Abstract
Virion capture assays, in which immobilized antibodies (Abs) capture virus particles, have been used to suggest that nonneutralizing Abs bind effectively to human immunodeficiency virus type 1 (HIV-1) primary viruses. Here, we show that virion capture assays, under conditions commonly reported in the literature, give a poor indication of epitope expression on the surface of infectious primary HIV-1. First, estimation of primary HIV-1 capture by p24 measurements shows a very poor correlation with an estimation based on infectivity measurements. Second, virion capture appears to require relatively low Ab affinity for the virion, as shown by the ability of a monoclonal Ab to capture a wild-type and a neutralization escape variant virus equally well. Nevertheless, in a more interpretable competition format, it is shown that nonneutralizing anti-CD4 binding site (CD4bs) Abs compete with a neutralizing anti-CD4bs Ab (b12) for virus capture, suggesting that the nonneutralizing anti-CD4bs Abs are able to bind to the envelope species that is involved in virion capture in these experiments. However, the nonneutralizing anti-CD4bs Abs do not inhibit neutralization by b12 even at considerable excess. This suggests that the nonneutralizing Abs are unable to bind effectively to the envelope species required for virus infectivity. The results were obtained for three different primary virus envelopes. The explanation that we favor is that infectious HIV-1 primary virions can express two forms of gp120, an accessible nonfunctional form and a functional form with limited access. Binding to the nonfunctional form, which needs only to be present at relatively low density on the virion, permits capture but does not lead to neutralization. The expression of a nonfunctional but accessible form of gp120 on virions may contribute to the general failure of HIV-1 infection to elicit cross-neutralizing Abs and may represent a significant problem for vaccines based on viruses or virus-like particles.
Neutralizing antibodies (Abs) can protect against primary human immunodeficiency virus type 1 (HIV-1) challenge in vivo (1, 7, 15, 16, 21, 25, 34) and therefore should ideally be induced by a candidate HIV-1 vaccine. However, although a range of HIV-1 immunogens elicit good Ab responses to HIV-1 envelope proteins, as measured, for example, by enzyme-linked immunosorbent assay (ELISA), this does not translate into neutralizing Ab responses. These responses, especially against conserved epitopes on the virus that are of greater interest to vaccine development, are generally rather poor (2, 3, 39). It then becomes critically important to understand what distinguishes neutralizing and nonneutralizing Abs to HIV-1 envelope.
A favored explanation has been that the difference lies in the ability of Abs to bind to mature oligomeric envelope as expressed on the surfaces of virions. Thus, for T-cell line-adapted (TCLA) viruses, it has been shown that there is a very good correlation between binding to mature oligomeric envelope as expressed on infected cells and neutralization (27, 31, 32). The large preponderance of Abs that bind to monomeric gp120 but do not neutralize do not bind effectively to mature oligomeric gp120. These experiments have been carried out most convincingly with infected cells on which CD4 has been down-modulated. This is necessary, since otherwise CD4 can capture shed gp120 and confound data interpretation.
The correlation between binding affinity for mature TCLA oligomeric envelope and neutralization for a range of Abs against different epitopes on gp120 suggests that occupancy of sites by Abs may be more important than the precise location of the site on the envelope spike (27). Coating of the virion surface by neutralizing Abs may interfere with HIV-1 attachment and/or fusion independently of the epitope recognized by the Ab (24, 28). Accordingly, studies with TCLA viruses show that a range of Abs inhibit attachment of HIV-1 to target cells (37). In summary, in this view, neutralizing Ab molecules coat the surface of HIV-1 and prevent infection. Nonneutralizing Abs do not coat the virion surface and cannot prevent infection.
Such findings have been difficult to extend to HIV-1 primary isolates, in particular due to technical difficulties. Moreover, recent results using virion capture assays appear to contradict this view. It has been shown that nonneutralizing Abs can capture infectious virions (22, 23, 40), and therefore it was concluded that nonneutralizing Abs can indeed bind to mature oligomeric envelope on the virion surface. An alternative view is then provided in which both neutralizing and nonneutralizing Abs bind to functional envelope spikes but only the neutralizing Abs interfere with the infectious process. The two views lead to different approaches to HIV-1 vaccine design. The first view suggests that a vaccine need only elicit Abs that bind well to mature oligomeric envelope, whereas the second view implies that this would be necessary but not sufficient.
Here, we have compared the abilities of Abs to capture virions, neutralize virus, and cross-compete with one another in both assays. We show that virion capture assays alone give a poor indication of epitope expression on the HIV-1 surface. More significantly, nonneutralizing anti-CD4 binding site (CD4bs) Abs compete with a neutralizing anti-CD4bs Ab (b12) for virion capture. However, they are not able to reduce the neutralizing activity of b12 even at high concentrations. This suggests that the nonneutralizing Abs are not interacting with envelope molecules required for viral entry. We propose that infectious virions can express a fraction, which may be very limited, of accessible nonfunctional forms of envelope. Ab binding to these forms is sufficient to permit virion capture but does not lead to neutralization. The results may have important implications for HIV vaccine design and for understanding the humoral response to HIV infection.
MATERIALS AND METHODS
Abs.
HIV-1 gp120-specific monoclonal Abs (MAbs) of human origin were kind gifts from the following sources: the anti-V3 loop Ab 19b was from James Robinson (20), the anti-V3 loop MAb 447-52D was from Susan Zolla-Pazner (8), and the anti-CD4bs Ab F105 was from Lisa Cavacini (29). The anti-CD4bs Abs b12 and Fab b6 were from our laboratory (4, 31). KZ52, an Ab specific for Ebola GP used as a negative control, was from our laboratory (14). The purified normal human immunoglobulin G (IgG) used as a control in some experiments was from our laboratory and was obtained using protein A chromatography. The D7324 Ab specific for a conserved region of the gp120 COOH terminus was obtained from Aalto Bioreagents, Dublin, Ireland.
Viruses.
Viruses pseudotyped with various HIV-1 envelopes and carrying the luciferase reporter gene were generated by cotransfection of 293T cells with the PNL4-3.luc.R−E− vector (provided by Nathaniel Landau) and the pSVIIIexE7 env-expressing vector. The pSVIIIexE7-89.6 and pSVIIIexE7-ADA vectors were provided by Joseph Sodroski. The pSVIIIexE7-JR-CSF vector was obtained by modification of the pSVIIIexE7 vector as reported previously (41). The pSVIIIexE7-p365qJR-CSF vector was obtained by introduction of a point mutation in pSVIIIexE7-JR-CSF using the Quick Change kit (Stratagene, La Jolla, Calif.).
HIV-1JR-CSF, a molecularly cloned primary isolate, was obtained from the AIDS Research Reagents Repository Program (12). Virus stock was made by infection of phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC). Virus was recovered after 5 to 7 days of culture, and the median tissue culture infectious dose per milliliter was determined by endpoint dilution.
Virus neutralization.
Pseudovirus neutralization was assessed by measuring infection of U87 target cells (obtained from the AIDS Research Reagents Repository Program; contributed by Hong Kuy Deng and Dan Littman) using the luciferase reporter gene. U87 CD4+ CCR5+ cells were used to measure infection by JR-CSF and ADA, and U87 CD4+ CXCR4+ cells were used to measure infection by 89.6. Briefly 2 × 104 U87 target cells in 100 μl of medium (Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 1 μg of puromycin/ml, 300 μg of G418/ml, glutamine, and penicillin-streptomycin) were added to microplate wells (96-well flat-bottom plates; Corning Inc., Corning, N.Y.) and incubated for 24 h at 37°C in 5% CO2. Fifty microliters of medium containing the equivalent of 500 to 1,500 pg of p24 of pseudotyped virus was incubated in triplicate with 50 μl of serial dilutions of anti-gp120 Abs for 1 h at 37°C. The virion-Ab mixtures were then added to the U87 cells. After a 3-day incubation, the cells were washed twice with phosphate-buffered saline (PBS) and lysed with 60 μl of luciferase cell culture lysis reagent (Promega, Madison, Wis.). Fifty microliters of lysate was transferred to round-bottom 96-well plates and centrifuged at 1,800 × g for 10 min at 4°C. Twenty microliters was transferred to an opaque assay plate (Corning, Acton, Mass.), and light was measured on a luminometer (EG&G Berthold; Perkin-Elmer, Gaithersburg, Md.) using a luciferase assay reagent (Promega). Neutralization was calculated as percent reduction in the average amount of luciferase activity in lysates from the test culture relative to that of untreated controls.
HIV-1JR-CSF neutralization was assessed using PHA-stimulated PBMC. Briefly, PBMC from three CCR5 wild-type donors were isolated, pooled, and stimulated with PHA (5 μg/ml) for 48 h, followed by interleukin-2 (40 U/ml) for 72 h. One hundred median tissue culture infectious doses of HIV-1JR-CSF was incubated with serial dilutions of Ab for 1 h at 37°C in triplicate. Virus-Ab mixtures were then incubated overnight with 5 × 104 activated PBMC in culture medium (RPMI 1640 supplemented with 10% fetal calf serum, 40 U of interleukin-2/ml, and penicillin-streptomycin). On the following day, the cells were washed extensively, and then they were cultured for 7 days. The cell supernatants were then harvested and tested for virus production by p24 antigen measurement using a commercial p24 ELISA kit (Alliance HIV-1 p24 ELISA kit; Perkin-Elmer Life Science, Boston, Mass.). Neutralization was calculated as percent reduction in the average amount of p24 in supernatants from the test culture relative to that of untreated controls.
Neutralization by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs was done using the protocol described above except for the following difference. Before incubation with the neutralizing Ab b12, virus was first preincubated with nonneutralizing competing Ab for 45 min at 37°C. The Ab b12 was then added, and the virus-Ab mixture was incubated for another 45 min before being added to cells. The assay was then carried out as described above.
Virus capture assay.
Ninety-six-well ELISA plates (Corning) were coated overnight at 4°C with goat anti-human IgG Fc-specific Ab (Sigma, St. Louis, Mo.) at 5 μg/ml in 50 μl of PBS (pH 7.5). The plates were washed twice in 25 mM Tris-HCl (pH 8.0)-150 mM NaCl-0.05% Tween 20 (ELISA wash buffer; EWB) and then saturated with 100 μl of 3% bovine serum albumin in PBS for 1 h at room temperature (RT). After two washes with EWB, the plates were incubated with anti-gp120 Ab at 10 μg/ml in 50 μl of PBS (pH 7.5) for 2 h at RT. After 10 washes with EWB and three washes with sterile PBS, 50 μl of medium containing the equivalent of 500 to 1,500 pg of p24 of pseudotyped virus was added and incubated for 4 h at RT. In some experiments, before being added to the plate, the virus was preincubated with competing anti-gp120 Ab or with a control Ab in sextuplicate for 1 h at 37°C. Following incubation with the virus, the plates were washed 10 times with PBS and processed for either p24 assay or infectious virus quantification experiments.
For p24 quantification, 200 μl of lysis buffer containing 0.5% Triton X were added to the wells. The quantity of p24 captured on the plate was measured using a p24 ELISA kit (Alliance HIV-1 p24 ELISA kit). To quantify infectious pseudovirus particles captured, plates with captured viruses were overlayed with U87 CD4+ CCR5+ or U87 CD4+ CXCR4+ cells (104/well) in a final volume of 200 μl of selective medium. The plates were then incubated for 3 days, and luciferase assays were conducted as described above.
Capture of HIV-1JR-CSF was carried out using the same protocol. After immobilization of the anti-gp120 Abs on the plate, 50 μl of medium containing the equivalent of 500 to 1,000 pg of p24 was added to the wells in triplicate. After a 4-h incubation, the plates were washed and p24 was quantified as explained above.
ELISA and MAb-gp120 binding assay.
Supernatants from transfected 293 T cells containing pseudotyped virus were treated with 1% NP-40 to provide a source of soluble gp120. Ninety-six-well ELISA plates (Corning) were coated overnight at 4°C with 50 μl of D7324 at 5 μg/ml in PBS (pH 7.5). The plates were washed twice with EWB and then saturated with 100 μl of 3% bovine serum albumin in PBS for 1 h at RT. After two washes with EWB, the plates were incubated with 50 μl of viral lysate for 4 h at RT. All subsequent incubations were performed in a final volume of 50 μl. After 10 washes, serial dilutions of Ab were added, and the plates were incubated for 2 h at RT. The plates were washed 10 times, and bound Ab was detected using a goat anti-human IgG conjugated to alkaline phosphatase (Pierce, Rockford, Ill.) in PBS for 1 h at RT. After extensive washing of the plates, detection was carried out with p-nitrophenyl phosphate tablets from Sigma-Aldrich following the supplier's recommendations.
RESULTS
Neutralization of HIV-1 JR-CSF, 89.6, and ADA pseudotyped particles by anti-gp120 Abs.
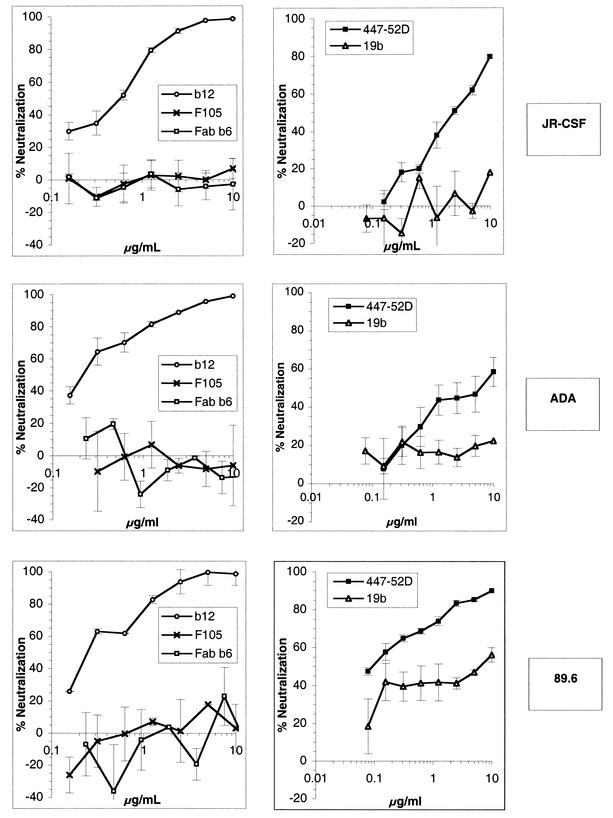
We first characterized the ability of a panel of anti-gp120 MAbs to neutralize a selection of primary HIV-1 isolates. As in most experiments described below, we used pseudotyped viruses and a luciferase-based assay in order to quantify viral infection. In this system, the viral envelope is the only parameter that varies between viruses. Furthermore, the single round of replication and a very broad dynamic range allow for a precise measurement of the infection level. Virions were pseudotyped with the envelope glycoproteins of the R5 HIV-1 isolates JR-CSF and ADA and with the glycoprotein of the R5X4 HIV-1 isolate 89.6. Neutralization assays were carried out with the anti-CD4 binding site Abs b12, F105, and Fab b6 and the anti-V3 loop Abs 447-52D and 19b. Our results showed that the Ab b12 was potent at neutralizing the three primary virions JR-CSF, ADA, and 89.6, as described previously (4, 5), with 90% neutralization at <10 μg/ml (Fig. 1). The Ab 447-52D was able to potently neutralize 89.6 and to neutralize JR-CSF at a high concentration but poorly neutralized ADA. The Abs F105, 19b, and Fab b6 were overall very poor neutralizers (Fig. 1).
FIG. 1.
Neutralization of HIV-1 JR-CSF, ADA, and 89.6 pseudotyped particles by anti-gp120 Abs. Pseudoviruses bearing the JR-CSF, ADA, or 89.6 envelope were incubated with anti-gp120 Abs at various concentrations for 1 h at 37°C. Following incubation, the virion-Ab mixture was added to U87 CD4+ CCR5+ cells (JR-CSF and ADA) or U87 CD4+ CXCR4+ cells (89.6). After a 3-day incubation, infection was assessed by measuring luciferase activity. Neutralization activity is expressed as a percentage of inhibition of infection. The neutralizing activities of the anti-V3 Abs 447-52D and 19b (right) and the anti-CD4bs Abs b12, F105, and Fab b6 (left) are shown. The error bars indicate standard deviations.
Binding of anti-gp120 Abs to monomeric gp120.
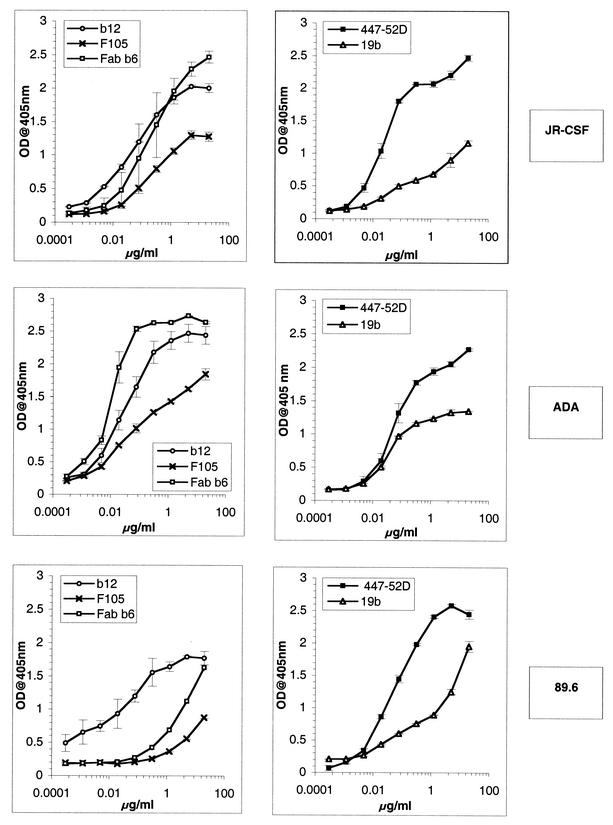
It was important for a better interpretation of the results of the virion binding assay to know the relative affinities of each Ab for the gp120 molecules of the three HIV-1 isolates studied. Therefore, we studied the binding of our panel of Abs to gp120 using a capture ELISA (Fig. 2). To estimate the relative affinities, the half-maximal binding values were determined for each Ab and each gp120, as presented in Table 1. The results showed that Abs b12 and 447-52D bound with high affinity to the gp120 molecules of the three different viruses. The Ab Fab b6 bound with high affinity to gp120 of ADA, with moderate affinity to gp120 of JR-CSF, and poorly to 89.6 gp120. The Ab F105 bound with moderate affinity to the gp120s of JR-CSF and ADA and poorly to 89.6 gp120. The Ab 19b bound poorly to 89.6 and JR-CSF gp120s and with a high affinity to ADA gp120.
FIG. 2.
Binding of anti-gp120 Abs to monomeric gp120 from HIV-1 JR-CSF, ADA, and 89.6. MAb binding to monomeric gp120 was measured using a gp120 capture ELISA. The binding curves of the anti-V3 Abs 447-52D and 19b (right) and the anti-CD4bs Abs b12, F105, and Fab b6 (left) are shown. The error bars indicate standard deviations. OD, optical density.
TABLE 1.
Half-maximal binding of anti-gp120 Abs to soluble gp120
| Ab | Binding (μg/ml) to gp120 of:
|
||
|---|---|---|---|
| JR-CSF | ADA | 89.6 | |
| b12 | 0.035 | 0.025 | 0.015 |
| F105 | 0.2 | 0.3 | 20 |
| Fab b6 | 0.2 | 0.009 | 2.5 |
| 447-52D | 0.02 | 0.05 | 0.05 |
| 19b | 20 | 0.03 | 5 |
These results are in accord with previous observations (19, 27, 31) that the ability of an Ab to neutralize does not necessarily correlate with its affinity for monomeric gp120. Indeed, some Abs bound to monomeric gp120 with high affinity but did not neutralize the corresponding gp120-bearing virus.
Capture of viral particles by immobilized anti-gp120 Abs as measured by p24.
Next, the abilities of the panel of neutralizing and nonneutralizing Abs to bind to viral particles as estimated by the capture of virions by immobilized Abs were determined. Virion capture was quantitated in terms of the amount of p24 captured, as described previously.
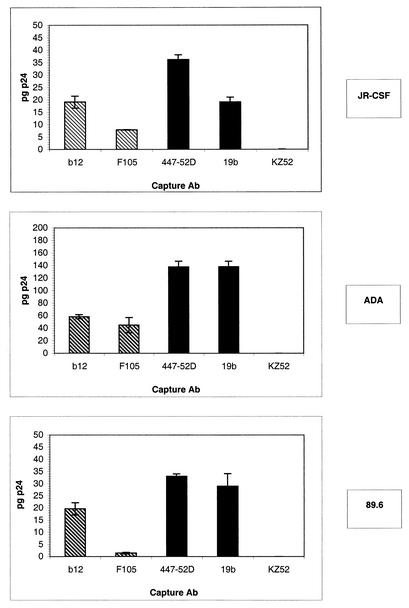
All the anti-gp120 Abs included in the study were able, with different efficiencies, to capture virions from the three HIV-1 isolates used, as detected by measurement of p24 (Fig. 3). (We refer to virions so detected as p24 and/or virions for reasons that will become apparent below). The anti-V3 loop Abs, in particular 447-52D, were, as described by Nyambi et al., the most efficient at capturing p24 and/or virions (447-52D captured ∼3, 8, and 6% of the p24 input for JR-CSF, ADA, and 89.6, respectively) (22). Analysis of the results revealed no correlation between the ability of an Ab to capture p24 virions and the ability of the Ab to neutralize, i.e., nonneutralizing Abs were as able to capture virions as neutralizing Abs. This was especially notable for the anti-V3 Abs 447-52D and 19b, which, for example, did not neutralize JR-CSF and ADA but captured amounts of p24 equal to or higher than the amounts captured by the neutralizing Ab b12. Surprisingly, the 19b Ab, which displayed a low affinity for monomeric gp120s from two of the three tested viruses in ELISA, was able to capture p24 virions as well as or better than the high-affinity Ab b12. This result suggested that there was no correlation between the affinity of an Ab for gp120 and the ability of the Ab to capture p24 and/or virion particles.
FIG. 3.
Capture of viral particles by immobilized anti-gp120 Abs as measured by p24. Anti-gp120 and control Abs were immobilized on ELISA wells via an anti-Fc Ab. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope glycoproteins were added, and the amount of p24 and/or virions captured was measured using a p24 ELISA kit. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. The error bars indicate standard deviations.
These results may be interpreted, as was done previously, as a demonstration of the capacity of not only neutralizing Abs but also certain nonneutralizing Abs to bind to gp120 at the surfaces of HIV-1 virions. However, estimation of p24 may not necessarily give an accurate reflection of infectious virus, since it is known that infectious particles represent only a low percentage of the total number of HIV-1 particles in a typical preparation. Neutralization implies binding of the Ab to infectious virions. Therefore, we decided to compare how neutralizing and nonneutralizing Abs can capture infectious virus. To assess whether the p24-based virus binding assay directly reflected the amount of infectious virus bound to the immobilized Ab, we further studied in parallel the amount of p24 and the number of infectious virions captured.
Capture of infectious viral particles by immobilized anti-gp120 Abs.
To quantify infectious virus captured by immobilized Abs, we took advantage of the luciferase gene carried by the pseudotyped viruses. As discussed previously, this reporter gene allows a sensitive and accurate detection of single-round viral infection. Following capture by immobilized Abs, as in the p24-based assay, virions were overlaid with target cells. Three days later, the cells were lysed and luciferase activity was measured. In parallel, levels of captured p24 were measured.
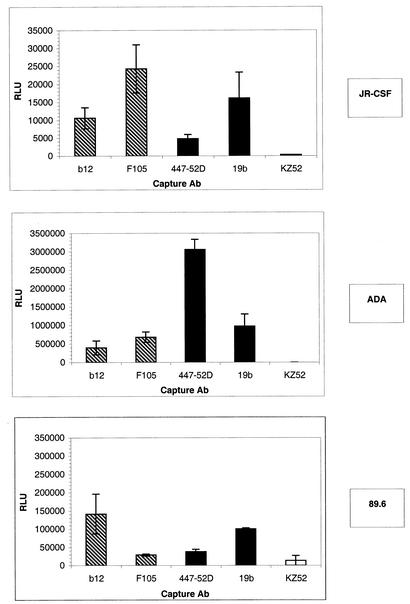
The results showed that there was no correlation between the amount of p24 and the amount of infectious virus captured in the assay used. The most striking example came from the anti-V3 MAb 447-52D. As seen in Fig. 4, 447-52D captured about twice as much JR-CSF virus as the Ab b12 as estimated by p24 but only half as much virus as estimated by infectivity. A similar result was seen with the 89.6 isolate. As another example of such a discrepancy, the Ab F105 captured less JR-CSF p24 but more infectious JR-CSF particles than the Ab b12. Overall, these results clearly demonstrate that p24 capture does not parallel infectious-virus capture and that therefore the p24-based virus binding assay cannot be used to infer the exposure of epitopes on primary infectious HIV particles.
FIG. 4.
Capture of infectious viral particles by immobilized anti-gp120 Abs. The quantification of infectious pseudoviruses bearing the JR-CSF, ADA, or 89.6 envelope captured by immobilized Abs was carried out by adding U87 target cells to the captured virions. Infection was quantified by measuring luciferase activity after a 3-day incubation. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. The error bars indicate standard deviations. RLU, relative light units.
We then looked for correlation between the ability of Abs to capture infectious virions and the ability to neutralize. Analysis of the results showed that there was no correlation between the ability of Abs to neutralize and the ability to capture infectious virus. For example, the nonneutralizing Abs F105 and 19b captured amounts of infectious JR-CSF virus similar to or greater than those captured by the neutralizing Ab b12. Similarly, 19b poorly neutralized 89.6 and did not neutralize ADA but was able in both cases to capture as much infectious virus as the potent neutralizing Ab b12. As another example, although the Ab 447-52D poorly neutralized ADA, it captured greater amounts of ADA virus than the neutralizing Ab b12. Conversely, 447-52D neutralized 89.6 at a low concentration but captured less 89.6 virus than the nonneutralizing Ab 19b. As a final example, the Ab F105 did not neutralize ADA or JR-CSF but captured as much infectious virus as the neutralizing Ab b12.
Therefore, both nonneutralizing and neutralizing Abs appear to capture infectious virus. This result may suggest that the epitopes of nonneutralizing Abs are well exposed on gp120 at the surface of infectious virus. However, we noted that, surprisingly, Abs with a relatively low affinity for gp120 could capture as much infectious virus as Abs with high affinity. For example, despite a low affinity for JR-CSF gp120, the Ab 19b captured as much JR-CSF infectious virus as the high-affinity Ab b12. Likewise, the low-affinity Ab F105 captured more infectious JR-CSF virus than the high-affinity Abs b12 and 447-52D. Similar observations were made for the other isolates. One possible explanation is that the epitopes of an Ab binding only weakly to monomeric gp120 are better exposed when gp120 is oligomerized on the virion surface. An alternative explanation is that, when immobilized on the plate, the array of Ab molecules has an increased avidity for the multivalently displayed gp120 molecules on the virion surface that permits capture even by Abs that have relatively low affinity for monomeric gp120.
Capture of infectious particles from a b12 escape JR-CSF variant.
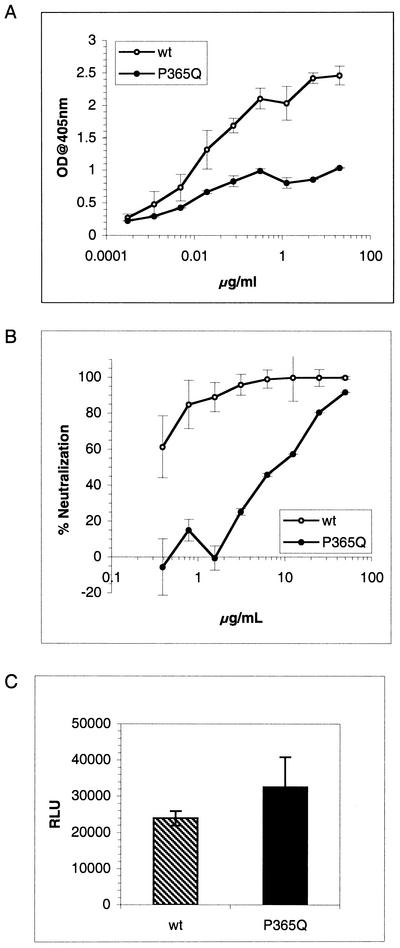
To understand whether immobilization of the Ab could lead to an artifactual increase in the Ab capacity to bind viral particles, we compared the ability of the b12 Ab to capture wild-type JR-CSF and a JR-CSF escape variant. This variant, which has been selected under b12 pressure in vitro as well as in vivo, contains a P-to-Q mutation at position 365 that strongly decreases b12 binding to gp120 (Fig. 5A ) (18, 28a). As a consequence, the Ab no longer binds efficiently to the virus and the neutralizing concentration is considerably higher: the 90% neutralizing concentration of b12 is about 1.5 μg/ml for the wild-type virus compared to 50 μg/ml for the escape variant (Fig. 5B).
FIG. 5.
(A) Binding of MAb b12 to wild-type (wt) JR-CSF gp120 and P365Q JR-CSF variant gp120. MAb binding to soluble monomeric gp120 was measured using a gp120 capture ELISA. OD, optical density. (B) Neutralization by MAb b12 of wild-type JR-CSF and P365Q JR-CSF variant. Pseudotyped viruses bearing the JR-CSF wild-type or the JR-CSF P365Q variant envelope were preincubated with the Ab before being added to U87 target cells. Infection was quantified by measurement of luciferase activity. Neutralization is expressed as a percentage of inhibition of infection. (C) Capture of infectious parti-cles from a b12 escape JR-CSF variant. Standardized amounts of infectious virus bearing either the wild-type or the JR-CSF P365Q variant envelope glycoprotein were added to immobilized b12. Captured infectious virions were quantified by adding U87 target cells and measuring luciferase activity after a 3-day incubation. The error bars indicate standard deviations. RLU, relative light units.
We normalized the numbers of wild-type and escape infectious particles and compared the amount of infectious virus captured by b12. Strikingly the Ab was able to capture as much escape variant as wild-type virus (Fig. 5C). This result, in addition to the ones described previously, clearly suggests that the virion binding assay, in the format described above, is not appropriate to quantify epitope exposure on gp120 at the surface of primary HIV-1 for the typical Abs studied.
We have shown that the capture assay can be misleading because of avidity effects. However, the data do not rule out the possibility that nonneutralizing Abs can bind significantly to the virus surface. To circumvent the problem of increased avidity, we decided to carry out experiments in which Ab and virus were first incubated in solution before being captured in a competition format.
Capture of infectious viral particles by immobilized anti-CD4bs Ab b12 and competition with nonneutralizing anti-CD4bs Abs.
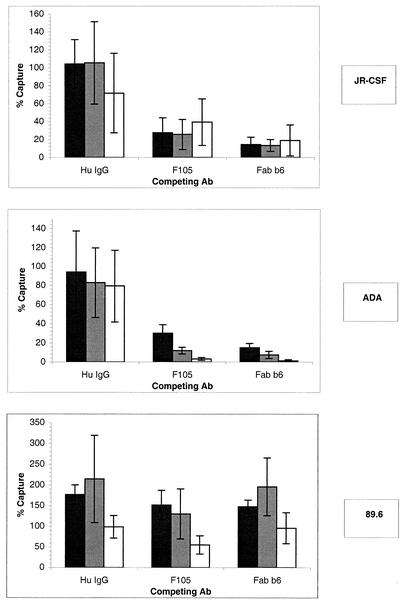
As described previously, the Ab b12 is directed against the CD4bs and neutralizes primary HIV-1. Therefore, it is clear that this Ab binds to envelope spikes on the surface of the virus. To understand whether nonneutralizing Abs could also bind to gp120 on virions, we preincubated the virus in solution with anti-CD4bs nonneutralizing Abs. We reasoned that if the nonneutralizing anti-CD4bs Abs could bind to the virus, they should be able to block capture by b12. We used the anti-CD4bs Abs F105 and Fab b6, which both compete with b12 for binding to gp120 in ELISA (reference 31 and data not shown). Interestingly, the nonneutralizing Abs F105 and b6 could efficiently block the b12-mediated capture of infectious virions, even at relatively low Ab concentrations in the cases of JR-CSF and ADA. Fab b6 was more efficient in competition with b12 than F105 (Fig. 6). This could be due to the greater affinity of Fab b6 than F105 for monomeric gp120, as shown in Fig. 2. Competition for 89.6 capture was weak and required higher concentrations of F105 and Fab b6 than competition for ADA and JR-CSF capture. This was in accord with the low affinity of both Abs for monomeric 89.6 gp120. Of note, a relatively weak enhancement of 89.6 capture by low concentrations of F105, Fab b6, and, more significantly, normal human IgG was detected. In most cases the enhancement may be partly explained by the variability of the assay. It can also be hypothesized that a low level of binding of Abs to the virus may nonspecifically favor capture by b12.
FIG. 6.
Capture of infectious viral particles by immobilized MAb b12 and competition with nonneutralizing anti-CD4bs Abs. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope were incubated in solution with cross-competing anti-CD4bs Abs before capture by immobilized anti-CD4bs MAb b12. The final competing Ab concentrations were 20 (open bars), 2 (shaded bars), and 0.2 μg/ml (solid bars). Captured infectious virions were quantified by adding U87 target cells and measuring luciferase activity after a 3-day incubation. The error bars indicate standard deviations. Hu, human.
These results demonstrate that nonneutralizing anti-CD4bs Abs can bind to gp120 at the surface of the virus. We then reasoned that if nonneutralizing anti-CD4bs Abs could bind to spikes at the surface of the virus, they should block access by neutralizing anti-CD4bs Abs (b12) and prevent neutralizing Ab binding and thus neutralization.
Neutralization by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs.
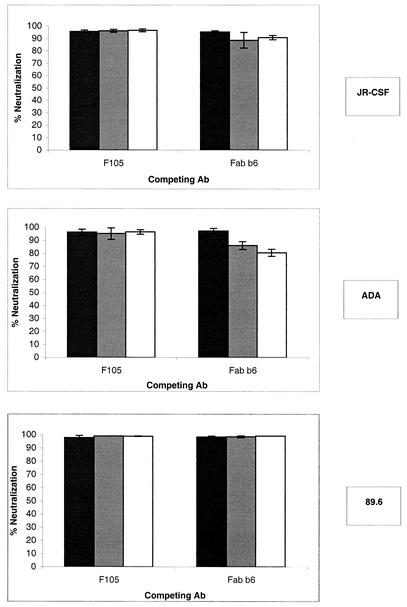
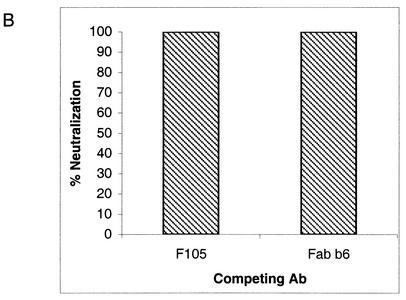
We performed neutralization with nonneutralizing and neutralizing cross-competing anti-CD4 binding site Abs. We used the same Abs as in the experiments described above. The Ab b12 was used at a final concentration of 5 μg/ml, corresponding to a ∼95% neutralization concentration for the three isolates used. The results show (Fig. 7) that in contrast to the capture competition format, the nonneutralizing Abs F105 and Fab b6 were not able to decrease the neutralizing activity of the Ab b12.
FIG. 7.
Neutralization by the MAb b12 in the presence of nonneutralizing anti-CD4bs Abs. Pseudotyped viruses bearing the JR-CSF, ADA, or 89.6 envelope were preincubated in solution with nonneutralizing anti-CD4bs MAbs for 45 min at 37°C. The final competing Ab concentrations were 30 (open bars), 3.75 (shaded bars), and 0.5 μg/ml (solid bars) for Fab b6 and 20 (open bars), 2.5 (shaded bars), and 0.3 μg/ml (solid bars) for F105. The neutralizing Ab b12 was then added (final concentration, 5 μg/ml), and the viruses were incubated for another 45 min. Following incubation, the virus-Ab mixtures were added to U87 target cells. Infection was assessed by measuring luciferase activity after a 3-day incubation. Neutralization is expressed as a percentage of infection inhibition. The error bars indicate standard deviations.
The fact that capture by the neutralizing Ab b12 was competed by nonneutralizing Abs directed against an overlapping epitope while neutralization was not competed by the same Abs suggest that (i) the capture of infectious viral particles is mainly mediated by gp120-bearing envelope molecules that are not involved in the neutralization process and therefore are not required for or essential to viral entry and (ii) the envelope molecules are heterogeneous at the surfaces of infectious virions. Thus, it would appear that the viral particles display on their surfaces envelope molecules that are not necessary to the infectious process, which can be bound by both nonneutralizing and neutralizing Abs, as well as envelope molecules that are required for the entry process and that can be bound only by neutralizing Abs.
Capture and neutralization of replication-competent PBMC-grown HIV-1JR-CSF.
The experiments described above were done using pseudotyped viruses, since as discussed, this system allows an accurate quantification of infection. Such an accurate quantification is not readily achieved for PBMC-grown virus. In order to investigate whether the results for pseudoviruses could be extended in a general way, we carried out experiments using HIV-1JR-CSF grown in PBMC.
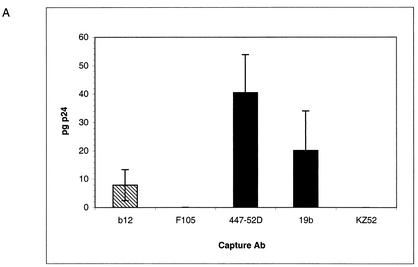
First, we studied the capture of HIV-1JR-CSF particles using our panel of Abs (Fig. 8A). Captured virus was quantified by measuring p24 as described above. The results obtained were similar to the ones obtained with the JR-CSF env pseudotyped virus. The nonneutralizing anti-V3 Abs were the best at capturing p24 and/or HIV-1JR-CSF virions. Of note, compared to pseudovirus, there was a greater difference between the quantity of p24 and/or virions captured by the anti-V3 Abs and the anti-CD4bs Abs. But again, there was no correlation between the ability of an Ab to neutralize and its ability to capture p24 and/or virions.
FIG. 8.
(A) Capture of PBMC-grown HIV-1JR-CSF by immobilized anti-gp120 Abs as measured by p24. Anti-gp120 and control Abs were immobilized on ELISA wells via an anti-Fc Ab. HIV-1JR-CSF was added to the wells, and the amount of p24 and/or virions captured was measured using a p24 ELISA kit. Capture by anti-CD4bs MAbs (hatched bars), anti-V3 loop MAbs (solid bars), and a control irrelevant MAb (open bars) is shown. (B) Neutralization of HIV-1JR-CSF by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs. HIV-1JR-CSF was preincubated in solution with nonneutralizing anti-CD4bs MAbs for 45 min at 37°C. The final competing Ab concentration was 20 μg/ml. The neutralizing Ab b12 was then added (final concentration, 15 μg/ml), and the viruses were incubated for another 45 min. Following incubation, the virus-Ab mixtures were added to PHA-activated PBMC. Infection was assessed by measuring p24 production after a 7-day incubation. Neutralization is expressed as a percentage of infection inhibition. The error bars indicate standard deviations.
As for the pseudoviruses, this result suggests that nonneutralizing epitopes may be exposed on the PMBC-grown virus particles. We then investigated, using a competition format as for the pseudoviruses, whether the nonneutralizing Abs could bind to the spikes involved in the infectious process of replication-competent virus. We performed a neutralization assay of HIV-1JR-CSF by the anti-CD4bs Ab b12 in the presence of nonneutralizing anti-CD4bs Abs. In this experiment, b12 was used at a final concentration of 15 μg/ml to achieve >90% neutralization, and the other Abs were used at a 20-μg/ml final concentration. The results showed that the nonneutralizing Abs did not interfere with the ability of b12 to neutralize JR-CSF (Fig. 8B). Therefore, as shown for pseudotyped viruses, the data suggest that nonneutralizing Abs do not bind to functional spikes at the surfaces of infectious PMBC-grown virions. As for the pseudoviruses, it would appear that the PBMC-grown virus bears functional and nonfunctional envelope molecules.
DISCUSSION
Virion capture assays using immobilized Abs have been used to provide information on the epitopes presented on the surface of HIV-1 and to hypothesize about mechanisms of neutralization, but the data presented here suggest there are a number of significant pitfalls. Our studies focused on pseudotyped viruses, where quantitative infectivity measurement can be made. However, the limited studies carried out here, together with other studies described above, suggest that the conclusions drawn are likely to apply to PBMC-grown viruses also.
First, among the pitfalls associated with the virion capture assay, it is apparent that estimates of the amount of virus captured based on p24 measurements correlate very poorly with infectious virus. In particular, some nonneutralizing MAbs capture relatively large amounts of p24-associated material but very little infectious virus. The nature of this p24-associated material is unclear, but presumably it must include Env to permit capture by anti-Env Abs. Possible candidates include vesicles containing p24 and having Env expressed at or attached to the membrane and noninfectious virion particles possessing some defect in replicative capacity or mostly bearing nonfunctional envelope molecules as discussed below.
Second, the ability of MAb b12 to capture infectious neutralization escape virus suggests that the requirements for virus capture and neutralization can be very different. This is likely to be related to the multivalent nature of the capture process. MAb b12 has a relatively low affinity for monomeric gp120 of the escape variant studied here: the affinity is at least 2 orders of magnitude lower than that for wild-type gp120. This almost certainly results in a greatly lowered affinity for envelope spikes and an inability of b12 to neutralize this virus at meaningful Ab concentrations. However, this affinity appears to be sufficient to capture virus, presumably through multivalent interaction of viral envelope with immobilized Ab on a surface. Clearly, even very weakly binding Abs, which consequently do not effectively neutralize virus, can capture infectious virions.
Virus capture assays, at least under conditions commonly used for primary HIV-1, thus cannot be used to quantify epitope exposure at the surfaces of virions or to show that nonneutralizing Abs bind effectively to virions. Of note, variations in the capture protocol, such as shorter incubation times and the use of various virus/Ab ratios, did not improve the ability of the test to discriminate between Abs (data not shown). A more persuasive case for the binding of nonneutralizing Abs to infectious virions is provided by data presented here showing, for three different primary HIV-1 envelopes, that nonneutralizing anti-CD4bs Abs in solution can compete with a neutralizing anti-CD4bs Ab (b12) for capture of infectious virus. This suggests that the nonneutralizing anti-CD4bs Abs are able to bind effectively to the envelope species that is involved in virion capture in these experiments. It should be noted that the nonneutralizing anti-CD4bs Abs do compete with b12 for binding to monomeric gp120. However the nonneutralizing anti-CD4bs Abs do not inhibit neutralization by b12 even in considerable excess. This suggests that the nonneutralizing Abs are unable to bind effectively to the envelope species required for virus infectivity.
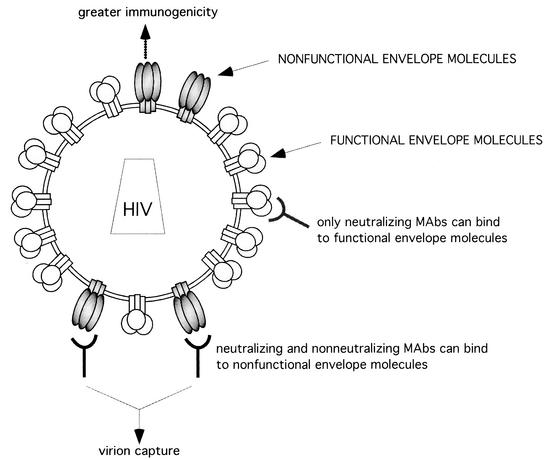
How can these data be reconciled? The explanation we favor is that the primary infectious virions studied here express envelope molecules of two distinct types. The first type, relatively accessible to Abs, is not required for virus entry; we propose to call this species nonfunctional envelope molecules. The second type of envelope molecules is necessary to the entry process, is of more limited accessibility to Abs, and can be called functional (Fig. 9). According to our hypothesis, both nonneutralizing and neutralizing Abs bind to nonfunctional envelope molecules with good affinity, whereas only neutralizing Abs bind to functional envelope molecules with significant affinity. The data suggest that, although neutralizing Abs can bind to both forms of the envelope, virion capture by neutralizing as well as by nonneutralizing Abs is mostly mediated by the nonfunctional envelope molecules. Thus, in contrast to neutralization, b12-mediated capture can be competed by nonneutralizing anti-CD4bs Abs, suggesting that, for the most part, functional envelope molecules are not involved in capture. We suggest that gp120 in the nonfunctional envelope molecules may be in a conformation resembling that of monomeric gp120. The affinity of b12 is generally considerably higher for monomeric gp120 than that deduced for primary envelope spikes, which may be consistent with virion capture being essentially mediated by nonfunctional envelope (26). This binding would then be well competed by nonneutralizing anti-CD4bs Abs, as they typically bind to monomeric gp120 with high affinity. Nevertheless, the lower affinity of b12 for functional envelope may not fully explain why functional Env would not mediate capture, as suggested by the experimental results. Indeed, the lower affinity of b12 for the functional Env may be compensated for by the multivalent interaction with the immobilized Ab, as shown above by the fact that, despite a low affinity, b12 can capture the P365Q escape mutant. An alternative explanation for the absence of capture through functional Env may be that the orientation of the b12 epitope on the functional Env molecules is not compatible with binding to immobilized b12. Another explanation may be that b12 binding to functional Env molecules leads to gp120 shedding, thus preventing capture. Finally, the data may be interpreted as suggesting that virions mostly bear nonfunctional spikes and that virus entry is mediated by a few functional spikes.
FIG. 9.
Model for proposed heterogeneity of envelope molecules at the surfaces of primary HIV-1 particles. Virions bear both functional and nonfunctional envelope molecules. Capture of virions is mainly mediated through nonfunctional envelope molecules. The presence at the viral surface of nonfunctional envelope molecules with greater immunogenicity could lead to the preferential elicitation of nonneutralizing Abs.
One caveat should be noted here. If the binding of b12 to envelope spikes were irreversible, although reversible to monomeric gp120, this could lead to diminished ability of nonneutralizing anti-CD4bs Abs to compete with b12 in neutralization assays. However, such irreversible binding would then appear to be inconsistent with the ability of nonneutralizing anti-CD4bs Abs to compete with b12 for capture. In addition, it may be that b12 binding leads to irreversible neutralization. However, under the conditions used, the nonneutralizing MAb should inhibit neutralization by b12, at least partially, whether the latter is reversible or irreversible.
We note that relatively few nonfunctional envelope molecules on the surfaces of infectious virions could permit capture on an Ab-coated surface because of multivalency effects. Binding of Abs to this minor population of envelope molecules need not impact upon infectivity and is consistent with an occupancy model of neutralization in which Ab sterically interferes with viral attachment and/or fusion (24, 27, 28). In the occupancy model, Ab is suggested to neutralize virus once coating exceeds ∼40% of the virion surface area (24, 33). There are a number of examples in the literature of Abs that bind to a minor population of virion surface-expressed proteins and do not result in virus neutralization (24). A classic case is Ab to influenza neuraminidase. The ratio of neuraminidase to hemagglutinin molecules on the virus surface is about 1:4, and the neuraminidase molecules appear to concentrate in local regions. Abs to neuraminidase bind to virus but do not neutralize (24). Another classic case is rabies virus, where an Ab to a minor conformation of envelope does not neutralize (24, 30). However, if the virus is subjected to conditions which convert most envelope molecules to this conformation, then the Ab does neutralize (30).
The presence on the virus of a fraction of nonfunctional envelope molecules would solve the paradox of having nonneutralizing anti-CD4bs Abs bound to virions without any impact on viral infectivity. Indeed, it had been proposed that these Abs can bind to the CD4 binding site without interfering with viral entry, a process that requires gp120-CD4 interaction (39). This implied that the Ab can dissociate from gp120 in an unclear manner during viral entry to allow gp120-CD4 interaction. Our studies reconcile the findings by suggesting that nonneutralizing anti-CD4bs Abs do not bind to functional spikes but bind only to a fraction of nonfunctional spikes and therefore do not interfere with viral entry.
Our work also does not favor the hypothesis of negative cooperativity discussed by Si et al. (35). According to this hypothesis, nonneutralizing Abs would be capable of binding to only two of three gp120 molecules of an envelope spike whereas neutralizing MAbs could bind to all three. Binding to all three gp120s would be required for neutralization. The binding of two nonneutralizing Abs per spike would not prevent virion surface CD4 from interacting with this open site and the initiation of infection. However, if this were true, it seems that, in contrast to our results, nonneutralizing MAbs would not inhibit capture by b12 via the third open site. There is some further evidence, both direct and indirect, that argues against the negative-cooperativity explanation. First, studies by Schönning et al. (33) show that a TCLA HIV-1 is completely neutralized at a neutralizing Ab coating density of between one and two Ab molecules bound per envelope spike. We have recently found a similar result for a primary HIV-1 (M. Franti and P. Poignard, unpublished observations). This result is fully consistent with the coating density found to neutralize a number of other viruses (24). Second, the weak activity of nonneutralizing anti-CD4bs Abs against TCLA viruses relative to b12 correlates well with their lower affinity for envelope oligomer on the surfaces of infected cells (19, 32). There is no indication of negative cooperativity of Ab binding, which would then be a phenomenon unique to primary-virus envelope. On the other hand, the envelope heterogeneity model requires no such fundamental difference between TCLA and primary viruses. Third, if the resistance of primary viruses to neutralization by the nonneutralizing anti-CD4bs Abs is due to a negative cooperative binding effect that is not operational for b12 or CD4, it is difficult to explain the resistance of primary viruses to neutralization by soluble CD4.
The interaction of Abs and envelope has been explored through binding of Abs to infected and transfected cells, as well as via virion capture (22, 40). In the case of transfected cells in particular, there is a potential for surface expression of uncleaved envelope that makes it very difficult to determine that one is studying Ab binding to functional envelope spikes. Thus, it is not unusual to have 75% of gp160 at the surfaces of transfected cells in an unprocessed form (40). In such cases, the binding of, for example, nonneutralizing anti-CD4bs Abs is expected and has little or no significance for understanding the involvement of Ab in virus neutralization, in which binding to functional processed envelope is likely to be critical. In the case of H9 cells infected with MN or IIIB TCLA virus, it appears that gp160 processing is complete, and the correlation between Ab neutralization and cell binding is consistent with binding to functional spikes on the infected cell surface (27, 32).
What are the natures of the proposed functional and nonfunctional envelopes? Functional gp120 molecules are believed to be associated with a heterotrimeric structure of the composition gp1203gp413, often referred to as envelope spikes (28). The nature of the proposed nonfunctional gp120 is less clear, although, as discussed above, a conformation that approximates monomeric gp120 would be consistent with much of the data. There are a number of formal possibilities, bearing in mind that essentially all folded presentations of gp120 or gp160 investigated to date apart from envelope spikes appear to bind nonneutralizing anti-CD4bs Abs as well as or better than b12. First, unprocessed gp160 of an unknown oligomerization state may be incorporated into virions, although such incorporation has been disputed in the literature (6, 11, 17) and our data do not favor this hypothesis; immunoprecipitations of PBMC-grown virus did not reveal gp160 incorporation (data not shown). Second, there may be spike heterogeneity where gp120 molecules are shed, leading to structural rearrangements that produce better exposure of epitopes of nonneutralizing Abs. Indeed the ability of MAbs to cryptic domains of gp41 to capture infectious viral particles (data not shown) suggests that free gp41 is present at the virus surface, probably as a consequence of gp120 shedding. Third, there may be misfolded envelope incorporated into virions in which the epitopes of nonneutralizing Abs of gp120 are unusually exposed. Finally, heterogeneity in the glycosylation of gp120 may lead to the presence at the viral surface of spikes with modified conformation and increased accessibility. It may be difficult to distinguish these possibilities, or indeed others not considered here, if the population of nonfunctional envelope molecules is relatively small.
The defective nonfunctional envelope molecules that we propose may help explain why only a small fraction of particles are infectious within a viral preparation (13). It can be hypothesized that noninfectious virions may represent particles that mostly bear nonfunctional spikes. Viral stocks may be made of a mixture of virions bearing, in various proportions, functional and nonfunctional envelope. Infectious particles may correspond to the small fraction of virions that display the minimal quantity of functional envelope molecules required for viral entry.
The presence of nonfunctional envelope on virions as proposed here may play a role in the trapping of viral particles on follicular dendritic cells (FDC). It has been suggested that HIV immune complexes can be captured on FDC, leading to virus persistence (10, 36). However, in many patients, neutralizing Ab responses are weak. As for the virus capture assay, binding of nonneutralizing Abs to nonfunctional spikes could then lead to viral trapping on FDC in infected individuals.
Importantly, the presence of nonfunctional envelope on virions could also contribute to the robust nonneutralizing but weak neutralizing Ab responses seen in HIV-1 infection (Fig. 9). Primary virions are readily captured by immobilized nonneutralizing Abs, suggesting that the nonfunctional envelope is accessible. Displayed as an array on a particle, such gp120 molecules are likely to be relatively immunogenic. Certainly, they can be expected to be more immunogenic than, for example, soluble gp120 generated by shedding, an alternative potential immunogen during HIV-1 infection. Furthermore, the presence of nonfunctional but accessible gp120 on the same particles as functional but poorly accessible gp120, as is thought to be the case for active envelope spikes (24, 27, 28), would act directly to inhibit the Ab response to functional envelope. B cells bearing immunoglobulin surface receptors for nonfunctional envelope will tend to adsorb virus particles and decrease the likelihood of virus stimulating B cells bearing receptors for functional envelope. In other words, the nonfunctional envelope will be immunodominant. As an additional possibility, if a B-cell antigen receptor recognizes an epitope present on both functional and nonfunctional Env molecules, affinity maturation is more likely to occur against the more accessible epitope on the nonfunctional envelope, further biasing the humoral immune response toward poor neutralization.
The presence of immunogenic nonfunctional envelope on HIV-1 particles would have important consequences for vaccines that seek to stimulate a neutralizing Ab response through immunization with killed virus or virus-like particles (9, 38). It may be of considerable value to block the nonfunctional envelope molecules, perhaps with nonneutralizing Ab, prior to immunization.
Acknowledgments
We are very grateful to Lisa Cavacini, James Robinson, Joseph Sodroski, and Susan Zolla-Pazner for providing reagents.
This study was supported by NIH grants AI45357 (P.P.) and AI33292 (D.R.B.). P.P. acknowledges a Scholar Award from the Pediatric AIDS Foundation (77370). M.M. was the recipient of a Philipps Foundation Fellowship.
REFERENCES
- 1.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D., and D. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87-S98. [PubMed] [Google Scholar]
- 3.Burton, D. R., and P. W. H. I. Parren. 2000. Vaccines and the induction of functional antibodies: time to look beyond the molecules of natural infection? Nat. Med. 6:123-125. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. H. I. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryston, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza, M. P., D. Livnat, J. A. Bradac, S. H. Bridges, et al. 1997. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J. Infect. Dis. 175:1056-1062. [DOI] [PubMed] [Google Scholar]
- 6.Dubay, J. W., S. R. Dubay, H. J. Shin, and E. Hunter. 1995. Analysis of the cleavage site of the human immunodeficiency virus type 1 glycoprotein: requirement of precursor cleavage for glycoprotein incorporation. J. Virol. 69:4675-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauduin, M. C., P. W. H. I. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 8.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 9.Griffiths, J. C., S. J. Harris, G. T. Layton, E. L. Berrie, T. J. French, N. R. Burns, S. E. Adams, and A. J. Kingsman. 1993. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral responses by a Gag:V3 fusion. J. Virol. 67:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath, S. L., J. G. Tew, A. K. Szakal, and G. F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377:740-744. [DOI] [PubMed] [Google Scholar]
- 11.Iwatani, Y., K. Kawano, T. Ueno, M. Tanaka, A. Ishimoto, M. Ito, and H. Sakai. 2001. Analysis of dominant-negative effects of mutant Env proteins of human immunodeficiency virus type 1. Virology 286:45-53. [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi, Y., S. Miles, R. Mitsuyasu, J. Merrill, H. Vinters, and I. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 13.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama, T., L. L. Rodriguez, P. B. Jahrling, A. Sanchez, A. S. Khan, S. T. Nichol, C. J. Peters, P. W. H. I. Parren, and D. R. Burton. 1999. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 73:6024-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 17.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53:55-67. [DOI] [PubMed] [Google Scholar]
- 18.Mo, H., L. Stamatatos, J. E. Ip, C. F. Barbas, P. W. H. I. Parren, D. R. Burton, J. P. Moore, and D. D. Ho. 1997. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J. Virol. 71:6869-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas III, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, J. P., A. Trkola, B. Korber, L. J. Boots, J. A. Kessler II, F. E. McCutchan, J. Mascola, D. D. Ho, J. Robinson, and A. J. Conley. 1995. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J. Virol. 69:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zolla-Pazner. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 72:9384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyambi, P. N., H. A. Mbah, S. Burda, C. Williams, M. K. Gorny, A. Nadas, and S. Zolla-Pazner. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 74:7096-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parren, P. W. H. I., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parren, P. W. H. I., H. J. Ditzel, R. J. Gulizia, J. M. Binley, C. F. Barbas III, D. R. Burton, and D. E. Mosier. 1995. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9:F1-F6. [DOI] [PubMed] [Google Scholar]
- 26.Parren, P. W. H. I., P. Fisicaro, A. F. Labrijn, J. M. Binley, W. P. Yang, H. J. Ditzel, C. F. Barbas III, and D. R. Burton. 1996. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type 1 envelope. J. Virol. 70:9046-9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parren, P. W. H. I., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poignard, P., E. Ollmann Saphire, P. W. H. I. Parren, and D. R. Burton. 2001. Gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19:253-274. [DOI] [PubMed] [Google Scholar]
- 28a.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. H. I. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 29.Posner, M. R., L. A. Cavacini, C. L. Emes, J. Power, and R. Byrn. 1993. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J. Acquir. Immune Defic. Syndr. 6:7-14. [PubMed] [Google Scholar]
- 30.Raux, H., P. Coulon, F. Lafay, and A. Flamand. 1995. Monoclonal antibodies which recognize the acidic configuration of the rabies glycoprotein at the surface of the virion can be neutralizing. Virology 210:400-408. [DOI] [PubMed] [Google Scholar]
- 31.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schönning, K., O. Lund, O. S. Lund, and J. E. Hansen. 1999. Stoichiometry of monoclonal antibody neutralization of T-cell line-adapted human immunodeficiency virus type 1. J. Virol. 73:8364-8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 35.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith-Franklin, B. A., B. F. Keele, J. G. Tew, S. Gartner, A. K. Szakal, J. D. Estes, T. C. Thacker, and G. F. Burton. 2002. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcγ receptors. J. Immunol. 168:2408-2414. [DOI] [PubMed] [Google Scholar]
- 37.Ugolini, S., I. Mondor, P. W. H. I. Parren, D. R. Burton, S. A. Tilley, P. J. Klasse, and Q. J. Sattentau. 1997. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J. Exp. Med. 186:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner, R., L. Deml, and H. Wolf. 1994. Polyvalent, recombinant HIV-1 virus-like particles: novel HIV-1 vaccine strategies. Antibiot. Chemother. 46:48-61. [DOI] [PubMed] [Google Scholar]
- 39.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 40.York, J., K. E. Follis, M. Trahey, P. N. Nyambi, S. Zolla-Pazner, and J. H. Nunberg. 2001. Antibody binding and neutralization of primary and T-cell line-adapted isolates of human immunodeficiency virus type 1. J. Virol. 75:2741-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]