Abstract
Induction of adaptive immunity to human immunodeficiency virus type 1 (HIV-1) at the mucosal site of transmission is poorly understood but crucial in devising strategies to control and prevent infection. To gain further understanding of HIV-1-specific T-cell mucosal immunity, we established HIV-1-specific CD8+ cytotoxic T-lymphocyte (CTL) cell lines and clones from the blood, cervix, rectum, and semen of 12 HIV-1-infected individuals and compared their specificities, cytolytic function, and T-cell receptor (TCR) clonotypes. Blood and mucosal CD8+ CTL had common HIV-1 epitope specificities and major histocompatibility complex restriction patterns. Moreover, both systemic and mucosal CTL lysed targets with similar efficiency, primarily through the perforin-dependent pathway in in vitro studies. Sequence analysis of the TCRβ VDJ region revealed in some cases identical HIV-1-specific CTL clones in different compartments in the same HIV-1-infected individual. These results clearly establish that a subset of blood and mucosal HIV-1-specific CTL can have a common origin and can traffic between anatomically distinct compartments. Thus, these effectors can provide immune surveillance at the mucosa, where rapid responses are needed to contain HIV-1 infection.
Human immunodeficiency virus type 1 (HIV-1) transmission occurs predominantly within the genital and rectal mucosa through sexual contact. Virus-specific T cells, known to control HIV-1 infection systemically (21, 29, 37), may also facilitate viral clearance in the mucosa. We previously identified class I major histocompatibility complex (MHC)-restricted CD8+ cytotoxic T lymphocytes (CTL) in the cervices of infected women that recognize and lyse HIV-1-infected cells. HIV-1-specific cervical CTL were more frequently detected in patients with preserved rather than decreased CD4+ T-cell counts, suggesting that the CD8+ effectors provide immune surveillance against local viral replication (28). More recently, we and others have extended these findings to semen and the rectal lymphoid tissue of infected men (19, 34, 38, 39).
The importance of understanding mechanisms to control infection locally is underscored by recent attention to the distinct properties of HIV-1 within the mucosa. Investigators comparing HIV-1 in blood and mucosal secretions (cervix and semen) have highlighted significant differences in viral phenotypes and genotypes as well as clearance following combination antiretroviral therapy (7, 12, 20, 32, 41, 43, 44). However, although mucosal antibody responses can differ from systemic responses (26), such discrepancies pertaining to HIV-1-specific T cells have not been elucidated. We previously demonstrated that most T cells in the female lower reproductive tract express T-cell receptor αβ (TCRαβ) (15, 28), similar to cells in the circulation and lymph nodes. Nevertheless, it is unclear whether HIV-1-specific CTL in the genital and gastrointestinal tracts emerge distinct from their systemic counterparts. Additionally, it is not known if mucosal CTL, similar to systemic CTL, preferentially utilize the perforin-dependent pathway to lyse infected cells (17, 23, 42).
Here we explore the functional properties of HIV-1-specific CTL in mucosal tissues of infected individuals through a detailed analysis of optimal epitope recognition and mechanisms of target cell lysis. The investigation also contrasts TCR usage and clonotypes between systemic and mucosal HIV-1-specific CTL, thus providing new information concerning the evolution and trafficking of the CD8+ memory response within the two compartments. We demonstrate that HIV-1-specific memory CTL are present in both blood and the mucosa, and that their ontogeny, epitope specificities, and function can be identical. These findings have utmost relevance in designing strategies to induce mucosal T cells by vaccination, since such effector activities may facilitate rapid viral clearance on sexual exposure to HIV-1.
MATERIALS AND METHODS
Study population.
Of 47 HIV-1-infected volunteers enrolled in a longitudinal mucosal immunity study, 12 were randomly selected for detailed investigation. Enrollment criteria and specimen collection were similar to those previously outlined (28). Blood and a cervical cytobrush, rectal biopsy specimen, and/or semen were obtained. Subjects provided written consent, and the study was approved by the University of Washington and Fred Hutchinson Cancer Research Center Human Subjects Committees.
Virological and T-cell subset analyses.
Blood plasma, seminal plasma, and cervical fluid HIV-1 RNA levels were determined by quantitative branched-DNA (bDNA) (Chiron, Emeryville, Calif.) and ultrasensitive reverse transcriptase PCR (RT-PCR), (Roche Molecular Systems, Branchburg, N.J.) assays (9, 27). Seminal plasma was pretreated with silica gel before being subjected to RT-PCR. Endocervical fluid was obtained using Sno-Strip wicks (36). Blood CD4+ T-cell counts were enumerated by standard consensus flow cytometry.
Isolation of blood and mucosal effector cells.
Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation using a Ficoll-Hypaque gradient. CD8+ T cells were positively selected using anti-CD8 monoclonal antibody (MAb)-coated microbeads (Miltenyi Biotec, Auburn, Calif.). Cervical T cells were isolated from endocervical cytobrush specimens (28); those with blood contamination were discarded. Males collected semen by masturbation; the semen was diluted 1:1 with phosphate-buffered saline and centrifuged (2,500 × g for 10 min). CD3+ T cells from the washed seminal cell pellet were positively selected using anti-CD3 MAb-coated microbeads (Miltenyi Biotec).
Three 3-mm punch rectal biopsy specimens, obtained 10 cm proximal to the anal verge, were placed in RPMI 1640 supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and Fungizone (2.5 μg/ml), repeatedly washed, and minced. Mononuclear cells were passed through a 140-μm screen to further purify lymphocytes from tissue debris and epithelial cells.
Generation and characterization of HIV-1-specific CTL clones.
Blood and mucosal HIV-specific T-cell clones were established as previously described (28). Briefly, adherent blood monocytes were infected overnight at a multiplicity of infection of 5 with a vaccinia virus recombinant virus vector expressing HIV-1 Env, Gag, and protease (vP1291) (kindly provided by J. Tartaglia, Virogenetics, Troy, N.Y.) and used as antigen-presenting cells. Blood and mucosal effector cells were plated by limiting dilution at 1 to 100 cells per well into 96-well round-bottom microtiter plates. The cells were provided with gamma-irradiated (3,300 rads) autologous antigen-presenting cells (3 × 103 infected adherent monocytes per well) and irradiated autologous feeder cells (5 × 104 per well). Human recombinant interleukin-2 (final concentration, 50 U/ml) was added after 48 h. After 14 to 21 days of culture, wells demonstrating cell growth were tested for cytolysis of autologous Epstein-Barr virus-transformed B lymphoblastoid cell lines (B-LCL) pulsed with rVV expressing HIV antigens (6) in a conventional CTL assay (28). Cells from wells with detectable HIV-specific cytolytic activity were further expanded to clones by stimulation with anti-CD3/4b (final concentration, 0.5 μg/ml) (kindly provided by Johnson T. Wong, Boston, Mass.) and 2 × 106 irradiated allogeneic PBMC per well as feeder cells. Expanded clones were further characterized for class I MHC restriction and fine epitope mapping by the chromium release assay (28). Peptides 20 amino acids (aa) in length and overlapping by 10 aa spanning the HIV-1MN env, HIV-1HXB-2 gag, and HIV-1IIIB pol genes (NIH AIDS Research and Reference Reagent Program, Bethesda, Md.) and 9 to 10-mers (Mimotopes, Clayton, Australia) were used for epitope mapping.
To determine the efficiency of cytolysis, autologous B-LCL were pulsed with serial dilutions of HIV-1 peptide and labeled with 100 μCi of 51Cr overnight at 37°C. Optimal effector TCR-MHC/peptide recognition was defined by the peptide concentration at which 50% maximal lysis was observed in a 4-h chromium release assay.
Mechanisms of cytolysis.
Prior to the CTL assay, effectors were pretreated with various concentrations of concanamycin A (CMA) (Wako Chemicals, Richmond, Va.), which blocks perforin-mediated lysis of target cells by CTLs, antagonistic anti-FasL MAb, and antagonistic anti-tumor necrosis factor alpha (TNF-α) MAb, alone or together (3). Anti-FasL MAb (CD95L; clone AHU-0212) and anti-TNFα MAb (clone B-C7) were purchased from Biosource International (Camarillo, Calif.). The susceptibility of B-LCL to apoptosis was determined after cell treatment with agonistic anti-Fas MAb (CD95; clone APO-1 [Biosource International]). The proportion of targets undergoing apoptosis was determined by measuring the uptake of acridine orange and ethidium bromide (Sigma Chemical Co., St. Louis, Mo.) under fluorescence microscopy at ×100 magnification (16).
TCR analysis of HIV-1-specific CTL clones.
Total RNA was extracted from 106 CTL clones by using a commercial kit (Qiagen Inc., Chatsworth, Calif.). cDNA was synthesized, and multiplex PCR amplifications were performed to identify variable genes (1). Amplified DNA fragments were separated on 2% agarose gels, excised, purified, and then sequenced using TCRBC primers (1) and ThermoSequenase (Amersham, Cleveland, Ohio). Sequences were aligned and compared with sequences published in GenBank to determine TCRB VDJ rearrangements. To identify mucosal clonotypes from archival PBMC specimens, clonal-specific TCRB VDJ rearrangements were analyzed from their cDNA by PCR using TCRBV6-specific and TCRBC-specific primers (1) followed by nested PCR using the TCRBV6 primer and clonal-specific primers for the cervical clone C20 (5′ TCATTGAGGCTGTCTGGG). The TCR Vβ family usage of HIV-specific CTL clones was also determined by flow cytometry using a FACScan flow cytometer and CELLQuest software (Becton Dickinson).
RESULTS
Induction of HIV-1-specific mucosal CTL.
To compare the specificities and functions of mucosal and systemic HIV-1-specific CD8+ T cells, we examined CTL responses from mucosal specimens (cervical cytobrushes, semen, and rectal biopsy specimens) and blood in 10 men and 2 women with chronic untreated HIV-1 infection (Table 1). The higher levels of CD4+ T cells (median, 691 cells/μl) and lower levels of plasma HIV-1 RNA (median, <400 copies/ml) indicated that HIV-1 infection was relatively well controlled in the absence of treatment; of note, eight patients were long-term nonprogressors. With the exception of NP007 and CHPIC1013, most patients had similar levels of HIV-1 RNA in the plasma and mucosa (Table 1).
TABLE 1.
Characteristics of the 12 HIV-1-infected study participants and the CD8+ CTL clones derived from blood and mucosa
| Study participant (HLA type) | Gender, duration of infection (yr) | CD4+ T cell count (cells/μl) | Amt. of HIV-1 RNA (no. of copies/ml) in:
|
No. of HIV-1-specific CD8+ CTL clones in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood
|
Mucosa
|
|||||||||||
| Blood | Mucosaa | Env | Gag | Pol | Total | Env | Gag | Pol | Total | |||
| MP 0471 (A24; B27, 62) | Male, 4 | 443 | 2,500 | 2,226 | 2 | 29 | 9 | 40 | 6 | 5 | 3 | 14 (semen) |
| CC 0710 (A24, 33; B14, 27) | Female, 9 | 500 | 3,000 | 10,000 | 7 | 15 | 31 | 53 | 0 | 10 | 3 | 13 (cervix) |
| NS 0909 (A31; B7, 57) | Female, 5 | 1,000 | 10,000 | 8,000 | 31 | 77 | 16 | 124 | 2 | 31 | 5 | 38 (cervix) |
| CHPIC 1013 (A3, 28; B35, 60) | Male, 4 | 336 | 18,750 | 868 | 1 | 3 | 5 | 9 | 0 | 2 | 3 | 5 (semen) |
| NP 001 (A30, 68; B57) | Male, 14 | 1,145 | <200 | NAb | 1 | 5 | 0 | 6 | 1 | 6 | 0 | 7 (rectum) |
| NP 002 (A2, 24; B27, 62) | Male, 13 | 716 | 414 | <400 | 0 | 34 | 11 | 45 | 0, 0 | 2, 8 | 0, 12 | 22 (semen, rectum) |
| NP 005 (A1, 66; B52, 57) | Male, 13 | 968 | 75 | NA | 0 | 47 | 0 | 47 | 0 | 17 | 0 | 17 (rectum) |
| NP 007 (A2, 3; B7, 13) | Male, 12 | 867 | 55 | 52,881 | 1 | 9 | 1 | 11 | 0 | 4 | 2 | 6 (rectum) |
| NP 012 (A2, 11; B44, 50) | Male, 14 | 590 | <200 | <400 | 0 | 2 | 1 | 3 | 0 | 2 | 1 | 3 (rectum) |
| NP 013 (A3, 31; B27, 38) | Male, 11 | 478 | <200 | <400 | 0 | 13 | 1 | 14 | 0 | 5 | 0 | 5 (rectum) |
| NP 014 (A3, 28; B53, 57) | Male, 13 | 1,129 | <200 | <400 | 3 | 51 | 13 | 67 | 1 | 7 | 11 | 19 (semen) |
| NP 015 (A2, 32; B27, 62) | Male, 15 | 666 | 5,857 | <400 | 1 | 24 | 37 | 62 | 0, 1 | 14, 3 | 0, 2 | 20 (semen, rectum) |
| Total (n = 12) | 10/2 (M/F), 12.5 (median) | 691 (median) | 307 (median) | 534 (median) | 47 (10%) | 309 (64%) | 125 (26%) | 481 | 11 (7%) | 116 (69%) | 42 (25%) | 169 |
Mucosal HIV-1 RNA measurements were performed with seminal plasma (male volunteers) or cervical Sno-strip (female volunteers) as described in Materials and Methods.
NA, not applicable.
Typically, the mucosal specimens contained fewer than 106 mononuclear cells, which precluded both phenotypic and functional analyses of CD8+ lymphocytes. For this reason, we chose to expand the antigen-specific memory cells in vitro into numbers sufficient to examine cytolytic function, epitope specificities, and TCRB clonotypes. Within 14 to 21 days, mononuclear cell expansion was observed in about 25% of the 900 culture wells from the blood and mucosal specimens. After 4 weeks of culture, HIV-1-specific CTL at low effector-to-target ratios (E:T) (≤5:1) were detected in a chromium release assay in approximately 30% of wells with cell growth. These cell lines were further expanded, and 481 class I MHC-restricted CD8+ CTL clones were generated from blood and 169 were generated from the mucosa (cervix, semen, or rectum) of our 12 study volunteers (Table 1). We refer to these CTL as clones, particularly since most emerged following prolonged expansion from wells plated with one cell and exhibited single specificities. However, we did not validate the single use of one TCR in every case of the 650 distinct populations, and we therefore recognize that a small proportion of these may be cell lines rather than clones. Of note, HIV-1-specific mucosal CD8+ CTL were established in all patients, including the five patients in whom mucosal HIV-1 RNA levels were below detection (Table 1). These findings indicate that local HIV-1-specific T-cell immunity is commonly induced in the genital and lower gastrointestinal tracts, sites of HIV-1 acquisition, and that CD8+ CTL may be present in the absence of detectable HIV-1 RNA.
Systemic and mucosal clones have similar HIV-1 epitope specificities.
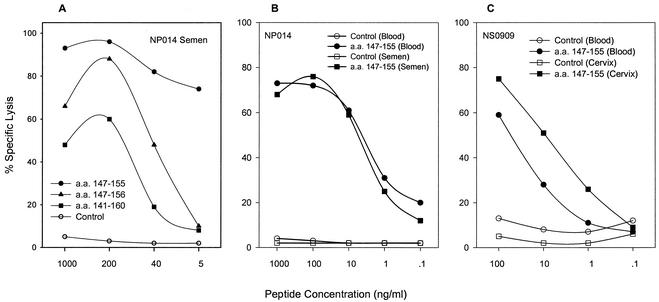
Recognition of HIV-1-expressing targets was remarkably similar in frequency between the two compartments. Gag-specific clones accounted for approximately two-thirds of the responses from both compartments (Table 1). Moreover, mucosal and blood CTL from five individuals had the same class I MHC restricting allele, regardless of the HIV-1 gene product recognized (data not shown). To compare the specificities of the blood and mucosal CD8+ T cells, we performed fine epitope mapping for samples from nine donors, focusing on Gag-specific CTL clones because of the higher proportion isolated in both compartments (309 of 481 [64%] in blood and 116 of 169 [69%] in mucosa) (Table 1). A representative example is depicted for donor NP014 (Fig. 1A), whose 12 clones from blood and semen were all B57 restricted and recognized the same Gag epitope within aa 147 to 155 (ISPRTLNAW).
FIG. 1.
HIV-1 Gag-specific cytolysis and functional avidity of blood and mucosal clones. (A) The optimal epitope of NP014 semen CTL clone is the Gag 9-mer (aa 147 to 155). Autologous B-LCL were pulsed with the 20-mer (aa 141 to 160), 10-mer (aa 147 to 156), and 9-mer (aa 147 to 155) at the peptide concentrations indicated, and specific lysis depicted was performed at an E:T ratio of 5:1. (B) Comparison of cytolysis of semen and blood clones from NP014 at different peptide concentrations of the optimal 9-mer (aa 147 to 155) expressed by autologous B-LCL. (C) Similar comparison of cytolysis by cervical and blood clones from NS0909 at an E:T ratio of 5:1.
Of the 425 Gag-specific clones established in these donors, we determined fine mapping and MHC restriction in 142 (33.4%) (Table 2). In every donor, Gag epitopes recognized in one component were also recognized in the other, and clones from several donors recognized the same Gag epitopes regardless of mucosal source. Many of the Gag epitopes restricted by B57, B27, and B62 fell within overlapping regions. Taken together, these findings demonstrate that HIV-1-specific CD8+ CTL derived from the systemic and mucosal compartments can have similar if not identical epitope specificities and MHC restriction patterns.
TABLE 2.
Epitope mappinga and class I MHC restriction analysis of HIV-1 Gag-specific CD8+ CTL clones
| Study participant | CTL clones (no. tested/ no. isolated) | Gag CTL epitope (aa residues)a | Class I restricting allele |
|---|---|---|---|
| NP002 | Blood (8/18) | GLNKIVRMY (269-277) | B62 |
| Rectum (4/8) | GLNKIVRMY (269-277) | B62 | |
| Semen (2/2) | GLNKIVRMY (269-277) | B62 | |
| NP005 | Blood (6/47) | YKTLRAEQASQEVKNWMTET (301-320) | B57 |
| Rectum (6/17) | YKTLRAEQASQEVKNWMTET (301-320) | B57 | |
| NP013 | Blood (3/3) | IYKRWIILGLNKIVRMYSPT (261-280) | B27 |
| Rectum (2/2) | IYKRWIILGLNKIVRMYSPT (261-280) | B27 | |
| NP014 | Blood (8/26) | ISPRTLNAW (147-155) | B57 |
| Semen (4/4) | ISPRTLNAW (147-155) | B57 | |
| Blood (8/25) | FSPEVIPMF (164-172) | B57 | |
| Semen (2/3) | FSPEVIPMF (164-172) | B57 | |
| NP015b | Blood (2/6) | GELDRWEKIRLRPGGKKKYK (11-30) | B62 |
| Semen (2/4) | GELDRWEKIRLRPGGKKKYK (11-30) | B62 | |
| Blood (5/8) | RWIILGLNK (264-272) | B27 | |
| Semen (7/10) | RWIILGLNK (264-272) | B27 | |
| Rectum (3/3) | HIV-1 Gag | B27 | |
| MP0471 | Blood (4/19) | IYKRWIILGLNKIVRMYSPT (261-280) | B27 |
| Blood (4/10) | IYKRWIILGLNKIVRMYSPT (261-280) | B62 | |
| Semen (3/3) | IYKRWIILGLNKIVRMYSPT (261-280) | B62 | |
| CC0710b | Blood (5/10) | RWIILGLNK (264-272) | B27 |
| Cervix (4/6) | RWIILGLNK (264-272) | B27 | |
| Blood (4/5) | DRFYKTRAE (298-307) | B14 | |
| Cervix (2/4) | DRFYKTRAE (298-307) | B14 | |
| NS0909 | Blood (15/77) | ISPRTLNAW (147-155) | B57 |
| Cervix (15/31) | ISPRTLNAW (147-155) | B57 |
Epitopes within HIV-1 Gag were defined by recognition of small peptides, 9 to 20 aa in length. Fine mapping to the optimal 9 to 10-mer was performed in those indicated, and the results of a representative experiment are shown in Fig. 1.
Although both the Gag 10-mer KRWIILGLNK (aa 263 to 272) and 9-mer RWIILGLNK (aa 264 to 272) were recognized, studies examining lysis by the clones of autologous B-LCL pulsed with serial peptide dilutions revealed a more avid response with the 9-mer.
Efficiency of cytolytic activity.
To compare the cytolytic efficiency of the clones, 16 pairs of blood and mucosal CTL clones from five subjects were tested for lysis of targets pulsed with serial 10-fold dilutions of the defined optimal 9-mer HIV-1 Gag epitope. In all cases, the efficiency of these interactions was high, with lysis of targets with as little as 0.1 to 1 ng of peptide per ml (Fig. 1B and C and data not shown). For example, both blood and semen B57-restricted clones from NP014 exhibited maximum target cell lysis of 75 to 80% with 100 ng of Gag peptide (aa 147 to 155) per ml and the minimal antigen concentration associated with effective cytolysis was 0.1 ng/ml (Fig. 1B). Taken together, the maximum, minimum, and optimal effector TCR-MHC/peptide recognition were similar in the majority of blood and mucosal (semen, rectal, and cervical) paired clones recognizing the same epitope. Differences, when noted, were minimal, as in the B57-restricted cervical and blood clones from NS0909 (Fig. 1C). These results indicate that mucosal CTL are highly efficient in lysing target cells and exhibit functional avidity similar to or greater than that of clones derived from blood.
Lysis pathways of HIV-1-specific mucosal CTL.
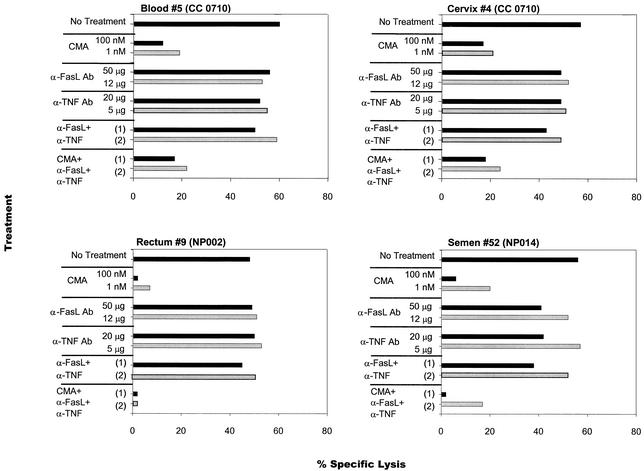
To determine if mucosal effectors lyse targets by the perforin-dependent granule exocytosis pathway, similar to blood-derived CTL (40), three mechanisms of lymphocyte cytotoxicity were evaluated. CD8+ CTL clones were pretreated with CMA, antagonistic non-cross-linking anti-FasL MAb, and antagonistic anti-TNF-α MAb, to selectively block perforin, Fas-FasL interactions, and TNF-α-induced cytotoxicity, respectively. In all cases, significant dose-dependent inhibition of target lysis occurred when effectors were pretreated with CMA (Fig. 2, representative examples). Even the lowest concentration of CMA (1 nM) reduced the lysis of autologous Gag-expressing targets by 63 to 83%. By contrast, the addition of the antagonistic antibodies for Fas and TNF activities had little impact on the efficiency of cytolysis (Fig. 2). When CTL were pretreated with all three inhibitors, reduction in lysis was similar to that observed with CMA alone (Fig. 2). An additional 12 pairs of blood, rectal, and cervical clones from volunteers NP002, CC0710, and NS0909 were tested for perforin-dependent lysis, and similar findings were observed (data not shown).
FIG. 2.
Selective inhibition of HIV-1-specific cytolytic activity by CMA but not anti-FasL or anti-TNF-α antagonistic antibodies. Blood and mucosal CD8+ clones were pretreated with CMA (1 to 100 nM), anti-FasL MAb (12 to 50 μg/ml), anti-TNF-α MAb (5 to 20 μg/ml), or control mouse immunoglobulin G for 2 h. In some experiments, a combination of two or three blocking agents at the highest (experiment 1) or lowest (experiment 2) concentrations was used. The figure depicts cytolysis of CD8+ clones isolated from patients CC0710 (blood and cervix), NP002 (rectum), and NP014 (semen). Blood and cervical clones from patient CC0710 recognized HIV-1 Gag within aa 264 to 272. Blood (data not shown) and semen HIV-specific CTL clones from patient NP014 recognized HIV-1 Gag within aa 164 to 172. Blood (data not shown) and rectal T-cell clones from patient NP002 recognized HIV-1 Gag within aa 269 to 277. Ab, antibody.
These results imply that mucosal CTL lysis is mediated primarily by the perforin-dependent pathway. Additional experiments determined if targets expressing HIV-1 epitopes were susceptible to lysis through Fas-FasL interactions. The majority of B-LCL (85 to 95%) expressed Fas by flow cytometric staining (data not shown). Moreover, in contrast to <5% of untreated B-LCL, a significant proportion (median, 40%; range, 10 to 50%) of B-LCL treated with cross-linking anti-Fas MAb (CD95) exhibited nuclear morphologic changes consistent with apoptosis, despite maintaining an intact cell membrane, when stained with acridine orange and ethidium bromide and examined microscopically. Additionally, <10% of mucosal and blood CD8+ CTL clones expressed FasL when stained with fluorescein isothiocyanate-conjugated anti-CD95L (data not shown). These results indicate that B-LCL serving as HIV-1-specific targets express the Fas receptor, which can be triggered to initiate cell death by apoptosis. However, the absence of FasL expression by most effector CD8+ T cells, together with the lack of an effect by antagonistic anti-FasL MAb, indicates that Fas-FasL interactions play a minor role, if any, in the killing of target cells. Overall, the more significant inhibition of perforin-mediated lysis of target cells by CMA alone suggests that the perforin-dependent pathway plays a major role in the cytolytic activity in vitro of systemic and mucosal effector cells.
HIV-1-specific blood and mucosal CTL clones can be derived from the same progenitor cell.
We have demonstrated that systemic and mucosal CD8+ CTL from an individual donor can recognize the identical class I-restricted HIV-1 epitope and can do so with the same efficiency and lytic function. This raises the possibility that such clones may originate from the same progenitor cell. To address this, the expressed TCRβ chains were analyzed and the VDJ segments within the CDR3 loops were sequenced in representative clones from blood and the mucosa in three donors.
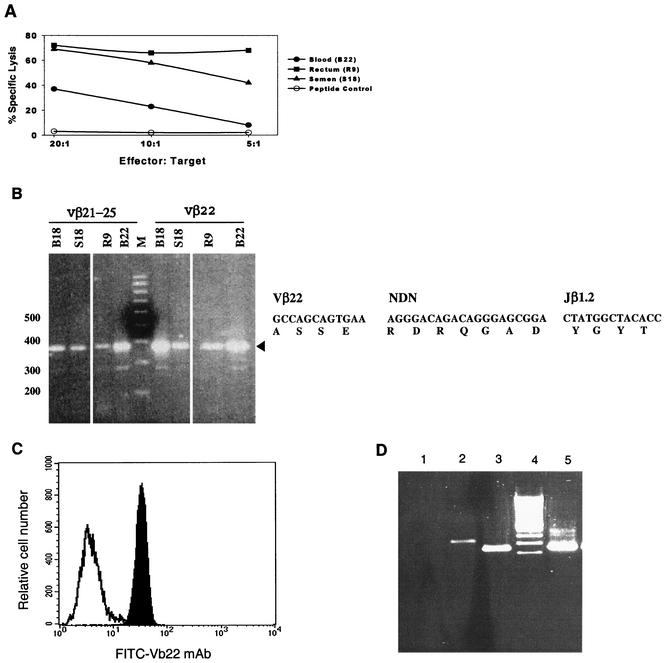
In subject NP002, HLA-B62-restricted CD8+ CTL clones derived from blood, semen, and rectal tissue recognized Gag peptide (aa 269 to 277) (Fig. 3A; Table 3). cDNAs from the clones were amplified by PCR with primers (tested in pools or alone) specific for each of the 23 TCR Vβ families. Amplification revealed usage of Vβ21 to Vβ25, specifically Vβ22, by the mucosal and blood clones (Fig. 3B). Expression of Vβ22 on each CTL clone was confirmed by flow cytometry (Fig. 3C). Next, the amplified TCR Vβ22 DNA PCR product was sequenced to precisely define rearrangements in the TCRβ VDJ region. All five clones (B18, B21, B22, S18, and R9) displayed the same TCRβ VDJ sequence, defined as Vβ22S1DJ1.2 (Table 3), whose amino acid sequence is specified in Fig. 3B. Thus, identification of the same sequence within the TCRβ rearrangement of the clones from NP002 indicates that virus-specific T cells found in the three distinct anatomical sites (blood, rectum, and semen) represent expansion from the same precursor.
FIG. 3.
Blood and mucosal Gag-specific CTL clones recognize the same epitope and display the same TCR β VDJ rearrangement. (A to C) Blood, rectal, and seminal CTL clones, established from volunteer NP002 from the same visit. (A) CTL clones derived from blood (B22), semen (S18), and rectum (R9) recognized the same HIV-1 Gag epitope within aa 269 to 277. (B) The four CTL clones (blood-derived B18 and B22, semen-derived S18, and rectum-derived R9) utilize TCR Vβ22 and present identical rearrangements of their TCR β VDJ segments. The four left lanes contain PCR products from the clonal cDNA amplified with pooled Vβ21 to Vβ25 primers. The four right lanes contain the PCR products from the clonal cDNA amplified with the Vβ22-specific primers. The coding and amino acid sequence of the five clones (including B21) TCR BVDJ region is presented to the right. (C) TCR Vβ22 usage was confirmed by flow cytometry. Expression of Vβ22 fluorescein isothiocyanate-conjugated MAb by the CTL clone B22 (solid peak) is shown in comparison to the isotype control (open peak). (D) PBMC from volunteer NS0909 were probed for the presence of TCR Vβ6 and C20 clone-specific VDJ segment. A positive response was detected with Vβ6 specific primers (lane 2) and C20 clone-specific primers (lane 3). Negative and positive control PCR assays were performed in the absence of Vβ6-specific primers (lane 1) or with the cDNA product from cervical clone C20 (lane 5).
TABLE 3.
TCRβ VDJ rearrangements of class I MHC-restricted Gag-specific CTL clones in three subjects
| Subject | CTL clonesa | TCR β rearrangement | Epitope specificity, MHC restriction |
|---|---|---|---|
| NP002 | Blood: B18, B21, B22 | Vβ22S1DJ1.2 | Gag aa 269-277, HLA-B62 |
| Semen: S18 | |||
| Rectum: R9 | |||
| NP014 | Blood: B19, B26 | Vβ6S2DJ2S2 | Gag aa 147-155, |
| Semen: S44 | HLA-B57 | ||
| Blood: B25 | Vβ6S2DJ1.1 | ||
| Semen: S42 | |||
| Semen: S50 | Vβ6.3DJ1S5 | ||
| Blood: B33 | Vβ21S3DJ1.2 | Gag aa 164-172, | |
| Semen: S52 | Vβ7S1DJ2.3 | HLA-B57 | |
| NS0909 | Blood: B4 | Vβ6S7DJ2.7 | Gag aa 147-155, |
| Cervix: C20 | Vβ6S3DJ1.4 | HLA-B57 | |
| Blood: B62 | Vβ6S3DJ2.1 | ||
| Blood: B71 | Vβ6.4DJ2.3 | ||
| Cervix: C37 | Vβ6S5DJ2.5 |
Clone origin and number are designated.
To examine if the second donor, NP014, had identical or distinct CTL clones circulating through the blood and mucosa, clones from blood (n = 4) and semen (n = 4) were evaluated. Of note, all CTL responses identified in this subject were B57 restricted (Table 2) and recognized Gag peptides (aa 147 to 155 or 164 to 172) (Tables 2 and 3). Among eight HLA-B57-restricted, Gag-specific CTL clones, five distinct TCR profiles were found (Table 3). Clones B19, B25, B26, S42, and S44 recognized the same epitope and expressed the same TCR Vβ6S2 but differed in their BD-BJ segments (Table 3). Of note, clones B19, B26, and S44 recognized the same epitope and had the same Vβ6S2 and DJ2S2 profile (Table 3) and identical sequences (data not shown). Thus, again, identity between semen and blood clones indicates that the mucosal and systemic CTL can be derived from common precursors.
Similarly, we examined three blood and two cervical CTL clones from volunteer NS0909. All clones showed distinct TCRβ rearrangements (Table 3). We then designed clone-specific primers based on the TCR VDJ region of cervical CTL clone C20 to investigate whether T cells with similar TCR VDJ rearrangements were present in blood at the same time that the cervical clone was identified. We performed nested PCR using Vβ6-specific primers followed by clone-specific primers. By this strategy, we demonstrated that cells with a TCRβ rearrangement similar to cervical clone C20 were present in PBMC (Fig. 3D). Taken together, these results provide the first evidence that virus-specific memory CTL circulate in vivo within peripheral blood and the mucosa, where they are primed to control HIV-1 at sites of sexual contact.
DISCUSSION
This study demonstrates that antigen-specific mucosal T cells are an integral part of the immune response to HIV-1 infection. Memory CD8+ CTL with similar epitope specificities and function reside in the mucosa of patients infected by sexual transmission and are thus positioned to contain HIV-1 at sites where most infections are acquired and transmitted. More importantly, our results provide the first evidence that CD8+ CTL associated with a naturally acquired, sexually transmitted infection recirculate between blood and the genital and gastrointestinal tracts.
Numerous studies have emphasized the fundamental role of mucosal immunity in controlling HIV-1 infection, yet few have actually examined T cells from the human genital and rectal mucosa. This is due in part to recovery of insufficient viable T cells by routine, noninvasive procedures. We circumvented this problem for women by obtaining endocervical cytobrushes from the transitional zone where T cells are more abundant (28) and for men by obtaining semen and small punch biopsy specimens from the rectum. The memory TCRαβ+ CD8+ CTL identified in the cervix and rectum likely represent a subpopulation of intraepithelial lymphocytes as well as T cells within submucosal lymphoid follicles (14). The origin of the seminal CTL is unidentified, but likely sources include the testis and prostate (20, 22, 24, 25, 33). We clearly demonstrate that despite continuous exposure to local antigens, memory CTL present in the genital and rectal mucosa are easily capable of antigen-specific clonal expansion in vitro. Thus, mucosal memory T cells become activated and proliferate similar to their systemic counterparts in persons with HIV-1 infection.
In numerous cases, HIV-1 epitope specificities and MHC restriction patterns were identical in peripheral blood and mucosal CTL, which suggests that a subset of lymphocytes involved in the immunological memory against HIV-1 at the genital and rectal mucosa evolves in concert with, rather than separately from, systemic responses. Of note, the mucosal and systemic CTL clones frequently recognized immunodominant Gag epitopes lying within more highly conserved regions of HIV-1. However, it is possible that recognition of subdominant epitopes or those falling within more variable regions of other gene products may differ among CTL within the two compartments. Such differences were perhaps less obvious in this study, with the use of vectors and peptides representing HIV-1 consensus strains rather than autologous viruses to express HIV-1 epitopes, as well as our cloning strategies and focus on Gag-specific responses in untreated donors who were predominantly long-term nonprogressors. There is also the possibility, although it was not examined in this study, that the HIV-1-specific T cells recruited to the mucosa may be present in response to continuing viral challenge following high-risk exposures. It is indeed possible that the clones generated in vitro may not be truly representative of those present within the initial specimen. However, we have noted persistence of many of these clones in subsequent time points in our longitudinal assessments (J. Cao, unpublished data). Also, discrepancies may emerge in persons infected with more than one HIV-1 strain within the same or different subtype. Nonetheless, the similarities in CTL responses derived from the blood and mucosa are striking.
Likewise, we observed similar CTL functional avidity for MHC-peptide complexes between the two compartments. These findings indicate that HIV-specific mucosal CTL, similar to those from blood, are capable of efficient target cell lysis. This may be particularly important in settings where the levels of viral replication and antigen expression are low, such as within days of infection in the genital tract and with use of antiretroviral therapy. Our in vitro studies also indicate that mucosal CTL, similar to blood CTL (3, 13, 18, 40, 42), utilize the granule exocytosis pathway to destroy HIV-1-expressing target cells. Inhibition of target lysis by CMA provides confirmation that killing is mediated primarily by direct contact between effectors and targets rather than through secretion of soluble factors (e.g., alpha interferon, gamma interferon, and chemokines). Conceivably, the culture conditions, the use of CTL clones rather than primary cells, and the use of the chromium release assay to evaluate cytolysis favored the detection of the perforin pathway (13). This concern led us to explore more carefully the expression of Fas on targets and FasL on effectors and the susceptibility of targets to lysis through the Fas receptor. In short, most target cells (>85%) expressed Fas and were lysed on cross-linking this receptor, but <10% of CTL expressed FasL and their cytolytic activities were maintained on blocking the Fas-FasL interaction. Thus, a role for Fas-FasL interactions in triggering cytolysis by mucosal memory CD8+ effectors cannot be entirely excluded, but its contribution to class I MHC-restricted CTL is probably minor (8). In addition, it remains unclear if mucosal CTL contain relatively low levels of perforin, as has been recently demonstrated for peripheral blood and lymphoid tissue (2, 8). Although the local environment may influence the efficiency of eliminating infected mucosal cells (4, 10), our results suggest that mucosal CTL have the capacity to respond as competently as those circulating in peripheral blood.
Perhaps the most compelling evidence for the importance of mucosal CTL in providing a local defense against HIV-1 is the demonstration, for the first time to our knowledge, that identical antigen-specific CD8+ T-cell clones reside in the peripheral blood, rectum, and genital tract of the same HIV-1-infected individual. This observation clearly establishes that T cells from these distinct compartments can have a common origin. Moreover, these results suggest that memory CD8+ T cells circulate between the mucosal and systemic compartments. Taken together, these results indicate that both common and distinct CTL clonal populations emerge mucosally and systemically following infection, and they are consistent with the results of previous murine studies examining TCR usage of gut T cells (35).
The question remains of where the priming of mucosal HIV-1-specific CTL occurs and how effectors track back to the mucosal tissue. Dendritic cells and other professional APCs present viral antigens to T cells in the regional lymph nodes. This heralds the transition from the early innate response to the onset of acquired immunity, and it is at this point that virus-specific T-cell memory is first established (31). Thus, mucosal CTL induced by HIV-1 infection are probably primed in the mucosa-associated lymphoid tissues (MALT) and regional nodes rather than from random stimulation within the mucosal tissue. However, others have observed that mutant mice lacking Peyer's patches or other lymphoid tissue displayed normal number of T cells in mucosal epithelium (5, 30), suggesting that T-cell priming by professional antigen-presenting cells can occur anywhere between the mucosal epithelium, the MALT, the regional lymph nodes, the blood, and the spleen. Antigen-specific T cells subsequently reach the blood to seed at different mucosal surfaces and MALT (11). For HIV-1-specific memory responses, we presume that CTL with common clonotypes were primed in the MALT and then circulate through blood and home back to the genital and rectal tissue. Because of the small number of cells isolated from the different mucosal tissues, we were limited in our effort to identify potent and selective adhesion molecules and chemoattractant receptors on the CD8+ CTL that may regulate the regional trafficking of mucosal lymphocytes in humans. Studies are in progress to identify phenotypes and homing markers that can better elucidate the induction and trafficking of the mucosal memory cells, but at least in the lower gastrointestinal tract the HIV-1-specific CTL will presumably express the α4β7 integrin (44). Since identical clonotypes were found in the blood, semen, and rectum, this suggests that mucosal lymphocytes can circulate between the reproductive and lower gastrointestinal tracts. However, less is known about the specialized trafficking mechanisms within the human genital mucosa, including the epithelial chemokines and adhesion molecules expressed by mucosal lymphocytes, as well as the differentiation state and homing properties of the CTL. With knowledge of the antigen-specific cells emerging from sexually exposed HIV-1-infected individuals, we can now more accurately define the characteristics of the local immune response ex vivo.
In conclusion, we demonstrate that HIV-1-specific memory CTL are present in both blood and mucosa and that their ontogeny, epitope specificities, and function may be identical. This has fundamental relevance to vaccine strategies, since it is possible that memory CTL identified in the circulation may home to the mucosa, where rapid responses are needed to contain HIV-1 infection. It is therefore possible that candidate HIV-1 vaccines can induce virus-specific cell-mediated immunity in different compartments and that the route of vaccine immunization can influence the level of immune responses present in different compartments or tissues. In future studies, it will be important to define factors required for the maintenance of virus-specific CTL at mucosal surfaces in infected patients as well as their potential efficiency in limiting HIV-1 transmission.
Acknowledgments
We express our appreciation to all our volunteers for their time and dedication, to Robert Coombs for the measurement of HIV-1 RNA loads, and to Micky Moerbe and Alicia Cerna for assistance with preparation of the manuscript.
This work was supported by NIH grants R37 AI35605 and P30A27757 and the Burroughs Wellcome Clinical Scientist Award in Translational Research (M.J.M.).
REFERENCES
- 1.Akatsuka, Y., E. G. Martin, A. Madonik, A. A. Barsoukov, and J. A. Hansen. 1999. Rapid screening of T-cell receptor (TCR) variable gene usage by multiplex PCR: application for assessment of clonal composition. Tissue Antigens 53:122-134. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T. E. Fehniger. 1999. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS 13:1295-1303. [DOI] [PubMed] [Google Scholar]
- 3.Ando, K., K. Hiroishi, T. Kaneko, T. Moriyama, Y. Muto, N. Kayagaki, H. Yagita, K. Okumura, and M. Imawari. 1997. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 158:5283-5291. [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arstila, T., T. P. Arstila, S. Calbo, F. Selz, M. Malassis-Seris, P. Vassalli, P. Kourilsky, and D. Guy-Grand. 2000. Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph. J. Exp. Med. 191:823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg, R. S., T. Paradis, R. Byington, W. Henle, M. S. Hirsch, and R. T. Schooley. 1987. Effects of human immunodeficiency virus on the cellular immune response to Epstein-Barr virus in homosexual men: characterization of the cytotoxic response and lymphokine production. J. Infect. Dis. 155:877-890. [DOI] [PubMed] [Google Scholar]
- 7.Coombs, R. W., C. E. Speck, J. P. Hughes, W. Lee, R. Sampoleo, S. O. Ross, J. Dragavon, G. Peterson, T. M. Hooton, A. C. Collier, L. Corey, L. Koutsky, and J. N. Krieger. 1998. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J. Infect. Dis. 177:320-330. [DOI] [PubMed] [Google Scholar]
- 8.Corazza, N., S. Muller, T. Brunner, D. Kagi, and C. Mueller. 2000. Differential contribution of Fas- and perforin-mediated mechanisms to the cell-mediated cytotoxic activity of naive and in vivo-primed intestinal intraepithelial lymphocytes. J. Immunol. 164:398-403. [DOI] [PubMed] [Google Scholar]
- 9.Dewar, R. L., H. C. Highbarger, M. D. Sarmiento, J. A. Todd, M. B. Vasudevachari, R. T. Davey, Jr., J. A. Kovacs, N. P. Salzman, H. C. Lane, and M. S. Urdea. 1994. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J. Infect. Dis. 170:1172-1179. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. C. 1995. Anatomical environment as a determinant in viral immunity. J. Immunol. 155:1023-1027. [PubMed] [Google Scholar]
- 11.Futterer, A., K. Mink, A. Luz, M. H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59-70. [DOI] [PubMed] [Google Scholar]
- 12.Gupta, P., C. Leroux, B. K. Patterson, L. Kingsley, C. Rinaldo, M. Ding, Y. Chen, K. Kulka, W. Buchanan, B. McKeon, and R. Montelaro. 2000. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasi species between blood and semen. J. Infect. Dis. 182:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Hadida, F., V. Vieillard, L. Mollet, I. Clark-Lewis, M. Baggiolini, and P. Debre. 1999. Cutting edge: RANTES regulates Fas ligand expression and killing by HIV-specific CD8 cytotoxic T cells. J. Immunol. 163:1105-1109. [PubMed] [Google Scholar]
- 14.Hayday, A., E. Theodoridis, E. Ramsburg, and J. Shires. 2001. Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2:997-1003. [DOI] [PubMed] [Google Scholar]
- 15.Hladik, F., G. Lentz, E. Delpit, A. McElroy, and M. J. McElrath. 1999. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J. Immunol. 163:2306-2313. [PubMed] [Google Scholar]
- 16.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagi, D., F. Vignaux, B. Ledermann, K. Burki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528-530. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678-3686. [PubMed] [Google Scholar]
- 19.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 20.Kiessling, A. A., L. M. Fitzgerald, D. Zhang, H. Chhay, D. Brettler, R. C. Eyre, J. Steinberg, K. McGowan, and R. A. Byrn. 1998. Human immunodeficiency virus in semen arises from a genetically distinct virus reservoir. AIDS Res. Hum. Retroviruses. 14(Suppl. 1):S33-S41. [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger, J. N., R. W. Coombs, A. C. Collier, D. D. Ho, S. O. Ross, J. E. Zeh, and L. Corey. 1995. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J. Urol. 154:1035-1040. [PubMed] [Google Scholar]
- 23.Lowin, B., M. Hahne, C. Mattmann, and J. Tschopp. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370:650-652. [DOI] [PubMed] [Google Scholar]
- 24.Miller, C. J. 1998. Localization of simian immunodeficiency virus-infected cells in the genital tract of male and female rhesus macaques. J. Reprod. Immunol. 41:331-339. [DOI] [PubMed] [Google Scholar]
- 25.Miller, C. J., P. Vogel, N. J. Alexander, S. Dandekar, A. G. Hendrickx, and P. A. Marx. 1994. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab. Investig. 70:255-262. [PubMed] [Google Scholar]
- 26.Moldoveanu, Z., M. W. Russell, H. Y. Wu, W. Q. Huang, R. W. Compans, and J. Mestecky. 1995. Compartmentalization within the common mucosal immune system. Adv. Exp. Med. Biol. 371:97-101. [DOI] [PubMed] [Google Scholar]
- 27.Mulder, J., N. McKinney, C. Christopherson, J. Sninsky, L. Greenfield, and S. Kwok. 1994. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J. Clin. Microbiol. 32:292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musey, L., Y. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 30.Nanno, M., S. Matsumoto, R. Koike, M. Miyasaka, M. Kawaguchi, T. Masuda, S. Miyawaki, Z. Cai, T. Shimamura, Y. Fujiura, et al. 1994. Development of intestinal intraepithelial T lymphocytes is independent of Peyer's patches and lymph nodes in aly mutant mice. J. Immunol. 153:2014-2020. [PubMed] [Google Scholar]
- 31.Neutra, M. R., N. J. Mantis, and J.-P. Kraehenbuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2:1004-1009. [DOI] [PubMed] [Google Scholar]
- 32.Ping, L. H., M. S. Cohen, I. Hoffman, P. Vernazza, F. Seillier-Moiseiwitsch, H. Chakraborty, P. Kazembe, D. Zimba, M. Maida, S. A. Fiscus, J. J. Eron, R. Swanstrom, and J. A. Nelson. 2000. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J. Virol. 74:8946-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pudney, J., and D. Anderson. 1991. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am. J. Pathol. 139:149-160. [PMC free article] [PubMed] [Google Scholar]
- 34.Quayle, A. J., W. M. Coston, A. K. Trocha, S. A. Kalams, K. H. Mayer, and D. J. Anderson. 1998. Detection of HIV-1-specific CTLs in the semen of HIV-infected individuals. J. Immunol. 161:4406-4410. [PubMed] [Google Scholar]
- 35.Regnault, A., A. Cumano, P. Vassalli, D. Guy-Grand, and P. Kourilsky. 1994. Oligoclonal repertoire of the CD8 alpha alpha and the CD8 alpha beta TCR-alpha/beta murine intestinal intraepithelial T lymphocytes: evidence for the random emergence of T cells. J. Exp. Med. 180:1345-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichelderfer, P. S., R. W. Coombs, D. J. Wright, J. Cohn, D. N. Burns, S. Cu-Uvin, P. A. Baron, M. H. Coheng, A. L. Landay, S. K. Beckner, S. R. Lewis, A. A. Kovacs, and the WHS 001 Study Team. 2000. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. AIDS 14:2101-2107. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 38.Shacklett, B. L., T. J. Beadle, P. A. Pacheco, J. H. Grendell, P. A. Haslett, A. S. King, G. S. Ogg, P. M. Basuk, and D. F. Nixon. 2000. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology 270:317-327. [DOI] [PubMed] [Google Scholar]
- 39.Shacklett, B. L., S. Cu-Uvin, T. J. Beadle, C. A. Pace, N. M. Fast, S. M. Donahue, A. M. Caliendo, T. P. Flanigan, C. C. Carpenter, and D. F. Nixon. 2000. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS 14:1911-1915. [DOI] [PubMed] [Google Scholar]
- 40.Shankar, P., Z. Xu, and J. Lieberman. 1999. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood 94:3084-3093. [PubMed] [Google Scholar]
- 41.Si-Mohamed, A., M. D. Kazatchkine, I. Heard, C. Goujon, T. Prazuck, G. Aymard, G. Cessot, Y. H. Kuo, M. C. Bernard, B. Diquet, J. E. Malkin, L. Gutmann, and L. Belec. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112-122. [DOI] [PubMed] [Google Scholar]
- 42.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 43.Zhang, H., G. Dornadula, M. Beumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]
- 44.Zhu, T., N. Wang, A. Carr, D. S. Nam, R. Moor-Jankowski, D. A. Cooper, and D. D. Ho. 1996. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 70:3098-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]