Abstract
We previously reported that a 350-bp region of the adeno-associated virus (AAV) type 2 rep gene contains a cis-acting element responsible for the Rep-dependent replication of a transiently transfected rep-cap plasmid. In this study, we further report that replicated rep-cap sequences can be packaged into AAV capsids in the absence of the inverted terminal repeats.
The usual procedure for recombinant adeno-associated virus (rAAV) assembly involves transfection of the vector and the rep-cap plasmid into cells which are either infected with adenovirus or cotransfected with an adenoviral helper plasmid (4, 11, 15). Despite the lack of homologous sequences between the rep-cap and vector sequences, the precise characterization of rAAV preparations indicated that they were contaminated to various extents with particles containing rep-cap AAV sequences. We designated these contaminating particles rep positive (rep+), because they were able to transfer a Rep function, as detected by a replication center assay (RCA) (10). Previous studies have indicated that most of these particles were replication competent and that they arose from nonhomologous recombination events between the rep-cap plasmid and the inverted terminal repeats (ITRs) in the rAAV vector (1, 14). Deletion of critical ITR sequences involved in the nonhomologous recombination events prevented the formation of such replication-competent particles. However, rAAV preparations remained contaminated by replication-defective AAV particles containing rep-cap genomes, further suggesting that these sequences had been packaged in the absence of the ITRs (14).
Altogether, these observations suggested that some additional cis-acting elements were present in the rep-cap sequences, allowing their replication and encapsidation. We and others have previously reported the presence of a cis-acting replication element (CARE) located in the 5′ portion of the rep gene (9, 12). The CARE was localized in a 350-bp region that included the p5 promoter and the 5′ portion of the rep coding sequence (nucleotides 190 to 540 of wild-type AAV), and it was demonstrated that this element behaved in vitro and in vivo as a Rep-dependent origin of replication in the absence of both ITRs (9).
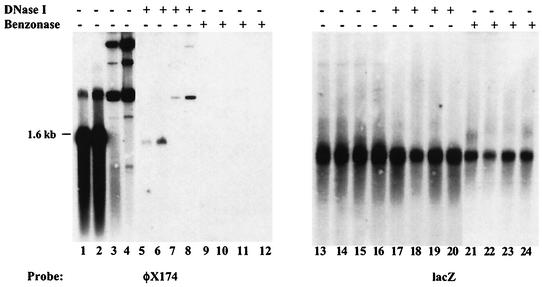
In the present study, we investigated whether the presence of CARE could also lead to the packaging of rep-cap sequences into AAV capsids in the absence of the viral ITRs. A critical element in this study was the need to distinguish between DNA truly packaged inside the particles and that simply contaminating the preparations. The conventional procedure to extract viral DNA from a cell lysate or a purified rAAV stock relies on the use of DNase I to digest contaminating DNA before extraction of packaged sequences. The activity of DNase I was checked by mixing purified rAAVLZ particles (rAAV encoding the nucleus-localized β-galactosidase) with up to 400 ng of φX174 DNA (either double or single stranded). After digestion for 1 h at 37°C with 50 U of DNase I (Roche) in 500 μl of Dulbecco modified Eagle medium, packaged DNA was extracted with proteinase K and phenol-chloroform and analyzed on a Southern blot by using either a φX174 or a lacZ probe. Incubation with DNase I did not completely digest the φX174 DNA (Fig. 1, lanes 5 to 8). In contrast, incubation of the samples with 100 U of Benzonase (Merck Eurolab) for 1 h at 37°C in 500 μl of a solution containing 50 mM Tris HCl (pH 8.0), 1 mM MgCl2, and 100 μg of bovine serum albumin/ml, completely removed the φX174 signal (Fig. 1, lanes 9 to 12), whereas viral DNA hybridizing to a lacZ probe was still observed (Fig. 1, lanes 21 to 24). This result indicated that Benzonase, not DNase I, was optimal in ensuring a complete digestion of DNA potentially contaminating the purified rAAV stock. As a consequence, all subsequent experiments involving extraction of packaged DNA were performed using Benzonase instead of DNase I.
FIG. 1.
Verification of digestion conditions by using either DNase I or Benzonase. One hundred microliters of purified rAAVLZ particles (approximately 3 × 109 particles) was mixed with different amounts of φX174 DNA (New England Biolabs) (lanes 1, 5, and 9, 200 ng of single-stranded DNA; lanes 2, 6, and 10, 400 ng of single-stranded DNA; lanes 3, 7, and 11, 200 ng of double-stranded DNA; lanes 4, 8, and 12, 400 ng of double-stranded DNA; lanes 13 to 24, the samples are in the same order as for lanes 1 to 12) and left undigested or digested with either 50 U of DNase I or 100 U of Benzonase for 1 h at 37°C. After digestion, viral DNA was extracted by proteinase K digestion and phenol-chloroform extraction and loaded on a 1% agarose gel. DNA was transferred under alkaline conditions and hybridized first to a φX174 probe and then to a lacZ probe after dehybridization.
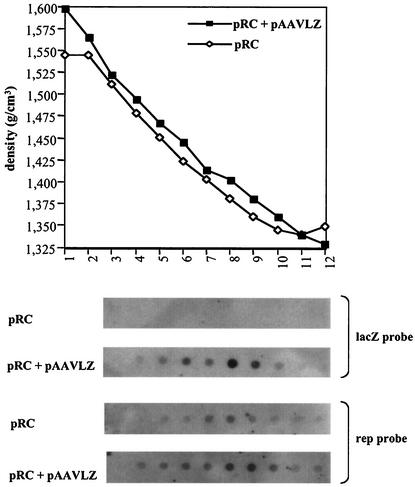
To examine whether rep-cap sequences could be packaged in the absence of the ITRs, adenovirus-infected 293 cells were transfected with the pRC plasmid, which contains the rep-cap genome with a deletion of the ITRs, either alone or in combination with the pAAVLZ plasmid encoding the AAV vector. At the time of the cytopathic effect, AAV particles were purified on a CsCl gradient. To detect the presence of particles containing lacZ or rep sequences, packaged DNA was extracted from each fraction of the gradient and analyzed by dot blot with a lacZ or rep probe. rAAV particles containing AAVLZ DNA peaked in fractions 8 and 9, having a buoyant density of 1.41 to 1.38 g/cm3 (Fig. 2). These same fractions were also positive when analyzed using a rep probe, although in this case, the rep-containing fractions appeared to spread throughout the gradient rather than being concentrated on a restricted number of points. This confirmed previous observations indicating that rAAV stocks prepared under these conditions are contaminated with particles containing rep DNA, with most of them arising from nonhomologous recombination events between the rep-cap genome and the AAV ITRs (1, 10, 14). The longer exposure time required to detect the rep signal, which resulted in a higher background, could explain why the rep-containing fractions appeared to be more dispersed through the gradient. Alternatively, it is possible that rep+ particles produced in the presence of the AAVLZ vector are more heterogeneous in density, further suggesting that they contain genomes of various sizes. Interestingly, a signal was also detected by the rep probe in the preparation made with plasmid pRC alone, and the signal peaked in fractions 7 and 8, having a buoyant density of 1.41 to 1.38 g/cm3, similar to that found for rAAVLZ particles (Fig. 2). The detection of DNA hybridizing to a rep probe was also made after purification of AAV particles by using iodixanol gradients and heparin columns (16) (data not shown). These results suggested that in the absence of the AAVLZ vector, AAV capsids containing rep sequences were generated.
FIG. 2.
Purification on a CsCl gradient of AAV particles produced in the presence or absence of pAAVLZ. Cell lysates prepared from adenovirus-infected 293 cells transfected with plasmid pRC alone or in combination with pAAVLZ were loaded on CsCl gradient prepared as previously described (10). After centrifugation, fractions were collected from the bottom of the gradient until slightly below the adenovirus band and their densities were calculated according to the refractive index measured. Fraction numbers are indicated below the graph. Five microliters each of fractions 3 to 12, recovered from each preparation, was analyzed for the presence of AAV particles containing lacZ or rep DNA by dot blot after extraction of the packaged DNA as indicated in the text. Note that a much longer exposure was necessary to detect the rep signal than the lacZ signal.
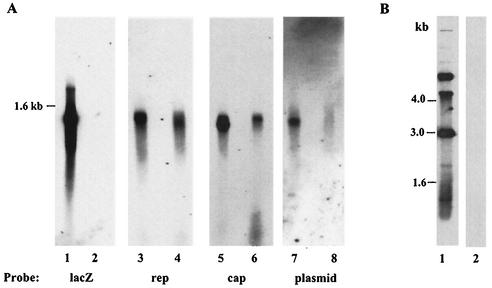
To further analyze the sequences packaged into AAV capsids, the CsCl fractions positive for lacZ or rep DNA and having a buoyant density of 1.41 to 1.38 g/cm3 (Fig. 2) were pooled and dialyzed. Packaged DNA was extracted and analyzed on a Southern blot. As expected, a lacZ signal was observed in the preparation made with pRC and pAAVLZ but not in the one made with pRC alone (Fig. 3A, lanes 1 and 2). When a rep or cap probe was used, a signal was detected in the rAAVLZ preparation, confirming the presence of rep-cap-containing particles (Fig. 3A, lanes 3 and 5). Again, as previously suggested by the results presented in Fig. 2, a rep-cap signal was also detected in the preparation made with pRC alone (Fig. 3A, lanes 4 and 6). Finally, when a plasmid probe hybridizing to a 500-bp sequence present in both pRC and pAVLZ backbones was used, a signal was clearly detected in the rAAVLZ and pRC preparation and, to a much lesser extent, in that obtained with plasmid pRC alone (Fig. 3A, lanes 7 and 8). Two observations established the single-stranded nature of these packaged molecules. First, the hybridization signal patterns were smears, such as the one observed for AAVLZ DNA (Fig. 3A), and migrated at similar positions in the gel. Second and more importantly, the DNA analyzed in this experiment was transferred under neutral conditions and thus the hybridization signal was restricted to single-stranded DNA. The use of a φX174 probe confirmed that under these conditions double-stranded circular φX174 DNA was not detected (Fig. 3B). Importantly, wild-type AAV contamination was also ruled out for the following reasons. (i) The adenoviral stocks and the cells were routinely tested by PCR for the absence of contaminating wild-type AAV. (ii) With a probe specific to the whole AAV ITR, no hybridization signal was observed in the case of particles produced by using the pRC plasmid alone whereas a signal was obtained with DNA extracted from rAAVLZ particles (data not shown). (iii) rep-cap particles generated in the absence of the rAAV vector were not competent for replication, as indicated by Southern blot analysis using a rep probe after sequential amplification on adenovirus-infected 293 cells (data not shown). Altogether, these results strongly suggested that the rep-cap and, to a lesser extent, the plasmid sequences could be packaged into AAV capsids as single-stranded DNA even in the absence of the ITRs. The presence of plasmid sequences packaged in AAV capsids was also recently suggested by Miller et al. (8), who found integrated pBR322 sequences following infection of HeLa cells with rAAV particles. In our study, since both the AAVLZ and pRC plasmids were derived from the same backbone, it was not possible to determine whether the plasmid signal in the AAVLZ preparation was derived mainly from the vector or from the replicating pRC construct. Therefore, it will be important in the future to determine whether plasmid sequences can also be detected in any rAAV stock produced by transient transfection or whether the packaging of such sequences can be prevented by using nonreplicating rep-cap constructs.
FIG. 3.
(A) Southern blot analysis of DNA extracted from purified AAV particles. 293 cells were transfected with plasmid pRC alone (lanes 2, 4, 6, and 8) or in combination with pAAVLZ (lanes 1, 3, 5, and 7) and then infected with adenovirus (Ad.dl324). AAV particles were purified from the cell lysate on a CsCl gradient. DNA extracted after Benzonase treatment of the particles (9 × 1010 AAVLZ and 1 × 109 rep+ particles as measured by dot blot using lacZ and rep probes, respectively) was run on a 1% neutral agarose gel and transferred under neutral conditions (20× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) to a membrane. The membrane was hybridized to a lacZ, rep, cap, or plasmid probe. (B) Transfer of double-stranded φX174 DNA under neutral or alkaline conditions. Five hundred nanograms of circular double-stranded φX174DNA (New England Biolabs) was run on a 1% agarose gel and transferred on a nylon membrane by use of either 0.4N NaOH (lane 1) or 20× SSC (lane 2). Both membranes were then hybridized to a φX174 probe.
In order to quantify the packaging of rep sequences, AAV particles produced in the presence or absence of the pAAVLZ vector were titered by dot blot with a lacZ or rep probe. Infectious particles were also measured by RCA. As indicated in Table 1, particles containing rep sequences were present at a significant level, representing 2% of the total number of AAVLZ particles. In the absence of the AAV ITRs, the number of rep-containing particles was reduced approximately 10-fold. Similar results were previously reported by Wang et al. (14). As expected, a much lower number of infectious rep+ particles was measured by RCA. The lower sensitivity of this titration method, which indirectly measured the transfer of a Rep function in the cell, might explain this difference.
TABLE 1.
Titration of AAVLZ and AAV rep+ particlesa
| Plasmid | Total no. of DNA-containing particles
|
Total no. of infectious particles
|
||
|---|---|---|---|---|
| AAVLZ | rep+ | AAVLZ | rep+ | |
| pAAVLZ plus pRC | 1.9 × 1011 | 4.2 × 109 | 3.5 × 109 | 1.6 × 106 |
| pRC | 5.5 × 108 | 1.0 × 104 | ||
AAV particles were produced by using a 6- × 15-cm dish of cells (approximately 108 293 cells) and the indicated plasmid, purified on CsCl gradients, and titered by dot blot with a lacZ or rep probe (to determine the number of DNA-containing particles) or by RCA with a lacZ probe (to determine the number of infectious particles). For rep+ particles produced in the presence of the AAVLZ vector, the RCA was performed as previously described (10). For rep+ particles produced with pRC alone, the RCA was performed as described in the legend to Fig. 5.
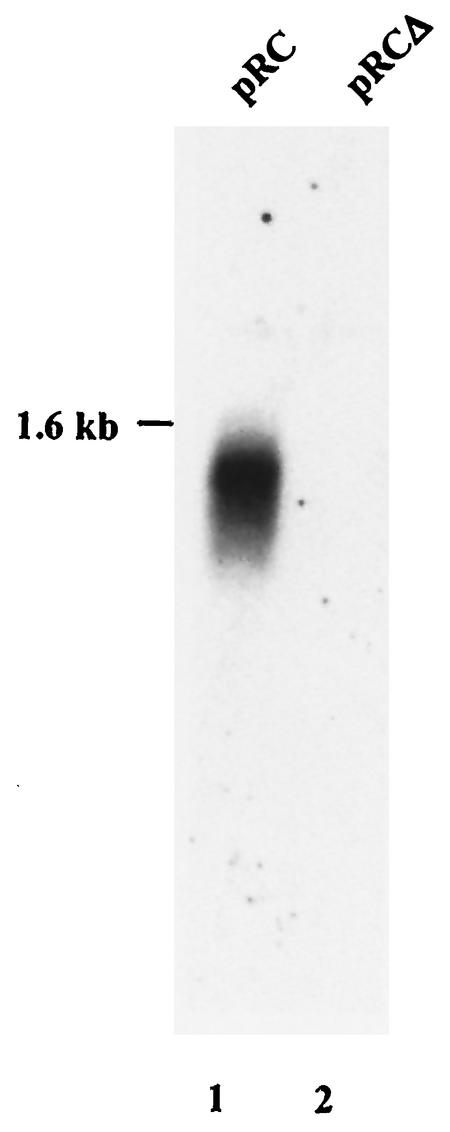
We next evaluated whether packaging of rep-cap sequences could be impaired by preventing the replication of plasmid pRC. For this purpose, we used plasmid pRCΔ, in which the CARE region of the rep gene had been deleted. A previous study demonstrated that this plasmid was unable to replicate when transfected into adenovirus-infected HeLa cells expressing the Rep proteins (HeRC32) (9). Similarly, HeRC32 cells were transfected with either pRC or pRCΔ and then infected with wild-type adenovirus. Packaged DNA was extracted from CsCl-purified particles and analyzed by Southern blot with a rep probe as previously described. In contrast to what was observed with plasmid pRC, a signal hybridizing to the rep probe was no longer detected upon transfection of the pRCΔ plasmid (Fig. 4). Similar results were obtained with either a cap or plasmid probe (data not shown). This indicated that deletion of the CARE region in the rep gene, which impaired replication, also resulted in the lack of encapsidation of single-stranded rep-cap and plasmid sequences despite the fact that functional Rep proteins and adenovirus helper functions were provided in trans. The observation that rAAV stocks produced by using the pDG plasmid, which provides the adenovirus and the AAV functions in trans (4), are not contaminated with rep+ particles is in accordance with this result (P. Nony, G. Chadeuf, P. Moullier, and A. Salvetti, unpublished data). Indeed, in pDG, the p5 promoter region of the rep gene has been replaced with the MMTV promoter (4). Nevertheless, it certainly cannot be concluded from these results that CARE, besides being an origin of replication, is a packaging element. This also concerns the viral ITR, for which the presence of a discrete packaging element has never been demonstrated. However, it is likely that encapsidation of AAV DNA is mediated by the interaction with Rep proteins, which have previously been shown to be able to bind empty capsids and to be involved in the packaging process (3, 5). Therefore, it is possible that CARE, and potentially other Rep binding site-containing regions in the AAV genome, act in synergy with the ITRs to ensure the efficient packaging of the viral genome.
FIG. 4.
Southern blot analysis of DNA extracted from purified AAV particles upon transfection by pRC and pRCΔ. HeRC32 cells (2) were transfected either with pRC (lane 1) or pRCΔ (lane 2) and then infected with adenovirus. DNA extracted from CsCl-purified AAV particles was analyzed in a Southern blot experiment by using a rep probe as described in the legend to Fig. 3.
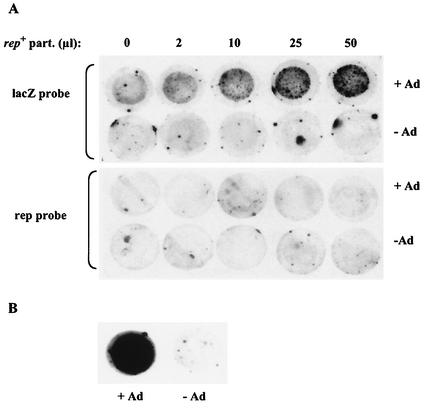
As a final step in these analyses, we asked whether rep+ particles produced with plasmid pRC alone could transfer a Rep function into infected cells. For this evaluation, we performed the following complementation experiment. HeLa cells were coinfected with (i) rAAVLZ particles, at a constant multiplicity of infection (MOI), that had been produced with pDG and were thus free of rep+ contaminants; (ii) an increasing volume of CsCl-purified rep+ particles, produced with plasmid pRC alone; and (iii) wild-type adenovirus. Twenty-four hours later, the cells were harvested, lysed on a membrane, and analyzed for replication of the rAAV vector by using a lacZ probe. In the absence of rep+ particles, replication of rAAVLZ was undetectable even in the presence of adenovirus (Fig. 5). This result confirmed that rAAV produced by using pDG was free of rep+ contaminants. In contrast, upon coinfection with adenovirus and rep+ particles, rAAVLZ replication centers became visible and increased in number along with the volume of rep+ particles used to coinfect the cells. The absence of specific signals following hybridization of the same filter with a rep probe confirmed the following: (i) the adenovirus stock used for this experiment was free of wild-type AAV contaminants and (ii) the rep+ particles produced with pRC were unable to replicate. This last result was also confirmed by the observation that these particles could not be sequentially amplified (data not shown). Altogether, these results indicated that rep+ particles were able to provide a functional Rep activity to the infected cells. This also implied that the single-stranded rep sequences packaged into the capsids were converted into a transcriptionally active double-stranded form. An alternative hypothesis to explain this result is that the Rep activity measured in this experiment was provided by functional Rep proteins present inside assembled capsids, as previously documented by Kube et al. (6). We do not favor this hypothesis since, as previously mentioned, Rep activity cannot be detected by using rAAV stocks produced with the pDG plasmid.
FIG. 5.
Analysis of rAAVLZ replication into adenovirus-infected HeLa cells following coinfection with rep+ particles. (A) HeLa cells were infected with 1.4 × 106 rAAVLZ infectious particles (MOI of 10) and various amounts of the pRC-purified preparation (the volumes are indicated at the top of the figure) and either coinfected or not with wild-type adenovirus (Ad) at an MOI of 50. Twenty-four hours later, cells were harvested and processed for analysis in an RCA, using either a lacZ or a rep probe, as previously described (10). (B) Positive control for rAAVLZ particles. HeRC32 cells were infected with 1.4 × 106 rAAVLZ infectious particles and either coinfected or not with adenovirus. Twenty-four hours later, rAAVLZ replication centers were detected with a lacZ probe.
In conclusion, this study demonstrated that rep-cap sequences and, to a lesser extent, plasmid sequences could be packaged into AAV capsids. This property was clearly linked to the presence of the previously characterized cis-acting element in the rep gene (CARE), which conferred to these sequences the ability to replicate in the presence of Rep proteins (9). A hypothetical model to explain these results predicts that replication of the rep-cap plasmid is initiated following binding of Rep to the Rep binding site present in the p5 promoter and cleavage at the terminal resolution site located downstream (7, 13), which initiates replication of the whole plasmid following a rolling-circle model. The displaced single-stranded sequences could then be translocated into AAV capsids either directly during replication or, more likely, after cleavage from the concatemer molecule due to the nicking activity of the large Rep proteins. Further characterization studies with rep+ particles will be performed to verify this model. Altogether, these findings extend the previous observations concerning the contamination of rAAV stocks with rep+ particles and contribute to the understanding of the AAV packaging process.
Acknowledgments
We thank Mark Haskins for critically reading the manuscript.
This work was supported by the Association Française contre les Myopathies, Vaincre les Maladies Lysosomales, Association Nantaise de Thérapie Génique, and the Fondation pour la Thérapie Génique en Pays de la Loire. P.N. was supported by a sponsored research agreement from Genopoietic Inc.
REFERENCES
- 1.Allen, J. M., D. J. Debelak, T. C. Reynolds, and A. D. Miller. 1997. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J. Virol. 71:6816-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadeuf, G., D. Favre, J. Tessier, N. Provost, P. Nony, J. Kleinschmidt, P. Moullier, and A. Salvetti. 2000. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2:260-268. [DOI] [PubMed] [Google Scholar]
- 3.Dubielzig, R., J. A. King, S. Weger, A. Kern, and J. A. Kleinschmidt. 1999. Adeno-associated virus type 2 protein interactions: formation of pre-encapsidation complexes. J. Virol. 73:8989-8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm, D., A. Kern, K. Rittner, and J. Kleinschmidt. 1998. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum. Gene Ther. 9:2745-2760. [DOI] [PubMed] [Google Scholar]
- 5.King, J. A., R. Dubielzig, D. Grimm, and J. A. Kleinschmidt. 2001. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 20:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kube, D. M., S. Ponnazhagan, and A. Srivastava. 1997. Encapsidation of adeno-associated virus type 2 Rep proteins in wild-type and recombinant progeny virions: Rep-mediated growth inhibition of primary human cells. J. Virol. 71:7361-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty, D. M., D. J. Pereira, I. Zolotukhin, X. Zhou, J. H. Ryan, and N. Muzyczka. 1994. Identification of linear DNA sequences that specifically bind the adeno-associated virus Rep protein. J. Virol. 68:4988-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, D., E. Rutledge, and D. Russell. 2002. Chromosomal effects of adeno-associated virus vector integration. Nat. Genet. 30:147-148. [DOI] [PubMed] [Google Scholar]
- 9.Nony, P., J. Tessier, G. Chadeuf, P. Ward, A. Giraud, M. Dugast, R. M. Linden, P. Moullier, and A. Salvetti. 2001. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 75:9991-9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvetti, A., S. Orève, G. Chadeuf, D. Favre, Y. Cherel, P. Champion-Arnaud, J. David-Ameline, and P. Moullier. 1998. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 9:695-706. [DOI] [PubMed] [Google Scholar]
- 11.Snyder, R. O., R. J. Samulski, and N. Muzyczka. 1990. In vitro resolution of covalently joined AAV chromosome ends. Cell 60:105-113. [DOI] [PubMed] [Google Scholar]
- 12.Tullis, G. E., and T. Shenk. 2000. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J. Virol. 74:11511-11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, X.-S., and A. Srivastava. 1997. A novel terminal resolution-like site in the adeno-associated virus type 2 genome. J. Virol. 71:1140-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, X.-S., B. Khuntirat, K. Qing, S. Ponnazhagan, D. M. Kube, S. Zhou, V. Dwarki, and A. Srivastava. 1998. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination. J. Virol. 72:5472-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao, X., J. Li, and R. J. Samulski. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zolotukhin, S., B. J. Byrne, E. Mason, I. Zolotukhin, C. Summerford, R. J. Samulski, and N. Muzyczka. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973-985. [DOI] [PubMed] [Google Scholar]