribution of PAR-6.
Determining the exact role for nucleoporins in orienting the mitotic spindle will be a challenge. It is possible that one or more of these proteins interacts directly with factors regulating the mitotic spindle and this interaction is required for proper spindle rotation. Alternatively, the spindle orientation defects could be an indirect effect caused by a partial failure in nuclear function during oogenesis or early embryogenesis.
Homologues of NPP-1, NPP-4, NPP-11, and NPP-13 are clearly involved in nucleo-cytoplasmic transport in vertebrates and yeast. Nsp1p, Nup49p, Nup57p, Nic96p, and Nup192 (homologues of NPP-11, NPP-4, NPP-1, NPP-13, and NPP-3, respectively) all show symmetrical localization to both the cytoplasmic and nucleoplasmic sides of the NPC in yeast (Rout and Aitchison, 2001) and mutations in Nsp1, Nup49, Nup57, and Nic96 all lead to defects in the nuclear import of reporter proteins (Doye et al., 1994; Grandi et al., 1995; Nehrbass et al., 1993). Nsp1p, Nup49p, and Nup57p form a core complex that is docked to the NPC via an interaction with Nic96p (Grandi et al., 1995). Similarly, the vertebrate nucleoporins Nup54, Nup58, Nup62 (homologues of NPP-1, NPP-4, and NPP-11, respectively) also form a complex that is involved in nuclear import (Finlay et al., 1991; Pante et al., 1994). When the Nup54/Nup58/Nup62 complex is immunodepleted from nuclear reconstruction extracts, reconstructed nuclei form with NPCs but are impaired in their ability to import a reporter protein. Adding the purified rat Nup54/Nup58/Nup62 complex to the extract can restore import activity. The conservation of function between yeast and vertebrate systems, together with our observations, suggests that in C. elegans, NPP-1, NPP-3, NPP4, NPP-11, and NPP-13 also are involved in nuclear import. Consistent with this, nuclear growth is inhibited when each of these genes are targeted by RNAi (Galy et al., 2003).
It is clear from the NPC clustering phenotype, the failure to exclude β-Tubulin::GFP, and the failure to import PIE-1::GFP in npp-1(RNAi) embryos that npp-1(RNAi) causes defects in NPC function. This suggests that the spindle orientation defects could be indirect. However, npp-1 seems not to be required for the localization of the inner nuclear membrane protein GFP::MAN1 nor for the nuclear import of CSN-5, YFP::LMN-1 and PCNA::GFP indicating that NPC function in protein import is not severely compromised. Similarly, terminally differentiated npp-1(RNAi) embryos express a number of cell type-specific GFP markers, indicating that their cells are capable of differentiation. Cells with completely compromised nuclei would likely not be able to differentiate. Therefore, catastrophic defects in NPC function have apparently not occurred in the npp-1(RNAi) embryos. If the spindle defects are indeed indirect effects of compromised NPC function, identifying the affected molecules would be of great interest.
In addition to nuclear import and export, nucleoporins have been implicated in a number of cellular processes including chromatin organization (Galy et al., 2000), chromosome segregation (Kerscher et al., 2001), and interaction with the spindle checkpoint machinery (Iouk et al., 2002). In addition to their nuclear envelope enrichment, some nucleoporins also localize to regions outside of the NPC. Vertebrate Nup98 is found concentrated in foci within the nucleus (Griffis et al., 2002). Yeast Ndc1 is an essential component of the spindle pole body and is required for its duplication (Chial et al., 1998; Winey et al., 1993). We have localized two C. elegans nucleoporins to the kinetochore during mitosis (V. Galy, P.A., I.W.M. unpublished). Vertebrate Nup62 interacts with a general transcription factor Sp1 and a putative transcription factor rtSox23 suggesting a requirement of Nup62 for proper transcription in certain situations (Han et al., 1998; Yamashita et al., 1998). Nup62 has also been suggested to be involved NF-kB signaling (Gamper et al., 2000). The diversity of functions ascribed to nucleoporins, especially homologs of NPP-11, raises the possibility that C. elegans nucleoporins could have roles away from the NPC and thus could play a direct role in orienting the mitotic spindle.
Evidence in both yeast and vertebrate models suggests the possibility of a more direct role for nucleoporins in spindle orientation. Nup57p, the yeast homologue of npp-1, interacts with Jnm1p, a member of the dynactin complex (Ito et al., 2001). jnm1 null mutants display defects in nuclear migration and defects in spindle orientation that are analogous to spindle orientation defects seen in npp-1(RNAi) embryos (McMillan and Tatchell, 1994). Jnm1p is the functional homologue of the vertebrate dynactin component p50/Dynamitin (Kahana et al., 1998). A recent study demonstrated that five nucleoporins, including nucleoporin Nup62 (NPP-11), co-immunoprecipitated with the dynactin p150(Glued) from bovine oocytes (Payne et al., 2003). Dynactin p150(Glued) also co-localizes to with nucleoporin Nup62 on the nuclear envelope in vertebrates (Payne et al., 2003). In C. elegans, depletion of the dynactin components DNC-1/p150(Glued) or DNC-2/dynamitin via RNAi led to a failed rotation of the mitotic spindle in P0 and P1 similar to the spindle orientation defects observed in npp-1(RNAi) embryos (Skop and White, 1998). The interaction between Jnm1p/dynamitin and Nup57p in S. cerevisiae predicts that NPP-1 may interact with DNC-2 in C. elegans. The interaction between vertebrate nucleoporin Nup62 and dynactin p150(Glued) predicts that NPP-11 and DNC-1 may also interact. If these interactions are required for the function of the dynactin complex, then depleting either NPP-1 or NPP-11 could lead to the spindle orientation defects observed in this study. Similarly, if the physical interactions between NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 are important for this function, then depletion of any of these components could lead to the observed spindle orientation defects. Although we were unable to detect an interaction with any of the nucleoporins and DNC-1or DNC-2 using the yeast two-hybrid system, such an interaction remains possible.
We can only speculate as to how these NPC components might contribute to spindle orientation. One simple model is a direct interaction with the dynactin complex to anchor the centrosomes to the nuclear envelope during rotation. Alternatively NPPs could influence microtubule dynamics either at the minus ends or at the plus ends by modifying proteins that migrate from the centrosomes to the cortex.
Analysis of the relationship between the NPPs and OOC-3 and OOC-5 could provide some insight. The spindle orientation defects, the failure to maintain cell polarity, and the smaller embryo size observed in npp-1(RNAi) embryos resembles phenotypes observed in ooc-3 and ooc-5 embryos (Basham and Rose, 1999; Rose and Kemphues, 1998). OOC-3 and the Torsin-related protein OOC-5 both localize to the endoplasmic reticulum and the nuclear envelope (Basham and Rose, 2001). A recent study demonstrated that the ATP-bound form of TorsinA localizes to the nuclear envelope in COS-7 cells while the ATP-free form is distributed throughout the endoplasmic reticulum (Naismith et al., 2004). This suggests that TorsinA binding partners are found at the nuclear envelope and may include nucleoporins or other nuclear envelope proteins. Thus OOC-5 could interact with nuclear envelope proteins including nucleoporins. OOC-5 activity at the nuclear envelope could then facilitate the nucleoporin activity required for proper rotation of the mitotic spindle.
In conclusion, we have shown that the C. elegans nucleoporins NPP-1, NPP-3, NPP-4, NPP-11, and NPP-13 physically interact and are required for spindle orientation in early embryos.
Supplementary Material
Acknowledgments:
We would like to thank Dr. Susan Strome for providing the PGL-1::GFP strain. Dr. Matthias Peter for providing the CSN-5 antibodies, Dr. Yuji Kohara for providing several ESTs, Dr. Donato Aceto for providing the PAR-6::GFP strain, Dr. Jun Kelly Liu for other necessary reagents and advice, and the Caenorhabditis Genetics Center for providing various strains. We also thank Mona Hassab for providing technical assistance. This work was supported by NIH Institute of Child Health and Development Grant HD27698. A.S. was supported by a NIH training grant in Genetics and Development #6M07617. P.A. was supported by the Deutsche Forschungsgemeinschaft and EMBL. A.S. is currently supported by the Cancer Prevention Fellowship Program, Division of Cancer Prevention, National Cancer Institute.
REFERENCES:
- Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol. 2000;149:863–74. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DG. Formation of the first cleavage spindle in nematode embryos. Dev Biol. 1984;101:61–72. doi: 10.1016/0012-1606(84)90117-9. [DOI] [PubMed] [Google Scholar]
- Ardizzi JP, Epstein HF. Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J Cell Biol. 1987;105:2763–70. doi: 10.1083/jcb.105.6.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P, Galy V, Hannak E, Mattaj IW. Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol Biol Cell. 2002;13:4355–70. doi: 10.1091/mbc.E02-06-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham SE, Rose LS. Mutations in ooc-5 and ooc-3 disrupt oocyte formation and the reestablishment of asymmetric PAR protein localization in two-cell Caenorhabditis elegans embryos. Dev Biol. 1999;215:253–63. doi: 10.1006/dbio.1999.9447. [DOI] [PubMed] [Google Scholar]
- Basham SE, Rose LS. The Caenorhabditis elegans polarity gene ooc-5 encodes a Torsin-related protein of the AAA ATPase superfamily. Development. 2001;128:4645–56. doi: 10.1242/dev.128.22.4645. [DOI] [PubMed] [Google Scholar]
- Belgareh N, Rabut G, Bai SW, van Overbeek M, Beaudouin J, Daigle N, Zatsepina OV, Pasteau F, Labas V, Fromont-Racine M, Ellenberg J, Doye V. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol. 2001;154:1147–60. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68:1061–75. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Baumer K, Gonczy P. Differential Activation of the DNA Replication Checkpoint Contributes to Asynchrony of Cell Division in C. elegans Embryos. Curr Biol. 2003;13:819–27. doi: 10.1016/s0960-9822(03)00295-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H, Strome S. A sperm-supplied factor required for embryogenesis in C. elegans. Development. 1996;122:391–404. doi: 10.1242/dev.122.1.391. [DOI] [PubMed] [Google Scholar]
- Cheng NN, Kirby CM, Kemphues KJ. Control of cleavage spindle orientation in Caenorhabditis elegans: the role of the genes par-2 and par-3. Genetics. 1995;139:549–59. doi: 10.1093/genetics/139.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chial HJ, Rout MP, Giddings TH, Winey M. Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J Cell Biol. 1998;143:1789–800. doi: 10.1083/jcb.143.7.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Tzur YB, Neufeld E, Feinstein N, Delannoy MR, Wilson KL, Gruenbaum Y. Transmission electron microscope studies of the nuclear envelope in Caenorhabditis elegans embryos. J Struct Biol. 2002;140:232–40. doi: 10.1016/s1047-8477(02)00516-6. [DOI] [PubMed] [Google Scholar]
- Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development. 2003;130:1255–65. doi: 10.1242/dev.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LI, Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci U S A. 1987;84:7552–6. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye V, Wepf R, Hurt EC. A novel nuclear pore protein Nup133p with distinct roles in poly(A)+ RNA transport and nuclear pore distribution. Embo J. 1994;13:6062–75. doi: 10.1002/j.1460-2075.1994.tb06953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–52. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Hurt EC, Aebi U, Pante N. Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes. J Cell Biol. 1998;143:577–88. doi: 10.1083/jcb.143.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DR, Meier E, Bradley P, Horecka J, Forbes DJ. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991;114:169–83. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Mario A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. Embo J. 2005 doi: 10.1038/sj.emboj.7600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell. 2003;14:5104–15. doi: 10.1091/mbc.E03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Olivo-Marin JC, Scherthan H, Doye V, Rascalou N, Nehrbass U. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403:108–12. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- Gamper C, van Eyndhoven WG, Schweiger E, Mossbacher M, Koo B, Lederman S. TRAF-3 interacts with p62 nucleoporin, a component of the nuclear pore central plug that binds classical NLS-containing import complexes. Mol Immunol. 2000;37:73–84. doi: 10.1016/s0161-5890(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–50. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Doye V, Hurt EC. Purification of NSP1 reveals complex formation with ’GLFG’ nucleoporins and a novel nuclear pore protein NIC96. Embo J. 1993;12:3061–71. doi: 10.1002/j.1460-2075.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Schlaich N, Tekotte H, Hurt EC. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. Embo J. 1995;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol Biol Cell. 2002;13:1282–97. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I, Roos MD, Kudlow JE. Interaction of the transcription factor Sp1 with the nuclear pore protein p62 requires the C-terminal domain of p62. J Cell Biochem. 1998;68:50–61. doi: 10.1002/(sici)1097-4644(19980101)68:1<50::aid-jcb5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Harel A, Orjalo AV, Vincent T, Lachish-Zalait A, Vasu S, Shah S, Zimmerman E, Elbaum M, Forbes DJ. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol Cell. 2003;11:853–64. doi: 10.1016/s1097-2765(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Harris TW, Lee R, Schwarz E, Bradnam K, Lawson D, Chen W, Blasier D, Kenny E, Cunningham F, Kishore R, Chan J, Muller HM, Petcherski A, Thorisson G, Day A, Bieri T, Rogers A, Chen CK, Spieth J, Sternberg P, Durbin R, Stein LD. WormBase: a cross-species database for comparative genomics. Nucleic Acids Res. 2003;31:133–7. doi: 10.1093/nar/gkg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Guan T, Gerace L. Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins. J Cell Biol. 1996;134:589–601. doi: 10.1083/jcb.134.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, White JG. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J Cell Biol. 1987;105:2123–35. doi: 10.1083/jcb.105.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iouk T, Kerscher O, Scott RJ, Basrai MA, Wozniak RW. The yeast nuclear pore complex functionally interacts with components of the spindle assembly checkpoint. J Cell Biol. 2002;159:807–19. doi: 10.1083/jcb.200205068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci U S A. 2001;98:4569–74. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–36. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J Cell Biol. 2002;156:595–602. doi: 10.1083/jcb.200110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana JA, Schlenstedt G, Evanchuk DM, Geiser JR, Hoyt MA, Silver PA. The yeast dynactin complex is involved in partitioning the mitotic spindle between mother and daughter cells during anaphase B. Mol Biol Cell. 1998;9:1741–56. doi: 10.1091/mbc.9.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher RL, Deacon SW, Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 2002;12:21–7. doi: 10.1016/s0962-8924(01)02184-5. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Shim YH, Kirchner J, Kaminker J, Wood WB, Strome S. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–45. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–38. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Kusch M, Wolf N. Maternal-effect lethal mutations on linkage group II of Caenorhabditis elegans. Genetics. 1988a;120:977–86. doi: 10.1093/genetics/120.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988b;52:311–20. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Hieter P, Winey M, Basrai MA. Novel role for a Saccharomyces cerevisiae nucleoporin, Nup170p, in chromosome segregation. Genetics. 2001;157:1543–53. doi: 10.1093/genetics/157.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosova B, Pante N, Rollenhagen C, Hurt E. Nup192p is a conserved nucleoporin with a preferential location at the inner site of the nuclear membrane. J Biol Chem. 1999;274:22646–51. doi: 10.1074/jbc.274.32.22646. [DOI] [PubMed] [Google Scholar]
- Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell. 2000;11:3089–99. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Goodstadt L, Dickens NJ, Doerks T, Schultz J, Mott R, Ciccarelli F, Copley RR, Ponting CP, Bork P. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 2002;30:242–4. doi: 10.1093/nar/30.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ben-Shahar TR, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:4598–603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiodice I, Alves A, Rabut G, Van Overbeek M, Ellenberg J, Sibarita JB, Doye V. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol Biol Cell. 2004;15:3333–44. doi: 10.1091/mbc.E03-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995;141:977–88. doi: 10.1093/genetics/141.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JN, Tatchell K. The JNM1 gene in the yeast Saccharomyces cerevisiae is required for nuclear migration and spindle orientation during the mitotic cell cycle. J Cell Biol. 1994;125:143–58. doi: 10.1083/jcb.125.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Draper BW, Krause M, Weintraub H, Priess JR. The pie-1 and mex-1 genes and maternal control of blastomere identity in early C. elegans embryos. Cell. 1992;70:163–76. doi: 10.1016/0092-8674(92)90542-k. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–70. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–2. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Miller DM, Shakes DC. Immunofluorescence Microscopy. In: Epstein HF, Shakes DC, editors. In ”Caenorhabditis elegans: Modern Biological Analysis of an Organism”. Academic Press; San Diego: 1995. pp. 365–394. [Google Scholar]
- Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–90. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Naismith TV, Heuser JE, Breakefield XO, Hanson PI. Proc Natl Acad Sci U S A. 2004. TorsinA in the nuclear envelope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrbass U, Fabre E, Dihlmann S, Herth W, Hurt EC. Analysis of nucleo-cytoplasmic transport in a thermosensitive mutant of nuclear pore protein NSP1. Eur J Cell Biol. 1993;62:1–12. [PubMed] [Google Scholar]
- Pante N, Bastos R, McMorrow I, Burke B, Aebi U. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J Cell Biol. 1994;126:603–17. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Rawe V, Ramalho-Santos J, Simerly C, Schatten G. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J Cell Sci. 2003;116:4727–38. doi: 10.1242/jcs.00784. [DOI] [PubMed] [Google Scholar]
- Piano F, Schetter AJ, Morton DG, Gunsalus KC, Reinke V, Kim SK, Kemphues KJ. Gene Clustering Based on RNAi Phenotypes of Ovary-Enriched Genes in C. elegans. Curr Biol. 2002;12:1959–64. doi: 10.1016/s0960-9822(02)01301-5. [DOI] [PubMed] [Google Scholar]
- Pintard L, Kurz T, Glaser S, Willis JH, Peter M, Bowerman B. Neddylation and deneddylation of CUL-3 is required to target MEI-1/Katanin for degradation at the meiosis-to-mitosis transition in C. elegans. Curr Biol. 2003;13:911–21. doi: 10.1016/s0960-9822(03)00336-1. [DOI] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–26. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose LS, Kemphues K. The let-99 gene is required for proper spindle orientation during cleavage of the C. elegans embryo. Development. 1998;125:1337–46. doi: 10.1242/dev.125.7.1337. [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD. The nuclear pore complex as a transport machine. J Biol Chem. 2001;276:16593–6. doi: 10.1074/jbc.R100015200. [DOI] [PubMed] [Google Scholar]
- Schlaich NL, Haner M, Lustig A, Aebi U, Hurt EC. In vitro reconstitution of a heterotrimeric nucleoporin complex consisting of recombinant Nsp1p, Nup49p, and Nup57p. Mol Biol Cell. 1997;8:33–46. doi: 10.1091/mbc.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95:5857–64. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga N, Mosammaparast N, Wozniak R, Goldfarb DS. Yeast nucleoporins involved in passive nuclear envelope permeability. J Cell Biol. 2000;149:1027–38. doi: 10.1083/jcb.149.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8:1110–6. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Leung-Chiu WM, Montgomery R, Orsborn A, Kuznicki K, Gressman-Coberly E, Mutapcic L, Bennett K. The GLH proteins, Caenorhabditis elegans P granule components, associate with CSN-5 and KGB-1, proteins necessary for fertility, and with ZYX-1, a predicted cytoskeletal protein. Dev Biol. 2002;251:333–47. doi: 10.1006/dbio.2002.0832. [DOI] [PubMed] [Google Scholar]
- Strome S, Wood WB. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1982;79:1558–62. doi: 10.1073/pnas.79.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Suntharalingam M, Wente SR. Peering through the Pore. Nuclear Pore Complex Structure, Assembly, and Function. Dev Cell. 2003;4:775–89. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–14. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- Wang X, Babu JR, Harden JM, Jablonski SA, Gazi MH, Lingle WL, de Groen PC, Yen TJ, van Deursen JM. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J Biol Chem. 2001;276:26559–67. doi: 10.1074/jbc.M101083200. [DOI] [PubMed] [Google Scholar]
- Watts JL, Etemad-Moghadam B, Guo S, Boyd L, Draper BW, Mello CC, Priess JR, Kemphues KJ. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development. 1996;122:3133–40. doi: 10.1242/dev.122.10.3133. [DOI] [PubMed] [Google Scholar]
- Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol. 1993;122:743–51. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Suzuki S, Fujitani K, Kojima M, Kanda H, Ito M, Takamatsu N, Yamashita S, Shiba T. cDNA cloning of a novel rainbow trout SRY-type HMG box protein, rtSox23, and its functional analysis. Gene. 1998;209:193–200. doi: 10.1016/s0378-1119(98)00047-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.