Abstract
Noroviruses are a major cause of epidemic acute nonbacterial gastroenteritis worldwide. Here we report our discovery that recombinant Norwalk virus virus-like particles (rNV VLPs) agglutinate red blood cells (RBCs). Since histo-blood group antigens are expressed on gut mucosa as well as RBCs, we used rNV VLP hemagglutination (HA) as a model system for studying NV attachment to cells in order to help identify a potential NV receptor(s). rNV VLP HA is dependent on low temperature (4°C) and acidic pH. Of the 13 species of RBCs tested, rNV VLPs hemagglutinated only chimpanzee and human RBCs. The rNV VLPs hemagglutinated all human type O (11 of 11), A (9 of 9), and AB (4 of 4) RBCs; however, few human type B RBC samples (4 of 14) were hemagglutinated. HA with periodate- and neuraminidase-treated RBCs indicated that rNV VLP binding was carbohydrate dependent and did not require sialic acid. The rNV VLPs did not hemagglutinate Bombay RBCs (zero of seven) that lack H type 2 antigen, and an anti-H type 2 antibody inhibited rNV VLP HA of human type O RBCs. These data indicated that the H type 2 antigen functions as the rNV VLP HA receptor on human type O RBCs. The rNV VLP HA was also inhibited by rNV VLP-specific monoclonal antibody 8812, an antibody that inhibits VLP binding to Caco-2 cells. Convalescent-phase sera from NV-infected individuals showed increased rNV VLP HA inhibition titers compared to prechallenge sera. In carbohydrate binding assays, the rNV VLPs bound to synthetic Lewis d (Led), Leb, H type 2, and Ley antigens, and these antigens also inhibited rNV VLP HA of human type O RBCs. Overall, our results indicate that carbohydrate antigens in the gut are a previously unrecognized factor in NV pathogenesis.
Norwalk virus (NV) was first isolated from an outbreak of winter vomiting disease, or acute gastroenteritis, at an elementary school in Norwalk, Ohio, in 1968 (1). Subsequent adult volunteer challenge studies with infectious stool filtrate from the Norwalk outbreak demonstrated that NV readily infects susceptible people, causing an acute illness associated with diarrhea, vomiting, myalgia, nausea, and fever within 15 to 24 h after exposure and lasting 24 to 72 h (16). Although these initial studies demonstrated that most people are highly susceptible to NV, presently no cell culture systems or animal models of infection exist, which limits the study of NV replication and pathogenesis.
NV is the prototype nonenveloped, positive-stranded RNA human virus in the genus Norovirus of the family Caliciviridae. Noroviruses are a major cause of acute gastroenteritis throughout the world and cause virtually all outbreaks of nonbacterial gastroenteritis in adults in the United States (18, 39). Furthermore, there are an increasing number of reports of gastroenteritis cases and outbreaks in children and the elderly caused by noroviruses, in part due to increased surveillance and better detection methods (15, 26, 39).
NV virions are detected in low numbers in infected stool. The virions are 27 to 38 nm in diameter, including 4.5-nm radial protrusions extending from the capsid shell that create the calix, or cup-like structures, apparent by electron microscopy (33, 44). The NV capsid proteins (open reading frames 2 and 3, which produce the structural viral proteins VP1 and VP2, respectively) spontaneously self-assemble into virus-like particles (VLPs) when synthesized in a recombinant baculovirus expression system. These recombinant NV VLPs (rNV VLPs) are structurally and antigenically similar to the capsids of native NV and are useful in modeling virus-cell interactions (27, 53).
Hemagglutination (HA) is one method that has been helpful in identifying cell-binding receptors for many viruses, such as influenza A virus and parvovirus B19 (7, 45). The VLPs from human parvovirus B19, JC human polyomavirus, and SA11 simian rotavirus have HA properties that are similar to those of their virions (8, 14, 42).
This is the first report of HA by VLPs from a human calicivirus. Our data demonstrate that the H type 2 histo-blood group antigen is the rNV VLP HA receptor on human type O red blood cells (RBCs) and that the rNV VLPs also bind to synthetic H and structurally related Lewis carbohydrate antigens.
MATERIALS AND METHODS
rNV VLP purification.
rNV VLPs were synthesized and purified by using methods described previously (53). Briefly, spinner flasks containing 3.5 × 106 Sf9 insect cells per 200 ml of Grace's insect cell medium were infected with pVL-NV ORF(2 + 3) recombinant baculovirus at a multiplicity of infection of 5. At 7 days postinfection the rNV VLPs were harvested from supernatants of spinner flask cultures. Cells were pelleted and discarded. The VLPs in the supernatant were pelleted by ultracentrifugation through a 30% sucrose cushion made with 0.2 M phosphate-buffered saline (PBS) for VLPs (PBS-V) (0.2 M sodium phosphate, 0.1 M NaCl, pH 6.0) in an SW28 rotor for 3 h at 120,000 × g and 4°C. The pellets were suspended in 0.39 g of CsCl (Invitrogen, Grand Island, N.Y.) per ml of PBS-V and subjected to isopycnic gradient centrifugation in an SW55 rotor for 18 to 24 h at 150,000 × g and 4°C. The visible band of rNV VLPs in the CsCl gradient was collected by micropipetting. CsCl was removed by diluting the VLPs in PBS-V and sedimenting by ultracentrifugation in an SW28 rotor for 3 h at 120,000 × g and 4°C. The VLP pellets were suspended in PBS-V containing protease inhibitors (aprotinin, leupeptin, and pepstatin [Sigma, St. Louis, Mo.] at 1 μg/ml each) and stored at 4°C. The rNV VLP protein concentrations were determined by the DC protein assay (Bio-Rad, Hercules, Calif.) microtiter plate method without detergent, and the rNV VLP structural integrity was ascertained by electron microscopic inspection of negatively stained (1.0% ammonium molybdate [Sigma], pH 5.0) VLPs on carbon-coated grids (Ted Pella, Reading, Calif.).
HA assay.
The HA assay methods were based on those described by the Centers for Disease Control and Prevention (9). Whole blood was collected in 2 volumes of Alsever's solution (2.05% [wt/vol] glucose [Sigma], 0.8% sodium citrate [Fisher, Fair Lawn, N.J.], 0.055% citric acid [Fisher], 0.42% NaCl [Fisher], pH 6.1) and stored at 4°C. Individual human RBC samples were graciously provided by volunteer donors at the Baylor College of Medicine's Influenza Research Center and the Methodist Hospital Blood Donor Center (Houston, Tex.). Pooled human RBC reagents from at least five donors were purchased from Immucor (Atlanta, Ga.). Human cord RBC samples were kindly provided by the Children's Nutrition Research Center's Leukocyte Biology Laboratory (Houston, Tex.). Human RBC samples with rare phenotypes were generously donated by Immucor/Gamma Biologicals (Houston, Tex.). Chimpanzee, baboon, spider monkey, and rhesus RBC samples were graciously provided by the Southwest Foundation for Biomedical Research (San Antonio, Tex.). Chicken, guinea pig, canine, and feline blood samples were provided by the Center for Comparative Medicine at Baylor College of Medicine (Houston, Tex.). Murine RBC samples were obtained during routine blood draws from normal control BALB/c mice. Bovine, porcine, and lapine blood samples were purchased from Lampire (Pipersville, Pa.). The RBCs were packed by diluting the cells in 0.01 M PBS (without Ca2+ or Mg2+; pH 7.2) (Invitrogen, Carlsbad, Calif.) and centrifuging for 15 min at 500 × g. The HA activity of rNV VLPs was tested in untreated 96-well V-bottom plates (Nunc, Naperville, Ill.). Equal volumes (50 μl each) of 0.5% packed human RBCs in 0.85% saline (pH 6.2) and rNV VLPs serially diluted in 0.01 M PBS for HA (PBS-H) (0.01 M sodium phosphate [EM Science, Gibbstown, N.J.], 0.15 M NaCl, pH 5.5; filtered with a 0.2-μm-pore-size filter) were combined at 4°C. The plates were covered with adhesive film (Excel Scientific, Wrightwood, Calif.) and incubated for 75 to 120 min in a cold room (at 4 to 8°C), unless otherwise indicated. Incubations at other temperatures were performed at room temperature (22 to 25°C) or in a tissue culture incubator (36 to 37°C). The HA titer was the reciprocal of the greatest rNV VLP dilution that did not allow sedimentation of the RBCs compared to negative control wells that contained only buffer. The positive control for HA was an influenza A virus (H1N1, A/Texas/36/91), grown in eggs, for which the titer had been previously determined. For each animal RBC sample, the percentage of packed RBCs in saline used for each HA assay was determined by the minimum concentration of RBCs able to sediment in negative control wells (PBS-H) within 6 h of incubation at 4°C.
Periodate treatment of RBCs.
Human type O RBCs were treated with freshly prepared 10.0 mM KIO4 (Sigma) for 20 min at room temperature and then rinsed and packed as described above. To test the RBCs' ability to be agglutinated, 40 μl of treated or control 2.0% RBCs was mixed with 10 μl of rNV VLPs (0.1 mg/ml), anti-D or anti-H monoclonal antibodies (MAbs) (Immucor) (0.1 mg/ml), or PBS-H on an ice-cold glass plate and incubated for 10 min at 4°C. The degree of HA was determined by visual comparison to controls and verified by an independent observer.
Enzyme treatment of RBCs.
All enzyme reactions were performed with 50 μl of packed RBCs in a total enzyme reaction volume of 125 μl. Control reaction mixtures were incubated without enzyme. Neuraminidase from Arthrobacter ureafaciens (6 μU; Sigma) in Dulbecco's PBS (D-PBS) (Invitrogen) was combined with the RBCs and incubated for 60 min at 37°C. l-(Toslylamido-2-phenyl)ethyl chloromethyl ketone-treated trypsin (1.25 ng; Sigma) in D-PBS was added to the RBCs and incubated for 30 min at 37°C. Trypsin activity was stopped by adding 0.5 μg of soybean trypsin inhibitor (Sigma) to the reaction mixture. After enzyme treatment, the RBCs were rinsed three times with 6 ml of PBS and packed by centrifugation as described above. After removal of the PBS supernatant, the packed RBCs were diluted with cold 0.85% saline (pH 6.2) to make a 0.5% RBC suspension for HA assays.
Enzyme-linked immunosorbent assay (ELISA)-based carbohydrate microtiter plate assay (CMA).
Multivalent carbohydrate-biotin reagents conjugated to polyacrylamide (CHO-PAA-biotin; Glycotech, Rockville, Md.) were immobilized onto streptavidin-coated plates as suggested by the manufacturer, with all reagents added in volumes of 100 μl/well, unless otherwise specified. Briefly, PVC microtiter flat-bottom plates (Dynex Technologies, Chantilly, Va.) were coated with 10 μg of streptavidin (Sigma) per ml in PBS at 4°C overnight, blocked by addition of 1% fatty acid-free bovine serum albumin (faf-BSA) (Sigma) in PBS at room temperature for 4 h, and then rinsed three times with 200 μl of PBS per well. Next, 0.1 μg of biotin per ml or 10 μg of CHO-PAA-biotin conjugate per ml in PBS with 0.25% faf-BSA was incubated at room temperature for 1 h and rinsed as described above. The rNV VLP-carbohydrate binding was optimized at 10 μg of rNV VLPs per ml in PBS (pH 7.5) with 0.25% faf-BSA and incubation at room temperature for 1 h before rinsing as described above. rNV VLPs bound to carbohydrate were detected by incubation with rabbit anti-rNV VLP serum (1:5,000) in PBS with 0.25% faf-BSA for 1 h at room temperature; rinsing as described above; addition of goat anti-rabbit immunoglobulin A (IgA), IgM, and IgG conjugated to horseradish peroxidase (ICN Biomedicals, Philadelphia, Pa.) (1:50,000) in PBS with 0.25% faf-BSA; incubation for 1 h at room temperature; and rinsing. Plates were developed with the TMB microwell peroxidase endpoint assay (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and absorbance was read at 450 nm (Spectromax 190; Molecular Devices, Sunnyvale, Calif.). Carbohydrate competition assays for rNV VLP binding to the microtiter plate-immobilized CHO-PAA-biotin were performed by preincubating the rNV VLPs with CHO-PAA reagents (without biotin) in PBS (pH 7.2) for 30 min at 4°C before addition to the microtiter plates prepared as described above. The plates were incubated for 1 h at 4°C and then rinsed and detected as described above.
HI assay.
Inhibition of rNV VLP HA was examined by using the multivalent CHO-PAA-biotin conjugates, human pre- and post-NV challenge sera (24), mouse hyperimmune serum IgG, and anti-rNV VLP MAbs (8812, 3901, and 3912) (30). The human sera were heat inactivated and kaolin treated for the HA inhibition (HI) assays as previously described (9). Mouse IgG from hyperimmune sera and monoclonal hybridoma ascites were purified by using UltraLink protein G columns (Pierce, Rockford, Ill.) according to the manufacturer's protocol and stored at 4°C in PBS with 0.02% NaN3. The HI activities of the carbohydrate conjugates, the treated human sera, and the mouse IgG were tested by serial dilutions of the inhibitors (starting concentrations of 100 μg/ml, 1:10, and 50 μg/ml, respectively) in a volume of 25 μl of PBS-H across 96-well V-bottom plates. An amount of rNV VLPs equal to 4 HA units (HAU) (1 HAU was defined as the amount of antigen present at the HA endpoint dilution) in a volume of 25 μl of PBS-H was added to each well, and the inhibitor was incubated with the rNV VLPs for 30 min at 4°C. (For the mouse IgG HI assays, the PBS-H contained 0.3% BSA fraction V [Calbiochem, San Diego, Calif.].) Next, 50 μl of 0.5% human type O RBCs in 0.85% saline was added to each well, and the plate was incubated as described above. Each inhibitor tested was negative for nonspecific HA of the RBCs at the highest concentration used in the assay. The HI titer was read as the last dilution of inhibitor that prevented HA of the RBCs by rNV VLPs.
RESULTS
HA by rNV VLPs is temperature and pH dependent.
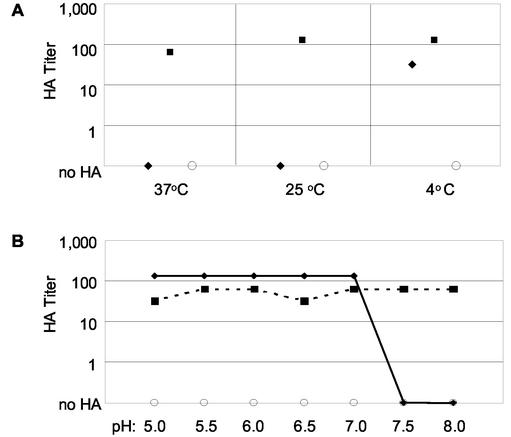
The rNV VLP HA of RBCs was tested at 4, 25, and 37°C in PBS-H (pH 7.2). The rNV VLPs were able to hemagglutinate human type O-positive RBCs at 4°C but not at 25 or 37°C (Fig. 1A). Microscopic inspection of the human type O-positive RBCs and HA of the RBCs by influenza A virus at all three temperatures demonstrated that the cells were intact at these temperatures. The HA of human type O-positive RBCs by rNV VLPs at 4°C was lost within 30 min when plates were warmed to room temperature. The loss of HA at room temperature was reversible. The HA titer was restored to a level equal to or up to fourfold lower than the initially observed titer when the plates were first cooled to 4°C, shaken to suspend the RBCs, and then incubated a second time at 4°C (data not shown). Because several enteric viruses have HA titers that are optimal at pHs of below 7.2 (9), we evaluated the effect of pH on rNV VLP HA of type O-positive RBCs. With pH increments of 0.5, the rNV VLPs hemagglutinated human type O-positive RBCs when diluted in PBS-H at pH 5.0 to 7.0 but not at pH 7.5 and 8.0 (Fig. 1B). HA by influenza A virus was pH independent between pH 5.0 and 8.0. Upon further examination of HA at pHs of between 7.0 and 7.5, rNV VLP HA was observed at pH 7.0 to 7.2 but was lost at pH 7.3 to 7.5 (data not shown). Therefore, for rNV VLP HA of human type O-positive RBCs, an incubation temperature of 4 to 8°C and a pH of between 5.0 and 7.2 were required. At the chosen conditions, i.e., incubation at 4°C and pH 5.5, an HA endpoint of 0.625 ng of rNV VLPs (3.6 × 107 particles) was regularly able to hemagglutinate approximately 3 × 106 human type O-positive RBCs. Thus, to further characterize the HA properties of rNV VLPs, we made our standard HA assay with 400 ng of rNV VLPs per ml as the initial VLP concentration that was serially diluted in PBS-H at pH 5.5 and incubated for 75 to 90 min at 4°C, unless otherwise noted.
FIG. 1.
HA titers of human type O RBCs by rNV VLPs. Fifty microliters of rNV VLPs (400 ng/ml) (♦), influenza A/TX/36/91 virus grown in eggs (▪), or PBS-H (○) was serially diluted in PBS-H and mixed with 50 μl of 0.5% RBCs. (A) The PBS-H was at pH 7.2, and the HA assay incubation times, as determined by sedimentation of RBCs in negative control wells, were 30 min at 37°C, 40 min at 25°C, and 90 min at 4°C. (B) The pH of the PBS-H ranged from 5.0 to 8.0 as indicated, and the HA assay incubation time was 90 min at 4°C.
rNV VLPs hemagglutinate chimpanzee RBCs.
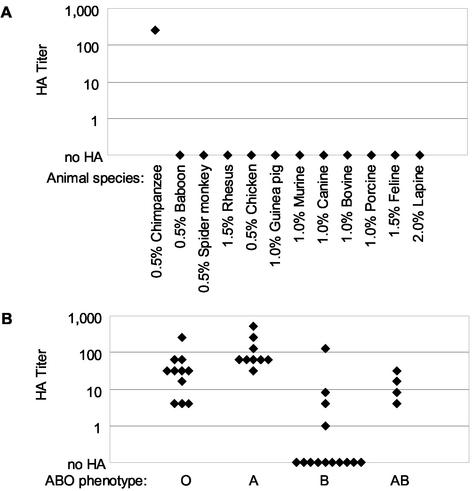
The ability of a virus to agglutinate RBCs depends on the expression of the HA receptor on the cells, and this expression may be limited to a subset of animal species RBCs. Therefore, we tested RBCs from different species to gain insight into the nature and expression pattern of the rNV VLP HA receptor. Additionally, if the rNV VLP HA was a biologically relevant interaction, we hypothesized that the narrow host restriction of NV infection, i.e., to most humans and some chimpanzees (24, 43, 54), would be reflected in the ability of the VLPs to agglutinate RBCs from susceptible species. A panel of RBC samples from 12 nonhuman animal species was chosen based on their susceptibility to other caliciviruses, evolutionary relatedness to humans, availability, and use in other viral HA assays. Of these RBC samples, only the chimpanzee RBCs (0.5%) were hemagglutinated by the rNV VLPs (Fig. 2A). This sample of chimpanzee RBCs also reacted with an anti-A histo-blood group typing reagent (Immucor), indicating the presence of A or A-like histo-blood group antigens on the chimpanzee RBCs (data not shown) (3). The HA titer for the chimpanzee RBCs was 256, similar to the high HA titers exhibited by several human type A RBC samples (Fig. 2B). The baboon (0.5%), spider monkey (0.5%), rhesus macaque (1.5%), chicken (0.5%), guinea pig (1.0%), murine (1.0%), canine (1.0%), bovine (1.0%), porcine (1.0%), feline (1.5%), and lapine (2.0%) RBC samples were not hemagglutinated by 400 ng of rNV VLPs per ml.
FIG. 2.
HA titers of individual RBC samples by rNV VLPs. Fifty microliters of rNV VLPs (400 ng/ml) serially diluted in PBS-H (pH 5.5) were combined with 50 μl of RBC samples (0.5 to 2.0%) from the indicated 12 animal species (A) and adult human RBC samples (0.5%) (separated by their ABO phenotypes) (B). The RBC concentrations used in HA assays were determined by sedimentation of RBCs in PBS-H (pH 5.5) negative control wells.
rNV VLPs hemagglutinate adult human type O, A (A1 and A2), and AB RBCs and a subset of adult human type B RBCs.
Many individual RBC samples and pooled human RBC reagent samples were tested for rNV VLP HA to determine the frequency of expression of the rNV VLP HA receptor on human RBCs. For convenience, the human RBC samples were grouped by their ABO and Rh phenotypes. The rNV VLPs hemagglutinated all adult human type O, A, and AB individual RBC samples tested (11, 9, and 4 individual samples, respectively) (Fig. 2B). However, only 4 of the 14 type B individual human RBC samples tested were hemagglutinated by 400 ng of rNV VLPs per ml (Fig. 2B). In HA assays starting at a much higher concentration of rNV VLPs (100 μg/ml), an additional six of the individual human type B RBC samples were hemagglutinated by the VLPs (data not shown). For all human RBC samples tested, the Rh factor did not affect rNV VLP HA of human RBCs. Additional rNV VLP HA assays were performed on pooled human A1, A2, B, and O RBC reagents. In agreement with our individual RBC sample data, 400 ng of rNV VLPs per ml hemagglutinated the pooled type A1, A2, and O RBCs but not the pooled type B RBCs (Table 1).
TABLE 1.
HA titers of untreated and neuraminidase-treated pooled human RBCs by rNV VLPs
| Pooled human RBCs | Neuraminidase treatmenta | HA titerb
|
|
|---|---|---|---|
| rNV VLPs | Rotavirus | ||
| O | − | 256 | 64 |
| + | 256 | 0 | |
| A1 | − | 256 | 64 |
| + | 256 | 0 | |
| A2 | − | 256 | 64 |
| + | 256 | 0 | |
| B | − | 0 | 16 |
| + | 6 | 0 | |
−, untreated RBCs; +, neuraminidase-treated RBCs.
0, no HA.
rNV VLP binding to RBCs is carbohydrate dependent.
Because the B antigen is a carbohydrate moiety, we tested the carbohydrate dependence of rNV VLP-RBC binding by periodate treatment, which oxidizes all carbohydrates on the cell surface. After periodate treatment of human type O-positive RBCs, HA by rNV VLPs and by anti-H, a carbohydrate-dependent antibody (Immucor), was lost (Table 2). This same treatment did not reduce anti-D HA, which recognizes an Rh factor protein on RBCs. Furthermore, trypsin treatment of type O RBCs did not affect the rNV VLP HA titer (data not shown), indicating that rNV VLP HA was not dependent upon a trypsin-sensitive protein on the RBC surface.
TABLE 2.
Agglutination of periodate-treated human type O+ RBCs by rNV VLPs
| Potassium periodate (mM) | HAa with the following antigen or antibody combined with RBCs:
|
|||
|---|---|---|---|---|
| rNV VLPs | Anti-H | Anti-D | PBS | |
| 10.0 | 0 | 0 | +++ | 0 |
| 0 | ++ | + | ++++ | 0 |
0, no HA; + to ++++, weakest to strongest HA.
Two cell surface carbohydrates to which viruses commonly bind are sialic acid and heparan sulfate. To test if the carbohydrate-dependent rNV VLP HA required sialic acid, pooled human RBC reagents were enzymatically treated with neuraminidase from A. ureafaciens, which removes both α-2,3 and α-2,6 linked sialic acid residues on the cell surface. This neuraminidase treatment of pooled human type O, A1, and A2 RBCs did not affect their rNV VLP HA titer (Table 1), whereas the sialic acid-dependent HA by rhesus rotavirus was lost. Thus, rNV VLP HA of human type O, A1, and A2 RBCs was sialic acid independent. Additionally, pretreating the rNV VLPs with heparan sulfate before performing the HA assay did not inhibit the VLPs' ability to hemagglutinate human type O RBCs (data not shown). Therefore, the rNV VLP binding was dependent upon a carbohydrate, and unlike many other viruses, this carbohydrate did not involve sialic acid or heparan sulfate.
An interesting observation was made when the pooled human type B RBCs were treated with neuraminidase and tested for rNV VLP HA (Table 1). As seen with the majority of individual type B RBC samples, the untreated pooled type B RBCs were not hemagglutinated by 400 ng of rNV VLPs per ml. However, after neuraminidase treatment, when the sialic acid-dependent rotavirus HA titer was abolished, the rNV VLPs were able to hemagglutinate the type B RBCs.
The rNV VLP HA is dependent upon H antigen.
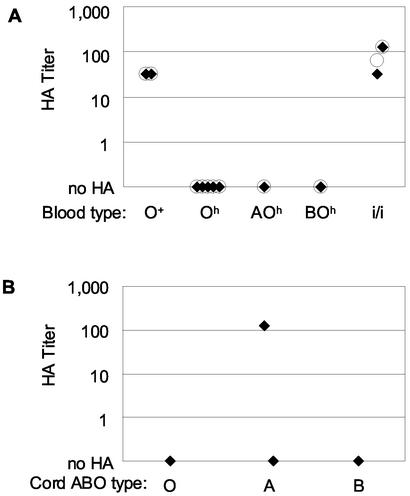
Individuals with the rare Bombay (Oh) blood type do not express the H histo-blood group antigen on their RBCs (35). Since the H antigen is the precursor substrate for A and B antigen expression, RBCs from Bombay-type individuals will appear to have an O phenotype regardless of their ABO genotype (35). Bombay-type individuals with an A or B and a secretor (Se+) genotype are A- or B-para-Bombay, respectively, where the A or B antigens may be expressed on additional carbohydrate substrates not normally expressed by RBCs (35). None of the five Bombay- or the two para-Bombay-type RBC samples assayed was hemagglutinated by 400 ng of rNV VLPs per ml (Fig. 3A). In fact, even 100 μg of rNV VLPs per ml did not hemagglutinate the seven Bombay-type RBC samples (data not shown). Furthermore, when monoclonal antibody BRIC 231 (Biogenesis, Poole, United Kingdom), which specifically recognizes the H type 2 antigen, was preincubated with human type O RBCs, the rNV VLPs did not hemagglutinate the RBCs (data not shown). These data strongly suggest that rNV VLP HA is dependent upon H antigen expression on human RBCs.
FIG. 3.
HA titers of rare-phenotype human RBC samples (A) and human umbilical cord blood samples (B) by rNV VLPs. Fifty microliters of rNV VLPs (400 ng/ml) (♦) or UEA-1 lectin (100 μg/ml) (○) was serially diluted in PBS-H (pH 5.5) and combined with 50 μl of 0.5% unbranched (i/i), Bombay (Oh), para-Bombay (AOh and BOh), or normal adult type O RBC samples (A) and with 50 μl of 0.5% umbilical cord RBCs in (B).
The rNV VLP HA receptor may be a developmental antigen.
Many RBC antigens, including the ABH antigens, are not expressed or are expressed at low levels on fetal and neonatal RBCs (37, 51). Four human umbilical cord RBC samples (neonatal RBCs) were obtained from full-term births and were tested for HA by rNV VLPs (Fig. 3B). One type A cord RBC sample showed an HA titer similar to that of some adult type A RBCs. The three additional cord blood samples (a second type A sample, a type O sample, and a type B sample) were not hemagglutinated by 400 ng of rNV VLPs per ml.
One developmental change in human RBC antigens is the increase in carbohydrate branching. Fetal RBCs have no branched carbohydrates (phenotype i), and adult levels of branching are attained by 2 years of age (phenotype I) (20, 52). RBCs from adults lacking the α-1,6 galactosyltransferase carbohydrate branching enzyme resemble fetal RBCs in their lack of branched-carbohydrate expression (4). The rNV VLP HA did not depend on expression of branched carbohydrates (Fig. 3A). Two type O (i/i) RBC samples were hemagglutinated by 400 ng of rNV VLPs per ml to an HA titer of 128, similar to the HA titer of normal type O RBCs.
rNV VLP HA of human type O RBCs is inhibited by mouse MAb 8812.
The previously characterized rNV VLP-specific MAbs 8812, 3901, and 3912 were tested for their ability to inhibit rNV VLP HA (30). Also, IgG purified from mouse anti-rNV VLP hyperimmune serum was tested for rNV VLP HI. Only MAb 8812 inhibited rNV VLP HA of human type O RBCs (Table 3).
TABLE 3.
HI titers of mouse anti-rNV VLP antibodies
Convalescent-phase sera from NV-infected volunteers show an increase in rNV VLP HI titer.
Prechallenge and convalescent-phase sera from seven NV-challenged volunteers were heat inactivated and kaolin treated to remove nonspecific hemagglutinating factors (9, 24). The treated sera were diluted (1:10) and had no nonspecific HA activity and lower HI titers than untreated sera. Increased HI titers were found in the convalescent-phase sera of NV-infected volunteers (n = 5; volunteers 1 to 5) compared to their prechallenge sera, while the HI titers did not increase in convalescent-phase sera from noninfected NV-challenged volunteers (n = 2; volunteers 6 and 7) (Table 4) (24).
TABLE 4.
HI titers of preimmune and convalescent-phase sera from volunteers given NVa
| Volunteer | Challenge outcomeb | HI titer
|
Fold HI titer increase | |
|---|---|---|---|---|
| Prechallenge | Postchallenge | |||
| 1 | Infected | <10 | 160 | 32 |
| 2 | Infected | 40 | 10,240 | 256 |
| 3 | Infected | 160 | 10,240 | 128 |
| 4 | Infected | 40 | 2,560 | 64 |
| 5 | Infected | 40 | 2,560 | 64 |
| 6 | Noninfected | 40 | 40 | 0 |
| 7 | Noninfected | 10 | 10 | 0 |
Heat-inactivated and kaolin-treated sera (1:10) were tested for their ability to inhibit rNV VLP HA by HI assay. Pre- and postchallenge sera were collected from human volunteers before and 2 to 4 weeks after NV (8fIIa) challenge and stored at −70°C (24).
Infected, seroresponse and/or antigen shedding with or without symptoms; noninfected, no seroresponse or antigen shedding with low symptom score (24).
The rNV VLPs specifically bind to synthetic H and Lewis carbohydrate antigens.
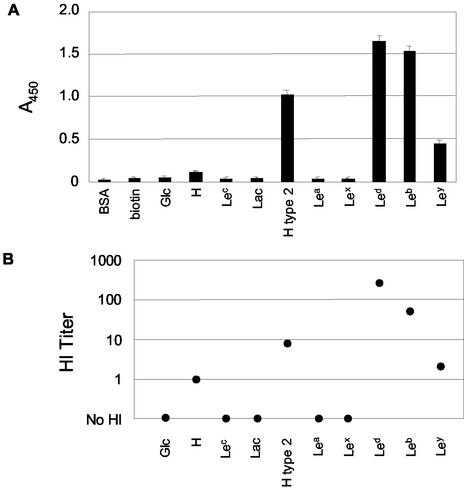
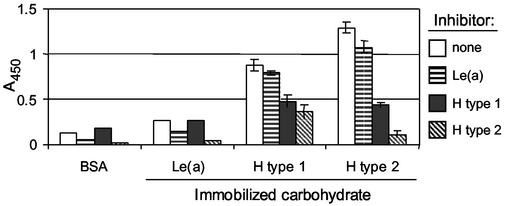
Based on the data that the H antigen may be the HA receptor for the rNV VLPs on human type O RBCs, the VLPs were tested for their ability to bind synthetic H and the structurally related Lewis carbohydrate reagents (Table 5). These synthetic H and Lewis antigens were chosen based on their relatedness to the H type 2 antigen found on human type O RBCs, their expression on gut mucosa, and their availability. When the rNV VLPs were incubated on carbohydrate-coated microtiter plates in the ELISA-based CMA in PBS (pH 6.0) at room temperature, the VLPs bound to the following carbohydrates, starting with the greatest rNV-specific detection: Lewis d (Led), Leb, H type 2, Ley, and H disaccharide (Fig. 4A). In HI assays, the same carbohydrates (CHO-PAA-biotin reagents) inhibited rNV VLP binding to human type O RBCs (Fig. 4B). The relative strength of detection of rNV VLPs bound to the immobilized carbohydrates in the ELISA-based CMA correlated with the relative magnitude of HI titers. Furthermore, when the free synthetic carbohydrates Led (H type 1), H type 2, and Lea were preincubated with the rNV VLPs before their addition to the ELISA-based CMA, Led and H type 2, but not Lea, inhibited rNV VLP binding to both the immobilized Led and H type 2 carbohydrates (Fig. 5).
TABLE 5.
Structures of carbohydrates and rNV VLP binding
| Symbol | Structurea,b | Name | Expression | rNV VLP bindingc |
|---|---|---|---|---|
| Glc | Glc-R | Glucose | —d (control) | 0 |
| H | Fucα-1,2-Galβ-R | H disaccharide | RBC + gut | (+) |
| Lec | Galβ-1,3-GlcNAcβ-R | Type 1 precursor | — | 0 |
| Lac | Galβ-1,4-Glcβ-R | Lactose | — | 0 |
| H type 2 | Fucα-1,2-Galβ-1,4-GlcNAcβ-R | H type 2 | RBC + gut | ++ |
| Lea | Galβ-1,3-(Fucα-1,4-)GlcNAcβ-R | Lea | Gut | 0 |
| Lex | Galβ-1,4-(Fucα-1,3-)GlcNAcβ-R | Lex | Gut | 0 |
| Led | Fucα-1,2-Galβ-1,3-GlcNAcβ-R | Led, H type 1 | Gut | ++++ |
| Leb | Fucα-1,2-Galβ-1,3-(Fucα-1,4-)GlcNAcβ-R | Leb | Gut | +++ |
| Ley | Fucα-1,2-Galβ-1,4-(Fucα-1,3-)GlcNAcβ-R | Ley | Gut | + |
| H type 3 | Fucα-1,2-Galβ-1,3-GalNAcβ-R | H type 3 | RBC + gut | Not tested |
Reviewed in references 29 and 31. Type 1 precursor has β-1,3 linkage, found in Lea, Led, and Leb; type 2 precursor has β-1,4 linkage, found in H type 2, Lex, and Ley.
Glc, glucose; R, polyacrylamide with or without biotin (-PAA-biotin or -PAA, respectively); Fuc, fucose; Gal, galactose; GlcNAc, N-acetylglucosamine; Lac, lactose; GalNAc, N-acetylgalactosamine.
0, no binding; (+), weak binding; + to ++++, lower to higher strength of binding.
—, not a terminal moiety.
FIG. 4.
rNV VLPs bind synthetic H and Lewis antigens. (A) Carbohydrate binding of rNV VLPs to synthetic H and Lewis antigens determined by ELISA-CMA. Multivalent CHO-PAA-biotin reagents were immobilized on microtiter plate wells and incubated with rNV VLPs in PBS (pH 7.0) at 25°C for 1 h. Bound rNV VLPs were detected with anti-rNV-specific rabbit serum, horseradish peroxidase-conjugated secondary antibody, TMB colorimetric development, and spectrophotometric absorption at 450 nm. Error bars indicate standard deviations. (B) HI titers of serially diluted multivalent CHO-PAA-biotin reagent (0.10 mg/ml) with 4 HAU of rNV VLPs and 50 μl of 0.5% human type O RBCs.
FIG. 5.
Synthetic Led (H type 1) and H type 2 compete for binding rNV VLPs. Multivalent Led (H type 1), H type 2, or Lea carbohydrates (CHO-PAA reagents) were preincubated with rNV VLPs in PBS (pH 7.0) for 30 min at 4°C. Carbohydrate and VLP mixtures were added to microtiter plate wells containing immobilized Led, H type 2, or Lea (CHO-PAA-biotin reagents) and incubated for 1 h at 4°C. The rNV VLPs bound to the immobilized carbohydrates were detected with anti-rNV-specific rabbit serum, horseradish peroxidase-conjugated secondary antibody, TMB colorimetric development, and spectrophotometric absorption at 450 nm. Error bars indicate standard deviations.
DISCUSSION
This is the first report demonstrating that recombinant VLPs from a human calicivirus can bind to and agglutinate RBCs. We employed HA and HI assays to determine properties of rNV VLP-RBC interactions, which may elucidate NV-host cell interactions for this noncultivatable gut virus. Many viruses hemagglutinate RBCs, including viruses that infect the gastrointestinal tract, such as some adenoviruses, enteroviruses, and rotaviruses. The HA reactions described for many of these enteric viruses are optimal at temperatures of 37, 24, or 4°C, and some have an optimal pH of between 5.8 and 7.4 (9). The HA of human RBCs by rNV VLPs was dependent on low temperature (4 to 8°C) and acidic pH (optimized at pH 5.0 to 7.0). The reversibility and repeatability of the rNV VLP HA assay suggest that most of the VLPs are not internalized by the RBCs but are instead released into the buffer at room temperature. The decrease in HA titer upon reincubation could be due to a low level of RBC or VLP degradation over the length of time for the assays.
Carbohydrates function as receptors or essential attachment factors for influenza and measles viruses, bacteria, and parasites (reviewed in reference 40). Detection of virus-binding carbohydrates on host cell and RBC surfaces can lead to the identification of cellular receptors required for infection. Many viruses that bind carbohydrates interact with or require negatively charged sialic acids or heparan sulfates (36). Although rNV VLP binding was determined to be carbohydrate dependent, based on the loss of RBC binding after periodate treatment, neuraminidase treatment of type A and O RBCs had no effect on their HA, and heparan sulfate did not inhibit HA. Therefore, sialic acids and heparan sulfates are not required by the receptor(s) for rNV VLP HA.
NV causes symptomatic infection in most humans and has a very limited host range (24, 43). Although chimpanzees do not develop symptomatic NV disease, some NV-challenged chimpanzees are infected because they produce NV-specific seroresponses and shed NV antigen (54). The rNV VLP HA of most human and chimpanzee RBCs could reflect the ability of the individual or animal to express the NV ligand(s). For instance, the susceptibility of an animal species to influenza A virus strains is reflected in the RBC binding specificity (13, 45).
Numerous NV volunteer challenge studies indicate that some individuals are not susceptible to NV infection (24, 43). The lack of rNV VLP HA for most type B RBCs indicates that a moiety involved in determining an individual's ABO phenotype may be important for VLP-cell interaction. However, the data showing that all type O, A, and AB but few type B RBC samples were hemagglutinated by 400 ng of rNV VLPs per ml were initially puzzling. The ABO phenotypes of human RBCs are determined by the presence or absence of A and/or B carbohydrate antigens on the surface of their RBCs (29). The minimum structure defining the A and B antigens are trisaccharide moieties synthesized by enzymatic transfer of an N-acetylgalactosamine (GalNAc) or a galactose (Gal) residue, respectively, in α-1,3 linkage to the terminal Gal residue of H antigens on glycolipids and glycoproteins (29, 55). The presence or absence of functional enzymes to synthesize the A and/or B antigens determines a person's ABO phenotype.
Since both the A and B glycotransferases add a carbohydrate residue to the same site, the absence of rNV VLP HA with type B RBCs is not easily explained by its terminal Gal residue blocking binding. However, the rNV VLPs gained the ability to hemagglutinate type B RBCs after neuraminidase treatment. This suggests that the rNV VLPs bind to an epitope on type B RBCs that may have been made accessible after removal of sialic acid from the cell surface and that a sialic acid-dependent interaction unique to the type B RBCs may block rNV VLP binding. Other protein-carbohydrate interactions have a similar sialic acid-dependent cryptic nature, including an antibody that recognizes the B antigen (50, 52). Alternately, the rNV VLP HA of type B RBCs may be a weak interaction that is detectable only after the loss of net negative charge on neuraminidase-treated RBCs. The reduced negative charge decreases cell-cell repulsion and thus enhances weak binding interactions (48). The HA of six additional individual human type B RBC samples by high concentrations of rNV VLPs can be interpreted as evidence for a weaker binding of the rNV VLPs to the type B RBCs. This weaker binding of the type B RBCs would not be primarily due to a reduction in potential binding sites, since α-galactosidase treatment of pooled type B RBCs to remove the terminal α-Gal residues, which converts B antigens to H antigens, also increased the HA titer (unpublished data). Thus, one possible explanation for the four human type B RBC samples that were hemagglutinated by 400 ng of rNV VLPs per ml is that these type B RBCs expressed less sialic acid. Alternatively, some B allele galactosyltransferases are less processive, leading to less H to B antigen conversion, which could lead to more H antigen being found on these type B RBCs (55).
This study shows that the H antigen is the HA receptor for rNV VLPs on human type O RBCs. None of the seven Bombay-type RBCs, which lack expression of the H antigen, were hemagglutinated by the rNV VLPs. Furthermore, preincubating the human type O RBCs with an antibody against H type 2 inhibited rNV VLP HA, and preincubating rNV VLPs with synthetic H antigen inhibited HA of human type O RBCs. H antigen is also expressed on chimpanzee RBCs but is not expressed on the RBCs of other species tested for rNV VLP HA (6). Thus, rNV VLP HA and H antigen expression on the RBCs are in agreement. Furthermore, ABH antigens are developmentally regulated (20), with little to no expression on fetal RBCs and expression increasing to adult levels after birth (37). Thus, the lack of rNV VLP HA of one type A umbilical cord RBC sample and one type O umbilical cord RBC sample is congruent with the developmental expression of ABH antigens. Another developmental change in carbohydrate structure that can affect lectin HA is branching (22). The fetal unbranched structure (type i) changes to an adult branched structure (type I) by developmentally regulated enzyme expression (4, 51). The rNV VLP HA of unbranched type i RBCs demonstrates that a branched carbohydrate structure is not required for rNV VLP HA. Without a cell culture system, the biological relevance of rNV VLP HA cannot be directly determined. However, the binding of other biologically relevant protein-carbohydrate pairs is also dependent on low temperature, such as the galactophillic Pseudomonas aeruginosa lectin PA-IL and the human cold agglutinating antibodies to I antigen (21, 22). The low-temperature dependence of rNV VLP-H type 2 antigen binding could reflect an enhanced affinity due to the stabilization of a VLP and/or carbohydrate conformation that promotes binding.
H type 2 is the only H antigen normally expressed on human RBCs (Table 5) (reviewed in reference 29). Our data indicate that H type 2 is the rNV VLP HA receptor on human type O RBCs. Another distantly related virus, rabbit hemorrhagic disease virus (RHDV), is the only other calicivirus VLP (and the only calicivirus virion) known to hemagglutinate human RBCs (46). Interestingly, H type 2 is the HA receptor for RHDV VLPs on human RBCs (47). Thus, the fact that RHDV VLPs bind to a carbohydrate expressed on mucosal cells of rabbits, humans, and other animals suggests that H type 2 antigen binding may be an initial step in calicivirus-host interaction, but without evidence for RHDV infection in humans, an additional host-restrictive step(s) must be necessary for productive RHDV infection. Unlike RHDV, feline calicivirus, and porcine enteric calicivirus, NV is not known to be a systemic pathogen (28, 34, 41). To date, all reports indicate that NV replication and pathogenesis are restricted to the gut, and NV has not been found in sera from infected humans. However, because of rNV VLP binding to RBCs, the possibility of systemic spread of NV should be reevaluated with more sensitive diagnostic tests.
The H type 2 antigen not only is found on RBCs but also is expressed on human enterocytes, mainly on glycoproteins (19). Many structurally related H and Lewis carbohydrate antigens, besides H type 2, are expressed in the gut and not on RBCs (23, 25). The H and Lewis antigens are highly expressed in the proximal small intestine, including the duodenal-jejunal junction, a site of NV-associated histopathology as seen in biopsies from NV-challenged volunteers (43).
A recent study demonstrated that the rNV VLPs bind to histo-blood group antigens present on gastrointestinal sections of secretor, but not nonsecretor, individuals (38). Our data show that in the ELISA-based carbohydrate microtiter plate assays the rNV VLPs bind to the following synthetic H and Lewis carbohydrates (greatest to least rNV VLP binding): Led (H type 1), Leb, H type 2, Ley, and H disaccharide. Our results showing rNV VLP binding to Leb differ from the data reported by Marionneau et al. (38). However, in subsequent assays this group also found that rNV VLPs bind to Leb (J. Le Pendu, personal communication). In the ELISA-based carbohydrate microtiter plate assay, the rNV VLPs were detected bound to the H type 2 reagents at room temperature and at 37°C in addition to 4°C, the temperature required for HA. This binding at increased temperature is probably due to differences in presentation of a single synthetic carbohydrate in the microtiter plate well versus in a heterogeneous carbohydrate population on RBCs. On enterocytes, the presence of the H and Lewis carbohydrates is dependent upon expression of specific glycotransferases (reviewed in reference 31). The expression of these enzymes depends on an individual's genetics and development, on tissue-specific patterns of expression, and on cellular differentiation (25, 31). The Lewis and H type 2 antigens are very similar terminal tri- and tetrasaccharides made by slight variations in linkages of the same three carbohydrate residues (Table 5). These antigens differ in their linkage between residues and/or in the presence of one or more fucose residues. For instance, Led (H type 1) differs from Leb by the absence of a second fucose on GalNAc (31). This suggests that the penultimate fucose residue found in Leb (and Ley) only slightly decreases rNV VLP binding compared to Led (and H type 2). The Led and Leb antigens are expressed on the surface of mucosal epithelial cells and in the mucous secretions of secretor individuals, who comprise approximately 80% of European-Americans. The nonsecretors do not express Led and Leb, and they may express Lea and Lex instead (5, 31). Since rNV VLPs bind Led and Leb, but not Lea and Lex, if NV infection is in part dependent upon specific Lewis antigen binding, as predicted by Marionneau et al., the secretor individuals may be more susceptible to NV infection, and nonsecretors may be less susceptible (38). However, there may be other factors that would make individuals resistant to NV challenge, such as previous infections with a homologous norovirus or the individuals' ABO phenotypes (32, 49).
The H type 3 antigen has also been reported to bind rNV VLPs (see structure in Table 5) (38). Our results support these data, based on the following observations. The GalNAc of the A type 2 antigen may be used as a substrate for synthesis of an H type 3 antigen (10, 11). Thus, there may be more H antigens available for rNV VLP binding to type A RBCs than to type O RBCs. In our HA assays, the rNV VLP HA titers for type A RBCs were consistently greater than or equal to those for type O RBCs. H type 3 may be an additional rNV VLP HA receptor on type A RBCs, but it is not required because it is not found on type O or desialylated type B RBCs.
Since several structurally related carbohydrate antigens bind to the rNV VLPs, it follows that these carbohydrates may interact at a common site on the rNV VLPs. The competitive inhibition experiments with synthetic carbohydrates indicate that free Led (H type 1) and H type 2 are able to inhibit rNV VLPs binding to both homologous and heterologous immobilized Led and H type 2 carbohydrates. These results suggest that Led and H type 2 bind at the same site(s) on the rNV VLPs. Structural studies of rNV VLP interactions with carbohydrates are needed to confirm these observations and to precisely locate the carbohydrate binding site(s) on the VLPs.
MAb 8812 recognizes a conformational epitope within the protruding domain on the rNV VLPs (amino acids 227 to 530) (30). This MAb inhibits both rNV VLP HA and binding to the Caco-2 human colonic adenocarcinoma cell line, which resembles small intestinal cells (53). The lack of HI by IgG purified from mouse rNV VLP hyperimmune serum suggests that MAb 8812 recognizes a nonimmunodominant epitope on rNV VLPs in immunized BALB/c mice. Additionally, the increased binding of rNV VLPs to more-differentiated Caco-2 cells agrees with increased expression of H antigens as Caco-2 cells differentiate in culture (2, 53). The identification of the carbohydrates to which rNV VLPs bind could be used to screen cells potentially permissive to NV infection. For the human parvovirus B19, identifying the P antigen as the HA receptor helped identify erythroblasts as a permissive cell for infection (7). However, for NV, expression of the H antigen on potential host cells is not sufficient for productive infection. Caco-2 cells express Led and H type 2 (2), yet these cells are not permissive to NV infection unpublished data. Nonetheless, H and Lewis antigen expression may prove to be required for or increase the efficiency of NV infection.
Without a cell culture system for NV, there is no means to directly determine if H or Lewis antigen binding plays a part in NV infection. An association between expression of the B antigen and resistance to NV infection and disease suggests that the lack of rNV VLP HA of most type B RBCs is biologically relevant (32). Indeed, the lack of symptomatic disease in any individual expressing type B antigen supports a model in which greater rNV VLP binding correlates with greater native NV binding and high virus binding is one factor contributing to severity of NV illness (32). Additionally, if the rNV VLP binding to RBCs is important in NV infection, infected individuals could develop antibodies to NV that block NV-carbohydrate binding, and an increase in rNV VLP HI titer could correlate with protection from subsequent NV challenges (17). Our examination of paired sera from NV-challenged volunteers shows that the postchallenge HI titers were greater than the prechallenge titers for the NV-infected individuals. Thus, infected individuals are able to develop NV-specific antibodies that block rNV VLP-H type 2 binding. The finding that HI titers increase in the convalescent-phase sera of NV-infected volunteers is of potential interest. With other viruses, increases in HI titer correlate with protection and/or neutralization better than increases in ELISA titer (12, 17). Currently we are retrospectively examining the relationship between pre- and postchallenge HI titers in relation to NV infection and disease. This and future studies should determine if the rNV VLP HI assay can serve as a surrogate for measuring protective immunity.
In conclusion, rNV VLP HA has proven useful in helping to identify carbohydrates as potential NV ligands that may be required for viral infection. Additional work is needed to understand norovirus-carbohydrate interactions. For instance, the specificity of carbohydrate antigen binding may be strain-specific. The VLPs from a genogroup II norovirus, Grimsby virus, did not produce the same HA profile as rNV VLPs. The recombinant Grimsby virus VLPs were able to hemagglutinate all normal human type O, A, B, and AB RBCs, and they also hemagglutinated one of the seven Bombay-type RBC samples (unpublished data). Understanding the nature of the potential initial norovirus-host cell interactions could lead to the development of antiviral agents and a greater understanding of norovirus pathogenesis.
Acknowledgments
We thank Marilyn Moulds and Immucor/Gamma Biologicals for the rare-type blood samples; Jose Garcia at the Methodist Blood Bank and volunteer donors at Baylor College of Medicine for human blood samples; Baylor Influenza Research Center for blood collection and influenza virus; Robert Lanford and the Southwest Biomedical Research Center for primate blood samples; Wayne Smith and the Children's Nutritional Research Center for cord blood samples; and Sue Crawford, Carl Zeng, Andrea Bertolloti-Ciarlet, Tracy Dewese-Parker, Max Ciarlet, the Margaret Conner lab, and Mark and Theresa Kuhlenschmidt for reagents and discussions.
This work was funded by grants AI-65299, AI-46581, DK-58955, and CA-09197 from the National Institutes of Health (Bethesda, Md.) and by grant DK-56338, which supports the Texas Gulf Coast Digestive Diseases Center.
REFERENCES
- 1.Adler, J. L., and R. Zickl. 1969. Winter vomiting disease. J. Infect. Dis. 119:668-673. [DOI] [PubMed] [Google Scholar]
- 2.Amano, J., and M. Oshima. 1999. Expression of the H type 1 blood group antigen during enterocytic differentiation of Caco-2 cells. J. Biol. Chem. 274:21209-21216. [DOI] [PubMed] [Google Scholar]
- 3.Apoil, P. A., F. Roubinet, S. Despiau, R. Mollicone, R. Oriol, and A. Blancher. 2000. Evolution of alpha 2-fucosyltransferase genes in primates: relation between an intronic Alu-Y element and red cell expression of ABH antigens. Mol. Biol. Evol. 17:337-351. [DOI] [PubMed] [Google Scholar]
- 4.Bierhuizen, M. F., M. G. Mattei, and M. Fukuda. 1993. Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev. 7:468-478. [DOI] [PubMed] [Google Scholar]
- 5.Bjork, S., M. E. Breimer, G. C. Hansson, K. A. Karlsson, and H. Leffler. 1987. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J. Biol. Chem. 262:6758-6765. [PubMed] [Google Scholar]
- 6.Blancher, A., M. E. Reid, and W. W. Socha. 2000. Cross-reactivity of antibodies to human and primate red cell antigens. Transfus. Med. Rev. 14:161-179. [DOI] [PubMed] [Google Scholar]
- 7.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 8.Brown, K. E., and B. J. Cohen. 1992. Haemagglutination by parvovirus B19. J. Gen. Virol. 73:2147-2149. [DOI] [PubMed] [Google Scholar]
- 9.Center for Disease Control. 1971. Recommended method for the use of adenovirus, enterovirus, myxovirus, reovirus, and vaccinia hemagglutination (HA) and hemagglutination inhibition (HI) reagents. Center for Disease Control, Atlanta, Ga.
- 10.Clausen, H., E. Holmes, and S. Hakomori. 1986. Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). II. Differential conversion of different H substrates by A1 and A2 enzymes, and type 3 chain H expression in relation to secretor status. J. Biol. Chem. 261:1388-1392. [PubMed] [Google Scholar]
- 11.Clausen, H., S. B. Levery, R. Kannagi, and S. Hakomori. 1986. Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). I. Isolation and chemical characterization. J. Biol. Chem. 261:1380-1387. [PubMed] [Google Scholar]
- 12.Clements, M. L., R. F. Betts, E. L. Tierney, and B. R. Murphy. 1986. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 24:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor, R. J., Y. Kawaoka, R. G. Webster, and J. C. Paulson. 1994. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205:17-23. [DOI] [PubMed] [Google Scholar]
- 14.Crawford, S. E., M. Labbe, J. Cohen, M. H. Burroughs, Y.-J. Zhou, and M. K. Estes. 1994. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 68:5945-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wit, M. A., M. P. Koopmans, L. M. Kortbeek, W. J. Wannet, J. Vinje, F. van Leusden, A. I. Bartelds, and Y. T. van Duynhoven. 2001. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am. J. Epidemiol. 154:666-674. [DOI] [PubMed] [Google Scholar]
- 16.Dolin, R., N. R. Blacklow, H. DuPont, R. F. Buscho, R. G. Wyatt, J. A. Kasel, R. Hornick, and R. M. Chanock. 1972. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc. Soc. Exp. Biol. Med. 140:578-583. [DOI] [PubMed] [Google Scholar]
- 17.Duvdevani, P., N. Varsano, R. Slepon, Y. Lerman, T. Shohat, and E. Mendelson. 1996. Determination of immunity to measles virus in young adults: comparative evaluation of a commercial enzyme immunoassay and the hemagglutination inhibition techniques. Clin. Diagn. Virol. 7:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 19.Finne, J., M. E. Breimer, G. C. Hansson, K. A. Karlsson, H. Leffler, J. F. Vliegenthart, and H. van Halbeek. 1989. Novel polyfucosylated N-linked glycopeptides with blood group A, H, X, and Y determinants from human small intestinal epithelial cells. J. Biol. Chem. 264:5720-5735. [PubMed] [Google Scholar]
- 20.Fukuda, M., M. N. Fukuda, and S. Hakomori. 1979. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J. Biol. Chem. 254:3700-3703. [PubMed] [Google Scholar]
- 21.Gilboa-Garber, N., and D. Sudakevitz. 1999. The hemagglutinating activities of Pseudomonas aeruginosa lectins PA-IL and PA-IIL exhibit opposite temperature profiles due to different receptor types. FEMS Immunol. Med. Microbiol. 25:365-369. [DOI] [PubMed] [Google Scholar]
- 22.Gilboa-Garber, N., D. Sudakevitz, and C. Levene. 1999. A comparison of the Aplysia lectin anti-I specificity with human anti-I and several other I-detecting lectins. Transfusion 39:1060-1064. [DOI] [PubMed] [Google Scholar]
- 23.Glynn, L. E., E. J. Holborow, and G. D. Johnson. 1957. The distribution of blood-group substances in human gastric and duodenal mucosa. Lancet ii:1083-1088. [DOI] [PubMed] [Google Scholar]
- 24.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34-43. [DOI] [PubMed] [Google Scholar]
- 25.Green, F. R., P. Greenwell, L. Dickson, B. Griffiths, J. Noades, and D. M. Swallow. 1988. Expression of the ABH, Lewis, and related antigens on the glycoproteins of the human jejunal brush border. Subcell. Biochem. 12:119-153. [DOI] [PubMed] [Google Scholar]
- 26.Green, K. Y., G. Belliot, J. L. Taylor, J. Valdesuso, J. F. Lew, A. Z. Kapikian, and F. Y. Lin. 2002. A predominant role for Norwalk-like viruses as agents of epidemic gastroenteritis in Maryland nursing homes for the elderly. J. Infect. Dis. 185:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, M., J. Hayes, K. O. Cho, A. V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 75:9239-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakomori, S. 1999. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim. Biophys. Acta 1473:247-266. [DOI] [PubMed] [Google Scholar]
- 30.Hardy, M. E., T. N. Tanaka, N. Kitamoto, L. J. White, J. M. Ball, X. Jiang, and M. K. Estes. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252-261. [DOI] [PubMed] [Google Scholar]
- 31.Henry, S., R. Oriol, and B. Samuelsson. 1995. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 69:166-182. [DOI] [PubMed] [Google Scholar]
- 32.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 33.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi, Y., Y. Tohya, T. Horimoto, K. Maeda, T. Miyazawa, and T. Mikami. 1994. Carrier-state infection of feline T-lymphoblastoid cells with feline calicivirus. Vet. Microbiol. 40:379-386. [DOI] [PubMed] [Google Scholar]
- 35.Kelly, R. J., L. K. Ernst, R. D. Larsen, J. G. Bryant, J. S. Robinson, and J. B. Lowe. 1994. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc. Natl. Acad. Sci. USA 91:5843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus. 2001. Fields virology, 4th ed., vol. 1 and 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 37.Kusnierz, G., and A. Leskiewicz. 1971. Studies on the ABH component of human newborn erythrocytes. Arch. Immunol. Ther. Exp. (Warsz.) 19:635-641. [PubMed] [Google Scholar]
- 38.Marionneau, S., N. Ruvoen, B. Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moulds, J. M., and J. J. Moulds. 2000. Blood group associations with parasites, bacteria, and viruses. Transfus. Med. Rev. 14:302-311. [DOI] [PubMed] [Google Scholar]
- 41.Ohlinger, V. F., B. Haas, G. Meyers, F. Weiland, and H. J. Thiel. 1990. Identification and characterization of the virus causing rabbit hemorrhagic disease. J. Virol. 64:3331-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ou, W. C., M. Wang, C. Y. Fung, R. T. Tsai, P. C. Chao, T. H. Hseu, and D. Chang. 1999. The major capsid protein, VP1, of human JC virus expressed in Escherichia coli is able to self-assemble into a capsid-like particle and deliver exogenous DNA into human kidney cells. J. Gen. Virol. 80:39-46. [DOI] [PubMed] [Google Scholar]
- 43.Parrino, T. A., D. S. Schreiber, J. S. Trier, A. Z. Kapikian, and N. R. Blacklow. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 297:86-89. [DOI] [PubMed] [Google Scholar]
- 44.Prasad, B. V. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 45.Rogers, G. N., and J. C. Paulson. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361-373. [DOI] [PubMed] [Google Scholar]
- 46.Ruvoen-Clouet, N., D. Blanchard, G. Andre-Fontaine, and J. P. Ganiere. 1995. Partial characterization of the human erythrocyte receptor for rabbit haemorrhagic disease virus. Res. Virol. 146:33-41. [DOI] [PubMed] [Google Scholar]
- 47.Ruvoen-Clouet, N., J. P. Ganiere, G. Andre-Fontaine, D. Blanchard, and J. Le Pendu. 2000. Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J. Virol. 74:11950-11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Springer, G. F., and P. R. Desai. 1985. Tn epitopes, immunoreactive with ordinary anti-Tn antibodies, on normal, desialylated human erythrocytes and on Thomsen-Friedenreich antigen isolated therefrom. Mol. Immunol. 22:1303-1310. [DOI] [PubMed] [Google Scholar]
- 49.Treanor, J. J., X. Jiang, H. P. Madore, and M. K. Estes. 1993. Subclass-specific serum antibody responses to recombinant Norwalk virus capsid antigen (rNV) in adults infected with Norwalk, Snow Mountain, or Hawaii virus. J. Clin. Microbiol. 31:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urdal, D. L., and S. Hakomori. 1983. Characterization of tumor-associated ganglio-N-triaosylceramide in mouse lymphoma and the dependency of its exposure and antigenicity on the sialosyl residues of a second glycoconjugate. J. Biol. Chem. 258:6869-6874. [PubMed] [Google Scholar]
- 51.Watanabe, K., and S. I. Hakomori. 1976. Status of blood group carbohydrate chains in ontogenesis and in oncogenesis. J. Exp. Med. 144:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe, K., S. I. Hakomori, R. A. Childs, and T. Feizi. 1979. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J. Biol. Chem. 254:3221-3228. [PubMed] [Google Scholar]
- 53.White, L. J., J. M. Ball, M. E. Hardy, T. N. Tanaka, N. Kitamoto, and M. K. Estes. 1996. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J. Virol. 70:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyatt, R. G., H. B. Greenberg, D. W. Dalgard, W. P. Allen, D. L. Sly, T. S. Thornhill, R. M. Chanock, and A. Z. Kapikian. 1978. Experimental infection of chimpanzees with the Norwalk agent of epidemic viral gastroenteritis. J. Med. Virol. 2:89-96. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, F. 2000. Molecular genetics of ABO. Vox Sang. 78(Suppl. 2):91-103. [PubMed] [Google Scholar]