Abstract
Viral envelope fusion proteins are important structural proteins that mediate viral entry and may affect or determine the host range of a virus. The acquisition, exchange, and evolution of such envelope proteins may dramatically affect the success and evolutionary divergence of viruses. In the family Baculoviridae, two very different envelope fusion proteins have been identified. Budded virions of group I nucleopolyhedroviruses (NPVs) such as the Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV), contain the essential GP64 envelope fusion protein. In contrast group II NPVs and granuloviruses have no gp64 gene but instead encode a different envelope protein called F. F proteins from group II NPVs can functionally substitute for GP64 in gp64null AcMNPV viruses, indicating that GP64 and these F proteins serve a similar functional role. Interestingly, AcMNPV (and other gp64-containing group I NPVs) also contain an F gene homolog (Ac23) but the AcMNPV F homolog cannot compensate for the loss of gp64. In the present study, we show that Ac23 is expressed and is found in budded virions. To examine the function of F protein homologs from the gp64-containing baculoviruses, we generated an Ac23null AcMNPV genome by homologous recombination in E. coli. We found that Ac23 was not required for viral replication or pathogenesis in cell culture or infected animals. However, Ac23 accelerated the mortality of infected insect hosts by approximately 28% or 26 h. Thus, Ac23 represents an important viral pathogenicity factor in larvae infected with AcMNPV.
The family Baculoviridae represents a large group of enveloped double-stranded DNA viruses that are restricted to invertebrate hosts, mainly insects of the order Lepidoptera (2). Baculoviruses are currently subdivided into two genera, the nucleopolyhedroviruses (NPVs) and the granuloviruses (GVs). Phylogenetic studies also indicate that NPVs can be further subdivided into two subgroups: groups I and II (9, 12, 33). During a single infection cycle, baculoviruses produce two virion phenotypes that play distinctly different roles. Virions of the occlusion-derived virus (ODV) mediate the transmission of infection from animal to animal, while virions of the budded virus (BV) mediate the cell-to-cell spread of infection within the infected animal and in cell culture. Virions of the ODV phenotype are formed very late in the infection cycle when nucleocapsids become enveloped within the nucleus. ODV are later encased in the crystallized occlusion body protein (polyhedrin in NPVs and granulin in GVs) to form occlusion bodies, which are released when the host cells lyse. When occlusion bodies of lepidopteran baculoviruses are consumed by susceptible larvae, the highly alkaline environment of the host's midgut triggers disassembly of the occlusion body and subsequent release of the ODV. ODV are highly infectious to midgut epithelial cells and initiate the infection cycle in the animal. In contrast to the ODV, virions of the BV phenotype are generated earlier in the infection cycle, when nucleocapsids bud through the host cell plasma membrane into the hemocoel. In the hemocoel, BV initially infects the tracheal epithelium and hemocytes and then infects the fat body, muscle, malpighian tubules, and other tissues, leading to the eventual death of the host (5-7).
BV enter cells via the endocytic pathway and appear to contain a very limited number of virus-encoded envelope proteins. GP64 is the major glycoprotein found in the BV envelope of Autographa californica multicapsid NPV (AcMNPV) and other members of the group I NPVs. Previous studies showed that GP64 was (i) involved in BV binding to host cells (8), (ii) necessary and sufficient for the low-pH-triggered membrane fusion that occurs during BV entry by endocytosis (3, 11, 20, 31), and (iii) essential for efficient virion budding from the cell surface (21, 23). Recent analyses of genomic sequences from group II NPVs and GVs indicate that they do not possess a gp64 gene. However, BV envelope proteins unrelated to GP64 have been identified from two group II NPVs; these envelope proteins include Ld130 from Lymantria dispar MNPV and Se8 from Spodoptera exigua MNPV (14, 16). Like GP64, both Ld130 and Se8 possess pH-triggered membrane fusion activity (15, 25) and were subsequently named F proteins (32). A recent study demonstrated that Ld130 (or LdF) and Se8 (or SeF) are capable of rescuing virion production and infectivity in a gp64-null AcMNPV (18). Thus, these F proteins are functionally analogous to GP64. While proteolytic cleavage does not appear to be required for activation of GP64, both LdF and SeF contain a conserved proprotein convertase (furin-like) cleavage site, and it was recently demonstrated that cleavage at that site is necessary for low-pH-triggered fusion activity of the SeF protein (18, 32). In addition, SeF cleavage is necessary for rescue of the gp64-null AcMNPV by this F protein (18).
F genes have been identified in all NPVs and GVs for which genomic sequences are available. Therefore, group I NPVs such as AcMNPV and the Orgyia pseudotsugata MNPV (OpMNPV) contain both a gp64 gene and a gene encoding an F protein homolog. Comparisons of available F protein sequences show that the conserved region containing the furin-like cleavage site is absent from the AcMNPV and OpMNPV F protein homologs Ac23 and Op21 (28). In addition, pH-triggered membrane fusion activity has not been detected in syncytium formation assays of transiently expressed Ac23 or Op21 (reference 27 and unpublished observations). These observations suggested that the F protein homologs in group I NPVs may play a role distinctly different from that of GP64. However, the presence of F homolog genes in baculovirus genomes suggests that they have a positive impact on viral success. We therefore examined the role of the F protein homolog (Ac23) in the context of the AcMNPV infection cycle. To accomplish this, we used a new PCR and bacmid-based recombination system to delete the Ac23 gene from the AcMNPV genome. Our results indicate that Ac23 is not essential for virus replication and that deletion of Ac23 has no detectable effect on yields of infectious budded virus from cultured cells, or from orally infected animals. However, Ac23 substantially accelerates the death of cultured insect cells and insect larvae.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda Sf9 cells and Trichoplusia ni Tn5B1-4 (High 5) cells were cultured at 27°C in TNM-FH complete medium containing 10% fetal bovine serum (10). Viruses were amplified and purified from bacmid-transfected Sf9 cells as described previously (18) and were subjected to titer determination by end-point dilution (24). Western blot analyses of budded virions and infected cell lysates were performed as previously described (18).
Gene disruption.
The Ac23 gene was deleted from an AcMNPV bacmid, bMON14272 (17), using the method of Datsenko and Wanner (4). A chloramphenicol resistance gene (chloramphenicol acetyltransferase or cat) was PCR amplified using plasmid pKD3 (4) as the template and primers that added terminal sequences corresponding to either the 5′ untranslated region (UTR) or the C-terminal coding region of the Ac23 gene. PCR amplification was performed with the primers 5′ORF23k and 3′ORF23k (Table 1) using Pfu Turbo DNA polymerase (Stratagene) and the following cycle parameters: 1 cycle (94°C for 10 min), 35 cycles (94°C for 1 min, 37°C for 2 min, and 72°C for 2 min), and 1 cycle (72°C for 10 min). The PCR product was gel purified with Qiaex II resins (Qiagen), digested with DpnI for 2 h, and repurified. Bacmid DNA (1 μg) and the DpnI-treated PCR product (≈100 ng) were cotransformed into Escherichia coli BW25113/pKD46 electrocompetent cells (4). The lambda RED recombinase was induced by addition of 1 mM arabinose approximately 4 h prior to preparation of electrocompetent cells. Transformed cells were incubated at 37°C for 4 h in SOC medium and recombinants were selected as resistant colonies on medium containing kanamycin and chloramphenicol.
TABLE 1.
Primers used in this study
| Primer namea | Primer sequence (5′-3′) |
|---|---|
| 5′ORF23k | TCATTTAAATGTTACGTCAGTAGTTAGTAT |
| ATAAGCCGTAGTGTAGGCTGGAGCTGCTTC | |
| 3′ORF23k | ATAATCATAGGGTACAACACAGGTTTATCA |
| TCTTTTAACACATATGAATATCCTCCTTAG | |
| 5′ORF23CR (A) | AATTACAATGACGTCAGAGGGCAG |
| 3′ORF23CR (B) | GATAGCCATTAAACATTTGTGTGC |
| 5′ORF23cd2 (C) | AGAACAGATCGAGTATCATCAAAGCTC |
| 3′ORF23cd2 (D) | ACATAATAGTTTTTGCTGTCTGTAATCC |
| 3′Ac23c-myc | CGCGGATCCCTACAGATCCTCTTCTGAGAT |
| GAGTTTTTGTTCTTTTATTCTTTCTATAATC | |
| 5′Hpalpolyhpro | GCGTTAACGTTGCTGATATCATGGAGATAA |
| 3′Hpalpolyhorf | GAGTTAACTTAATACGCCGGACCAGTG |
| AcEcoH5′3036 (E) | CAAGGCGACAAGGTGCTGATGC |
| AcEcoH3′2662 (F) | TGTGAATAAAGGCCGGATAAAACT |
| 5′LEF-5CR | CCCAACAACGTCAAAAACAAAACG |
| 3′LEF-5CR | ACTAAGCCCGCTAAGCTCAAATAG |
| 5′6248FBgusR | CTCCCACACCTCCCCCTGAACCT |
Primer designations in Fig. 1C are shown in parentheses after primer names.
To confirm the structure of the Ac23 locus in the selected bacmids, PCR amplifications were performed using Taq DNA polymerase (Promega) and five different primer pairs. Primer pair I corresponded to sequences upstream and downstream of open reading frame 23 (ORF23) (5′ORF23CR and 3′ORF23CR [Table 1]). Primer pairs II [5′ORF23CR and AcEcoH3036(CAT)] and III [3′ORF23CR and AcEcoH2662(CAT)] were used to detect and confirm the predicted recombination junctions at the Ac23 locus. Primer pair IV (5′ORF23cd and 3′ORF23cd) was used to confirm the loss of the Ac23 coding region. Figure 1C shows the relative positions of the four primer pairs. One recombinant bacmid containing the desired modification was selected and transformed into E. coli DH10B cells containing a helper plasmid (pMON7124) that encodes a Tn7 transposase (18) to generate strain Ac23null bacmid-DH10B+pMON7124.
FIG. 1.
(A) Schematic of Ac23 disruption strategy. A PCR product (middle construct) containing a cat cassette flanked by FRT (flip recombinase target site) sequences and sequences homologous to the Ac23 region at both ends was generated using two 60-mer primers (P5′ORF23k and P3′ORF23k [Table 1]). Each primer consisted of a 20-nt P1 or P2 priming-site sequence flanking the cat cassette on pKD3 (4) and a 40-nt Ac23 homology region complementary to sequences upstream of the Ac23 coding region (H1) or from the 3′ end of the Ac23 coding region (H2). The PCR product (middle construct) was cotransformed with AcMNPV bacmid DNA into E. coli cells expressing the Red recombination system. Colonies resulting from recombination between the bacmid and the linear PCR product sequences were resistant to both chloramphenicol and kanamycin and were selected on the appropriate media. (B) Diagram of donor plasmid constructs used for introduction of genes (GUS reporter, envelope protein genes, and the polyhedrin gene) into the polyhedrin locus of a bacmid by Tn7-mediated transposition. Four types of donor plasmids were generated. Each contained a GUS reporter gene under the control of a p6.9 promoter. Construct type I contains only a GUS reporter gene and does not contain baculovirus genes. Construct type II contains an envelope protein gene in addition to the GUS reporter gene. Two different envelope protein genes were inserted separately: the Ac23 gene (under the control of its own promoter) or the SeMNPV F gene (SeF) (under the control of the AcMNPV gp64 promoter). Construct type III contains the polyhedrin gene under the control of its own promoter. Construct type IV contains both the polyhedrin gene and an envelope protein gene. Viruses derived from polyhedrin gene-negative donor plasmids (I and II) were used for the initial transfection-infection assays and for one-step growth curve studies with Sf9 cells (see Fig. 2 to 4), while viruses derived from polyhedrin gene-positive donor plasmids (III and IV) were used for all other studies (see Fig. 5 to 8). A donor plasmid from construct type III was also inserted into the original bacmid (bMON14272) as a positive control for insect-feeding experiments. SV40, simian virus 40. (C) Confirmation by PCR analysis of the presence or absence of sequence modifications in bacmid constructs. The positions of primer pairs used in the analysis of the Ac23 locus are indicated by arrows designated A through F. The names and sequences of the primers are described in Table 1. The upper diagram shows the structure of the wild-type Ac23 locus, and the lower diagram shows the structure of the Ac23 locus in the recombinant Ac23null bacmid. Representative PCR analysis of five recombinant bacmids using four pairs of primers are shown in the panels below. The original Ac23 knockout bacmid (Ac23null, lane 3) isolated from BW25113/pKD46 cells was used to generate all other Ac23null bacmids by transposition. Ac23 knockout bacmids are designated Ac23null/GUS (lane 4), Ac23null/GUS+SeF (lane 5), and Ac23null/GUS+Ac23 (lane 6). Primer pairs used for PCR analysis are indicated beside each panel, and the size of the expected PCR product is shown under the primer pair. Numbers in parentheses represent the size of the expected PCR product when the Ac23 gene is present. Lane 1 corresponds to the 1-kb DNA ladder (Promega) used as molecular size standards. The bands corresponding to the 1- and 2-kb standards are designated by open and solid circles, respectively. Lane 2 shows a lef5 knockout bacmid (Aclef5−) generated in parallel and used as a control.
Donor plasmids and transposition into an Ac23-null bacmid.
Several donor plasmids (18) were generated for insertion of genes into the Ac23null bacmid by Tn7-mediated transposition. All donor plasmids contained a β-glucuronidase (GUS) reporter gene under the control of the p6.9 promoter, and some also contained an F gene (Ac23 or SeMNPV F). Since bacmid bMON14272 has a deletion in the polyhedrin gene, the AcMNPV polyhedrin gene was inserted into all the above-mentioned donor plasmids for use in insect-feeding assays that required occlusion bodies. Figure 1B shows four types of donor plasmids that were generated for those studies.
As an example, to generate the Ac23null-repair bacmid as a positive control, a donor plasmid containing the Ac23 gene was used for reintroduction of the Ac23 gene into the Ac23null bacmid. The Ac23 promoter (nucleotides [nt] 1 to 302 upstream of the initiator ATG codon), ORF, and 3′UTR (nt 1 to 108 downstream of the ORF, including polyadenylation signals) regions were PCR amplified using bacmid bMON14272 as template and cloned into pCR-Blunt II TOPO (Invitrogen). The Ac23 gene was then transferred as a KpnI-NotI fragment to plasmid pFASTBAC1-lacZ to generate pFASTBAC1-lacZ ORF23. pFASTBAC1-lacZ is a plasmid derived from pFASTBAC1 (Invitrogen) by deletion of the polyhedrin promoter and insertion of a lacZ cassette into the SalI site of the polylinker. The Ac23 gene was then released from pFASTBAC1-lacZ ORF23 by NotI digestion and subcloned into the NotI site of pΔFBgus(R) (18), adjacent to a GUS reporter gene (under the control of an AcMNPV p6.9 promoter) to generate pΔFBgus(R)-Ac23.
To generate a c-myc epitope-tagged Ac23 protein, the Ac23 ORF was PCR amplified using primers 5′ORF23cd2 and 3′Ac23c-myc (Table 1) and plasmid pFASTBAC1-lacZ ORF23 as the template. The PCR product was subcloned into vector pCR4-TOPO (Invitrogen) and digested with BamHI and NarI to release the 3′ end of the gene, which was used to replace the 3′ end of the Ac23 gene in plasmid pΔFBgus(R)Ac23. The resulting plasmid was named pΔFBgus(R)Ac23c-myc.
To insert the polyhedrin gene into bacmid constructs for occlusion body production, the polyhedrin gene coding sequences and promoter region were amplified by PCR using primers 5′HpaIpolyhpro and 3′HpaIpolyhorf (Table 1) and AcMNPV DNA as the template. The polyhedrin gene was cloned into pCR4Blunt-TOPO (Invitrogen), and a 0.7-kb fragment resulting from a partial HpaI digest was subcloned into the HpaI site in a series of pΔFBgus(R) vectors. Plasmids containing the polyhedrin gene in the same orientation as the GUS gene (Fig. 1B) were selected and used for transposition into the Ac23null bacmid (see below). All donor plasmids were transformed into E. coli strain Ac23null bacmid-DH10B+pMON7124 electrocompetent cells. The resulting bacmids were screened and transposition was confirmed by diagnostic PCR using primers pUC/M13 reverse, gus-jm, and P5′6248FBgusR (Table 1) as previously described (18). All PCR-derived fragments were completely sequenced to ensure that no errors were introduced.
Insect rearing and larval feeding assays.
Analyses of insect mortality and viral pathogenesis in insect larvae were performed with cabbage looper (T. ni) larvae. T. ni rearing, egg collection, and sterilization were performed as reported previously (13). Occlusion bodies (OB) were prepared (24) from the following viruses that contained a polyhedrin gene: wild-type AcMNPV, an AcMNPV derived from bacmid bMON14272 (Acbacmid/GUS+PH), Ac23null virus (Ac23null/GUS+PH), Ac23null-repair virus (Ac23null/GUS+Ac23+PH), and SeF-pseudotyped Ac23null virus (Ac23null/GUS+SeF+PH). In each case except for wild-type AcMNPV, a polyhedrin gene was inserted by transposition into the polyhedrin locus as described above. To dissociate any clumped OB, resuspended OB were vortexed for 2 h, counted on a hemocytometer, and diluted to the desired concentration in water containing 1 mg of FD&C no. 1 blue food coloring (Hilton-Davis) per ml.
Droplet feeding assays (13) were performed in a semiblind manner with coded samples to measure neonate mortality after OB feeding. Orally infected larvae were maintained individually in the dark at 26°C, and larvae that died or were injured were removed from the assay and discarded at 24 h postfeeding, to eliminate the low level of non-virus-induced neonate mortality that was occasionally observed. Virus-induced mortality was scored at various time points thereafter, and insects were scored as dead when there was no response to manual stimuli with the tip of a toothpick.
For assays of fourth-instar T. ni larvae, individual insects were fed 0.5 μl of deionized H2O containing 250 OB and kept individually in diet cups at 26°C. In initial mortality assays, fourth-instar larvae were orally infected within 15 h (see Fig. 5A) or 5 h (see Fig. 5B) after the third- to fourth-instar molt. In subsequent experiments (see Fig. 5C and D and Fig. 8), larvae were orally infected within 2 h of molting into the fourth instar and used for mortality assays, hemolymph collection, and in situ staining. Mortality was determined as described above for neonate assays. For hemolymph collection at various times after OB feeding, insects were surface sterilized and a portion of a proleg was surgically removed. Exactly 2 μl of hemolymph was collected at the wound site, added to 750 μl of TNM-FH complete medium containing antibiotic, mixed, and centrifuged for 10 min at 5,000 × g. A 50-μl aliquot of the clarified supernatant was transferred to a fresh tube, and the viral titer was determined by measuring the 50% tissue culture infective dose (TCID50) on Sf9 cells. In situ staining was performed on insects dissected at various times after OB feeding, as described previously (21) with minor modifications.
FIG. 5.
Analysis of survival times in neonatal and fourth-instar T. ni larvae infected with OB from Ac23null, Ac23null-repair, and wild-type AcMNPV. The percent mortality of larvae orally infected with 6 OB/neonate (A), 60 OB/neonate (B), and 250 OB/fourth-instar larva (C and D) were plotted against the time after oral infection. Larvae were either mock infected or orally infected with either vAc23null/GUS+PH virus (Ac23null), vAc23null/GUS+Ac23+PH repair virus (Ac23repair), wild-type AcMNPV, or the SeF-pseudotyped Ac23null virus (Ac23null/GUS+SeF+PH). Note that mortality of larvae fed OB derived from Ac23null virus (vAc23null/GUS+PH) was delayed compared with that for the wild-type AcMNPV. The delay was rescued by reintroducing the Ac23 gene into the polyhedrin locus of the Ac23null bacmid (vAc23null/GUS+Ac23+PH). Results shown are the average for two independent experiments, with error bars representing SE, except for doses of 6 OB/neonate, in which the results for Acbacmid/GUS+PH are derived from a single experiment.
FIG. 8.
Viral BV loads in the hemolymph of orally infected T. ni larvae were similar in fourth-instar T. ni larvae infected with Ac23null virus and Ac23null-repair virus. Fourth-instar T. ni larvae were orally infected with Ac23null virus (Ac23null) or Ac23null-repair virus (Ac23repair) at a dose of 250 OB/insect. Hemolymph samples were collected at various times p.i. (0, 12, 24, 30, 36, 42, and 48 h p.i.), and infectious BV titers were determined on Sf9 cells. At 0 and 12 h postfeeding, BV titers were not detectable in hemolymph of any of the eight larvae collected at each time point for each treatment. At 24 h postfeeding the average BV titer was determined from two of the eight insects that had viral titers above the detection limit (2 × 107 IU/ml). Error bars represent SE. The sample size (N) for each data point is listed below the graph.
Progression of the infection cycle and cell death.
For examination of cells that were in the late or very late stages of infection, 106 Sf9 or High 5 cells were infected at a multiplicity of infection (MOI) of 5 for 1 h, washed twice with phosphate-buffered saline (pH 7.4), and placed in fresh TNM-FH, complete medium. At various time points, images of the infected cells were captured with a charge-coupled device camera mounted on a Olympus IX70 inverted microscope to determine the percentages of OB-containing cells or cells stained for GUS reporter activity (18). Captured representative images were enhanced in Adobe Photoshop 6.0, printed on a color laser jet HP 4500 printer (Hewlett-Packard), and scored manually. For scoring of dead cells, 105 cells (Sf9 or High 5 cells) were infected at an MOI of 10 for 1 h (in a 24-well plate), washed once with phosphate-buffered saline, and incubated in TNM-FH complete medium. At various time points, ethidium homodimer-1 (Molecular Probes) was added to a final concentration of 5 μM. After 15 min, images were captured as described above. For examination of the progression of the infection cycle and for determination of the percentages of dead cells, 500 to 1,500 cells were counted for each of two replicate experiments.
Sequence comparison of F proteins.
F homolog gene sequences were obtained from genomic baculovirus sequences in GenBank. The F protein sequence accession numbers are as follows: Ac23, NP054052; Bm14, NP047428; Op21, NP046177; Ha133, NP075202; Px26, NP067765; Xc27, NP059715; Se8, NP037768; Ld130, NP047767; S1136, AAL01818; Ep19, AAK85583; Cp31, AAK70691; and Mc9, AAM09117. Signal sequence prediction was performed with SignalP (22). Multiple-sequence alignment of all mature protein sequences (without the predicted signal sequences) was performed with ClustalW using MegAlign v5.03 (DNAstar Inc.) and default parameters except for a gap penalty of 6 and Gonnet protein weight matrix. ClustalW (29) was also used to obtain a neighbor-joining tree of mature F homologs.
RESULTS
Generation of Ac23null viruses.
To determine whether Ac23 is an essential AcMNPV gene, we deleted Ac23 from an AcMNPV bacmid (bMON14272) by the method of Datsenko and Wanner (4). Two 60-mer oligonucleotides were synthesized and used to generate a linear PCR product from plasmid pKD3 (Fig. 1A). The PCR product contained a chloramphenicol resistance gene (cat) flanked by sequences homologous to (i) a region immediately upstream of the Ac23 initiator ATG codon (H1 in Fig. 1) and (ii) a region overlapping the Ac23 coding region immediately upstream of the stop codon (H2 in Fig. 1). The PCR product was purified and cotransformed, along with bacmid DNA, into electrocompetent cells of E. coli strain BW25113/pKD46 expressing the λ phage recombination genes exo, beta, and gam. Colonies harboring recombinant bacmids were selected on medium containing kanamycin and chloramphenicol. The selected bacmids were expected to contain a deletion of the Ac23 ORF nt 18512 to 20570 in the AcMNPV genome (1). Replacement of the Ac23 ORF with the cat cassette was confirmed by PCR with primers specific for detection of predicted recombinant junction regions (Fig. 1C). One Ac23null bacmid was chosen for introduction into E. coli DH10B cells to serve as the isogenic bacmid for all subsequent introductions of reporter genes and generation of a “repair” bacmid. To introduce a GUS reporter gene under the transcriptional control of the AcMNPV p6.9 late promoter and to reinsert the Ac23 gene into the Ac23null bacmid (to generate a “repair” virus as a positive control), four different donor plasmids were constructed. These donor plasmids were then used to introduce genes into the polyhedrin locus by Tn7-mediated transposition (18). Thus, four recombinant bacmids were generated for our initial studies: Ac23null/GUS (an Ac23null bacmid with a GUS reporter gene), Ac23null/GUS+Ac23 (a “repaired” Ac23null bacmid with a GUS gene and the Ac23 gene), Ac23null/GUS+Ac23cmyc (a repaired Ac23null bacmid containing a GUS gene and an Ac23 gene that was tagged with a c-myc epitope), and Ac23null/GUS+SeF (an Ac23null bacmid containing the GUS gene and an SeMNPV F gene). Transposition of donor plasmid sequences into the bacmids was confirmed by diagnostic PCR (Fig. 1C).
Ac23 detection in BV.
To determine if the AcMNPV F protein homolog (Ac23) is expressed in infected cells and is present in AcMNPV budded virions, we performed Western blot analysis on purified budded virions generated from a virus (Ac23null/GUS+Ac23cmyc) expressing an Ac23 protein with a C-terminal c-myc epitope tag. A band corresponding to a protein with the predicted molecular mass for the Ac23-c-myc protein (approximately 81 kDa) was detected with an anti-c-myc antibody (Fig. 2). This band was not detected in controls isolated from viruses expressing the wild-type Ac23 protein (data not shown). In addition, as a positive control for the anti-c-myc antibody, a GP64-cmyc protein was detected. These results indicate that Ac23 is expressed in AcMNPV-infected cells and that it is incorporated into AcMNPV budded virions along with GP64. This result also suggests that Ac23 found in BV is not proteolytically cleaved like F proteins from SeMNPV and LdMNPV; this observation is consistent with the absence of a predicted furin cleavage site in Ac23.
FIG. 2.
Western blot analysis of Ac23 expression and localization to AcMNPV budded virions. Lane 1 shows purified budded virions isolated from bacmid Ac23null/GUS+Ac23cmyc, which expresses a c-myc epitope-tagged Ac23 protein. Lane 2 shows a control of infected-cell extracts isolated from virus vAcgp64null/gp64cmyc (72 h p.i.) which expresses a c-myc epitope-tagged GP64 protein. Duplicate blots were incubated with an anti-c-myc monoclonal antibody (top panel) and an anti-GP64 monoclonal antibody (lower panel). A band corresponding to the approximate predicted size (81 kDa) of the c-myc-tagged Ac23 protein is indicated by an arrow.
Ac23 is not essential for AcMNPV infection, propagation, or BV production in cell culture.
The GP64 protein is essential for viral replication in cell culture and in infected animals (21, 23). In baculoviruses without a gp64 gene, F proteins appear to play a similar role to GP64 (18). Viruses such as AcMNPV have both GP64 and a homolog of the F protein. Therefore, we asked whether the AcMNPV F homolog, Ac23, was necessary for viral propagation in cultured insect cells. We first used a transfection-infection assay to determine whether the Ac23null bacmid was capable of propagating an infection in Sf9 cells. Sf9 cells were transfected with the Ac23null or Ac23null-repair (Ac23null/GUS+Ac23) bacmid. After 3 days of incubation, supernatants were collected from transfected cells and used to infect Sf9 cells, which were then stained for GUS reporter activity (Fig. 3). Our results show that the Ac23null bacmid, like the repair bacmid (Ac23null/GUS+Ac23), was able to generate a spreading infection in Sf9 cells. We therefore conclude that Ac23 is not essential for propagation of viral infection in cultured Sf9 cells.
FIG. 3.
Analysis of viral late promoter-reporter (GUS) activity in Sf9 cells. (Top panels) Sf9 cells were transfected with the indicated bacmids (C, E, and G) or mock treated (A), incubated for 72 h, and stained for GUS activity. (Bottom panels) Supernatants from transfected cells were used to infect Sf9 cells, and the infected cells were stained for GUS activity at 48 h p.i. (B, D, F, and H). Stained cells in the lower panels indicate that infectious virions were generated from transfected cells in the panels above.
The presence of Ac23 on budded virions suggested that it may play a role in virus budding or production of infectious virions. To determine whether deletion of Ac23 affected infectious virus (BV) production, we compared an Ac23null virus to wild-type AcMNPV and Ac23null-repair viruses in one-step growth curve experiments with Sf9 cells (Fig. 4). Although the titers of Ac23null and Ac23null-repair viruses differed (by approximately 10-fold) at 24 h postinfection (p.i.), it is unclear whether this has any significance, since (i) both viruses were in log-phase production of BV at this time and (ii) BV titers of the Ac23null virus were indistinguishable from those of control viruses by 48 h p.i. (Fig. 4). Thus, our results from one-step growth curve experiments show that deletion of Ac23 may reduce the initial rate of BV production but that Ac23 has no substantial effect on the yield of infectious BV production by 48 h p.i. in cultured Sf9 cells.
FIG. 4.
Analysis of BV production by one-step growth curve analysis. BV production by the Ac23null virus is indistinguishable from that for wild-type virus. Sf9 cells were infected at an MOI of 5 with each of the following virus preparations: wild-type AcMNPV (AcMNPV), Ac23null/GUS virus (Ac23null), Ac23/GUS+Ac23 virus (Ac23repair), and Ac23null virus expressing the SeMNPV F protein (Ac23null/GUS+SeF). Supernatants harvested at the indicated time points p.i. were subjected to titer determination on Sf9 cells, and each data point represents the average titer derived from three independent infections. Error bars represent stardard deviation from the mean.
Ac23 accelerates mortality in T. ni larvae.
To determine whether Ac23 plays a role in the AcMNPV infection cycle in animals, we examined the effect of the Ac23 deletion on AcMNPV-induced mortality in insect larvae. Polyhedrin is essential for the production of the occluded form of the virus, which is orally infectious and is responsible for the spread of infection among insects in nature. Therefore, we inserted the polyhedrin gene into all bacmids to be tested in insects. The polyhedrin gene was inserted into donor plasmids without and with the Ac23 gene to generate an Ac23null/GUS+PH bacmid and an Ac23null/GUS+Ac23+PH repair bacmid, respectively (Fig. 1B, constructs III and IV). These polyhedrin gene-containing bacmids were used to generate and amplify viruses and OB in Sf9 cells. With purified OB to infect T. ni neonate larvae in sensitive droplet feeding assays, initial experiments were performed to determine whether bacmid-derived OB had any negative consequences on insect mortality in T. ni compared to wild-type AcMNPV OB. In a semiblind neonate mortality assay, we determined that wild-type AcMNPV and bacmid-derived OB (vAcbacmid/GUS+PH) resulted in similar total mortalities and response times when used to orally infect T. ni neonates (Fig. 5A). With a dose of 6 OB/insect, the highest degree of larval mortality occurred between 72 and 90 h p.i., and the calculated arithmetic mean response time or median survival time (ST50) (30) was 90 h (standard error [SE] = 0.7 h) for wild-type AcMNPV and 93 h (SE = 3.49 h) for vAcbacmid/GUS+PH (Table 2). Similar results were seen with a dose of 0.6 and 3 OB/insect in parallel experiments (data not shown). Thus, larval mortality of T. ni neonates was similar when infected with OB from either a bacmid-derived virus or wild-type AcMNPV, and the use of bacmids had only a slight, insignificant effect. To examine the effects of the Ac23 gene on larval mortality, insects were fed OB from Ac23null (vAc23null/GUS+PH) and control viruses. No significant difference in total mortality was observed when insects fed the Ac23null virus were compared with those fed control viruses containing the Ac23 gene. However, insects fed the Ac23null virus exhibited a substantial delay in mortality. Insects orally infected with a dose of 6 Ac23null OB/insect showed 50% mortality at approximately 114 h p.i. (Fig. 5A). This represents an ST50 of 119 h (SE = 1.4 h) (Table 2). Thus, compared with the control viruses, for which the ST50 values were approximately 93 to 94 h, mortality resulting from the Ac23null virus was delayed by at least 26 h. At a lower dose of 3 vAc23null/GUS+PH OB/insect, 50% mortality was delayed another 12 h to about 126 h p.i. (data not shown). Furthermore, the delay in mortality caused by the Ac23null virus was also observed at a substantially higher dose of 60 OB/insect (Fig. 5B). At this higher dose, the mortality curve was shifted to the left by approximately 6 h (compare Fig. 5A and B) and the calculated ST50 for the Ac23null virus was 112.6 h (SE = 1.45 h [Table 2]). At all four doses tested, the delayed-mortality phenotype of the Ac23null virus was rescued to near wild-type levels by reintroducing the Ac23 gene into the Ac23null bacmid (vAc23null/GUS+Ac23+PH) (Fig. 5, Table 2, and data not shown). The ST50 for the repair virus at 6 OB/insect was 93.8 h (SE = 1.6 h), which is very close to that for the bacmid carrying a polyhedrin gene, Acbacmid/GUS+PH (ST50 = 93 h; SE = 3.5 h) and the wild-type AcMNPV (ST50 = 90 h; SE = 0.7 h). Thus, the delayed mortality observed with the Ac23null virus results from the loss of the Ac23 gene. This delayed mortality is independent of the OB dose since the effect was observed with all four concentrations of ODV assayed. Thus, while Ac23 did not affect the final mortality, mortality was accelerated when Ac23 was present.
TABLE 2.
Survival times after OB infection of larvae
| Virus | Dose (OB/insect) | ST50 (SE) (h) | No. | Fig. |
|---|---|---|---|---|
| AcMNPV | 6/neonate | 90 (0.7) | 94 | 5A |
| Acbacmid | 6/neonate | 93 (3.5) | 40 | 5A |
| Ac23null | 6/neonate | 119 (1.4) | 99 | 5A |
| Ac23repair | 6/neonate | 93.8 (1.6) | 89 | 5A |
| AcMNPV | 60/neonate | 83.5 (1.1) | 54 | 5B |
| Ac23null | 60/neonate | 112.6 (1.5) | 62 | 5B |
| Ac23repair | 60/neonate | 86.9 (1.1) | 61 | 5B |
| Ac23null | 250/4th instar | 128.2 (1.6) | 73 | 5C |
| Ac23repair | 250/4th instar | 95.8 (1.2) | 72 | 5C |
| Ac23null | 250/4th instar | 125.9 (2.5) | 42 | 5D |
| Ac23repair | 250/4th instar | 97.5 (2.7) | 43 | 5D |
| Ac23null/Se F | 250/4th instar | 149.7 (3.1) | 58 | 5D |
In addition to studies of neonate larvae, we also examined mortality in fourth-instar T. ni larvae (Fig. 5C). Mortality was scored for animals infected with 250 OB of either the Ac23null virus (vAc23null/GUS+PH) or the Ac23null-repair virus (vAc23null/GUS+Ac23+PH) per insect. While insects infected with the Ac23null-repair virus showed an ST50 of 95.8 h (SE = 1.2 h), larvae infected with the Ac23 knockout virus (vAc23null/GUS+PH) had an ST50 of 128.2 h (SE = 1.6 h) (Table 2), a delay of 32 h. Thus, analysis of larval mortality in both neonate and 4th instar T. ni larvae showed that Ac23 did not affect the viral dose required for larval mortality but significantly accelerated mortality in infected animals.
To examine the likely cause of the Ac23-mediated acceleration of mortality, we examined (i) the progression of the infection cycle in cultured cells, (ii) the timing of death of infected cultured cells, (iii) the BV load in the hemolymph of infected insects, and (iv) the systemic spread of viral infection in various tissues.
Ac23 accelerates the death of infected cultured insect cells.
To determine whether the Ac23-mediated acceleration of insect mortality might result from the accelerated death of infected cells, we infected two cell lines derived from two insect species (Sf9 cells from S. frugiperda and High 5 cells from T. ni) with the Ac23null virus (vAc23null/GUS+PH) and the Ac23null-repair virus (vAc23null/GUS+Ac23+PH). At 66 and 96 h p.i., the cells were stained with ethidium homodimer-1, which identified cells incapable of maintaining the integrity of the plasma membrane. In comparisons of cells infected with either the Ac23null or control (Ac23null-repair) virus, we found that for both cell lines a higher percentage of cells were stained with ethidium homodimer-1 when infected with the control Ac23null-repair virus (Fig. 6). Thus, analysis of cell viability suggested that Ac23 accelerates the death of AcMNPV-infected S. frugiperda (Sf9) and T. ni (High 5) cells. Interestingly, at 96 h p.i., large numbers of Ac23null-repair virus-infected High 5 cells have detached or lysed, suggesting that progression into the late stages of the infection cycle occurs earlier in High 5 cells than in Sf9 cells (data not shown).
FIG. 6.
Analysis of cell death in virus-infected cells: Ac23 accelerates the death of infected cultured insect cells. (A and B) Sf9 cells (A) and T. ni High 5 cells (B) were infected with either the Ac23null virus or the Ac23null-repair virus (Ac23repair) and examined for viability by their ability to exclude the dye ethidium homodimer-1. The number of cells stained with ethidium homodimer-1 was determined, and each bar represents the average percentage of dead cells from two independent infection experiments. Error bars represent standard deviations from the mean. (C and D) Representative fields of Sf9 cells observed under epifluorescence after treatment with ethidium homodimer-1 at 96 h p.i. for Ac23null virus (C) or the Ac23null-repair virus (D).
Ac23 accelerates the progression of the infection cycle in cultured insect cells.
In initial studies, we noted that OB were observed earlier in Ac23null-repair virus-infected cells than in Ac23null virus-infected cells (data not shown). Because OB production is a marker of the very late phase of the infection cycle, this suggested that cells infected with a virus expressing Ac23 might progress more rapidly into the very late phase. Quantitation of OB-positive cells at various times after initiation of virus infection confirmed that, on average, OB were generated more rapidly in cells infected with Ac23-containing viruses, suggesting that Ac23 accelerates progression into the very late stages of the infection cycle (Fig. 7B and D). Similar results were observed when we used a marker of late-stage infection (expression of a GUS reporter gene under the control of a late viral p6.9 promoter) to monitor the percentage of cells in the late stage of the infection cycle at various time points after infection (Fig. 7A and C). These results suggest that Ac23 may accelerate the death of infected cells and the host insect by accelerating the rate of the infection cycle at the level of the individual cell. Similar to the results of cell viability assays described above, at 72 h p.i. a significant number of High 5 cells infected with the Ac23null-repair virus had detached, making accurate quantitation difficult (data not shown). Interestingly, our results also indicate that progression into the late stages of the infection cycle was more rapid in High 5 cells than in Sf9 cells (compare times in Fig. 7A and B).
FIG. 7.
Analysis of markers for AcMNPV infection cycle progression. Ac23 accelerates the progression of the infection cycle in infected cultured insect cells. Sf9 cells (A and B) and High 5 cells (C and D) were infected with either the Ac23null virus or the Ac23null-repair virus. (A and C) Percentages of cells that expressed the late-stage marker (GUS activity); (B and D) percentages of cells scored positive for the very late marker (OB formation). Each bar represents the average of two independent infections, with error bars representing the SE.
Ac23 does not appear to affect viral load in infected insects.
To determine if the delayed-mortality phenotype observed in Ac23null virus-infected larvae was correlated with a delay in the accumulation of infectious BV virions within the hemolymph, we measured BV titers in the hemolymph of larvae infected with Ac23null and control Ac23null-repair viruses. For these studies, fourth-instar larvae were orally infected with 250 OB/larva within 2 h after the molt to the fourth instar. Hemolymph samples were collected at 12, 18, 24, 30, 36, 42, and 48 h p.i., and titers of infectious virions were determined on Sf9 cells using standard TCID50 assays. Using these methods, the minimal detectable virus titers were approximately 2 × 104 IU per μl of hemolymph. Circulating infectious virus (BV) was first detected at 24 h p.i. in the hemolymph of insects infected with either the Ac23null or Ac23null-repair virus, and no substantial differences in hemolymph titers were observed throughout the course of the experiment except for a slight difference observed at 30 h p.i. (Fig. 8). At 30 h p.i., the hemolymph titer of the Ac23null virus was 2.5 times greater than that of the Ac23null-repair virus. These data are consistent with the results of one-step growth curve experiments performed with cultured Sf9 cells, which suggest that BV production is not substantially affected in the Ac23null virus (Fig. 4). Although we did not detect differences in the hemolymph titers of these viruses at the times examined, we cannot rule out the possibility that differences may have occurred at earlier times (12 to 20 h p.i.), when we were unable to measure BV titers in our assays.
To determine whether the delayed mortality observed in insects infected with the Ac23null virus was due to a slower spreading of the secondary infection in hemocytes, tracheal epithelial cells, muscle, fat body, etc., we examined the tissues of infected larvae by staining for GUS reporter activity at various times postinfection. Infected larvae were examined at 24, 30, 36, 48, and 54 h p.i. Replicate larvae (six to eight larvae) were examined at each time point. We observed no temporal differences in the detection of primary (midgut specific) and systemic (non-midgut tissues) infection in insects infected with the Ac23null or Ac23null-repair viruses (data not shown). Thus, using this GUS reporter system, we detected no correlation between the observed differences in mortality and gross tissue pathology between 24 and 54 h p.i.
SeMNPV F does not complement an Ac23 deletion in AcMNPV.
We previously demonstrated that F proteins from certain group II NPVs (SeMNPV and LdMNPV) were able to rescue an AcMNPV virus containing a gp64 deletion. To determine whether the SeMNPV F protein could also rescue the Ac23null phenotype, we generated and examined a recombinant Ac23null virus containing an inserted SeMNPV F gene (vAc23null/GUS+SeF+PH). When vAc23null/GUS+SeF+PH occlusion bodies were fed to fourth-instar T. ni larvae, the delayed-mortality phenotype that was observed in Ac23null virus-infected larvae was exacerbated (Fig. 5D, Table 2). Furthermore, approximately 30% of the insects that were orally-infected with vAc23null/GUS+SeF+PH OB developed to the pupal stage, thus escaping the infection. In contrast, insects infected with the Ac23null or Ac23null-repair virus in parallel experiments showed nearly 100% mortality. These results were confirmed using a second, independently isolated vAc23null/GUS+SeF+PH virus (data not shown). Thus, under these conditions, the SeMNPV F protein was unable to rescue the delayed-mortality phenotype that resulted from the deletion of Ac23.
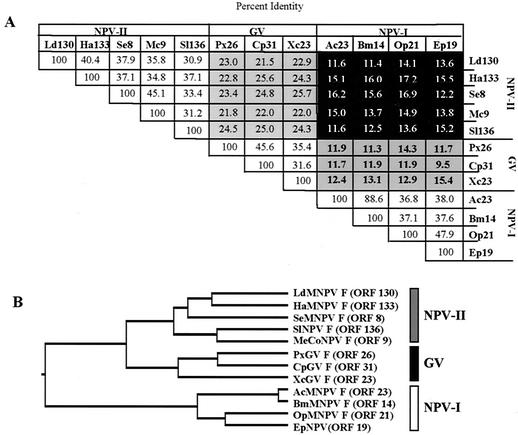
Group I NPV F homologs have low similarity to F proteins from group II NPVs and GVs.
Although the F proteins from all baculoviruses have similar structural features such as conserved cysteine residues, coil-coiled domains, and transmembrane domains, data presented here and elsewhere (18) show that F homologs of group I NPVs are functionally different from F proteins of group II NPVs and GVs. To further examine this relationship, we compared the sequence identities of mature F proteins, excluding the predicted signal peptides. Our results showed that F proteins within the three major baculovirus subgroups (group I and II NPVs and GVs) were most similar to members of the same group (>31% identity within each group [Fig. 9 ]). It is well established that group I and II NPVs are evolutionarily more closely related to each other than to GVs (9). However, F proteins do not follow this general trend. F proteins from group II NPVs are more closely related to GVs (range = 21.5 to 25.7% identity) than they are to the F protein homologs of Group I NPVs (range = 11.4 to 17.2% identity) (Fig. 9). These results, in combination with functional data, suggest that F homologs from baculoviruses with a gp64 gene (group I NPVs) are under different selection pressures from those from viruses without a gp64 gene (group II NPVs and GVs).
FIG. 9.
CLUSTAL W analysis of F proteins and F homologs from group I and II NPVs and GVs. F homologs from group I NPVs are less similar to F proteins from group II NPVs and GVs than group II NPVs and GVs are to each other. (A) Comparison matrix of percent identities of mature Lepidopteran baculovirus F proteins. Percent identities between F homologs from group I NPVs (NPV-I) and F proteins from group II NPVs (NPV-II) and GVs are shaded black and dark grey with bold text, respectively. Percent identities between F proteins from group II NPVs and GVs are shaded light grey. (B) Neighbor-joining tree of mature F proteins from group II NPVs and GVs and F homologs from group I NPVs.
DISCUSSION
Analysis of genomic sequences from viruses belonging to each of the three major subgroups (group I and II NPVs and GVs) within the Baculoviridae revealed the following. While all baculoviruses appear to have an F gene, gp64 genes have been identified only in closely related viruses belonging to group I NPVs (reviewed in reference 26). It was hypothesized that F proteins may be the prototypical baculovirus envelope fusion protein and that GP64 represents a recent acquisition (25). The fact that gp64 is an essential gene in AcMNPV (a group I NPV) but can be functionally replaced by F proteins from group II NPVs (which do not have a gp64 gene) provides strong evidence that GP64 and these F proteins are functionally analogous (18). Interestingly, group I NPVs such as AcMNPV and OpMNPV (which contain gp64 genes) also contain an F gene. However, the F protein (Ac23) in AcMNPV is unable to compensate for the loss of gp64 (18, 21, 23), suggesting that Ac23 is not functionally analogous to GP64 and F proteins from group II NPVs. We have therefore used the term “F homolog” to describe the F proteins from the group I NPVs. Pearson and Rohrmann (26) distinguished between the F proteins as Fa proteins (fusion competent) and Fb proteins (fusion incompetent). The presence of an F homolog gene in the AcMNPV genome (and in the genomes of other gp64-containing baculoviruses) suggested that the F homolog may provide a selective advantage for the virus. To determine the significance of F homologs in gp64-containing baculoviruses, we used a highly efficient PCR and E. coli-based recombination system (4) to delete the Ac23 gene in AcMNPV. Our results show that Ac23 is not essential for viral replication in cultured insect cells or in T. ni larvae. Deletion of Ac23 from the AcMNPV genome had no apparent effect on the timing or production of infectious BV in Sf9 cells. Furthermore, comparisons of Ac23null and Ac23null-repair viruses showed that Ac23 is not essential for viral pathogenesis in T. ni larvae. Indeed, the Ac23null virus was just as lethal in infected T. ni larvae as was the Ac23null-repair virus. However, differences were observed in host survival times. Host mortality was accelerated by more than 28% (or 26 h) in T. ni larvae orally infected with viruses carrying the F homolog (Ac23) gene. Even though Ac23 is not an essential gene, the maintenance of F protein homologs in the genomes of group I NPVs could be promoted by at least two general factors: (i) the Ac23-mediated acceleration of host mortality may offer a selective advantage to the virus in nature, and (ii) the close proximity of Ac23 to an essential gene may decrease the likelihood that it would be deleted. Within the group I NPVs, the F homolog genes are flanked on one side by a core gene (Ac22 in AcMNPV; Op18, Bm13, and Ep18 in other group I NPVs). Although it is not known whether Ac22 and the Ac22 homologs are essential, their presence in all baculoviruses examined to date suggests that they may serve a critical or very important function in the baculovirus infection cycle. Since the 3′UTR of Ac22 may overlap with the Ac23 ORF that was deleted in this experiment, it is possible the regulation of the Ac22 gene may be effected in the Ac23null virus. However, the Ac23null virus and the Ac23null-repair virus used in this study are isogenic except for the insertion of the Ac23 gene into the polyhedrin locus (i.e., the Ac23 locus and surrounding genes are identical in the Ac23null and Ac23null-repair viruses). Thus, the Ac23null mutant phenotypes we described in comparison to the Ac23null-repair virus are due to the lack of Ac23 gene and not due to misregulation of adjacent genes such as Ac22.
Analysis of predicted amino acid sequences from all available Lepidopteran baculoviruses revealed that F proteins from group II NPVs are more closely related to F proteins from GVs than they are to F proteins of group I NPVs. That is, the F proteins from group I NPVs are more divergent than those from other subgroups. In the absence of functional data, this would be unexpected since group I and II NPVs are more closely related evolutionarily than are NPVs and GVs, which represent separate genera. However, in combination with the functional data described above, F protein sequence comparisons suggest that F proteins in gp64-containing baculoviruses (i.e., group I NPVs) are under different selection pressures from those in other baculoviruses (group II NPVs and GVs).
Three pieces of evidence suggest that Ac23 (and probably other F homologs from group I NPVs) plays a role different from that of GP64 and group II NPV F proteins. First, while the F protein from SeMNPV (SeF) was able to substitute for the unrelated GP64 protein in the context of an AcMNPV infection (18), it did not rescue the deletion of a related gene Ac23 in the Ac23null virus. Second, the GP64 and F proteins from group II NPVs have pH-mediated membrane fusion activity (3, 15, 25), while no such activity has been detected in F homolog proteins from gp64-containing viruses (reference 27 and unpublished data). Third, a conserved furin-like cleavage site found in F proteins from group II NPVs (Ld130 and Se8) and GVs (Xc27, Px26) (15) is absent from F homologs from group I NPVs (Ac23 and Op21) (19, 28). Functional studies of the SeMNPV F protein showed that cleavage at the furin-like cleavage site is essential for (i) pH-triggered membrane fusion activity (32) and (ii) rescue of a gp64null AcMNPV virus by the SeMNPV F protein (18). Thus, the furin cleavage site serves as an indicator of the functional divergence of F proteins from group I and group II NPVs. The observation that the SeMNPV F protein did not rescue the delayed-mortality phenotype caused by an Ac23 deletion (and exacerbated the phenotype) suggests several possible explanations. One possibility is that proteins that normally interact with Ac23 may interact nonproductively with SeF, resulting in a more dramatic phenotype. Alternatively, because it is also an envelope fusion protein, the SeF protein may interact with GP64 or other components of the entry or fusion apparatus, disrupting normal functions of GP64. Thus, while the SeF protein may also accelerate mortality in the context of an SeMNPV infection, we were unable to demonstrate this by using the current heterologous system.
A mechanistic explanation for the effect of Ac23 on the acceleration of host mortality is lacking. However, our analysis of infectious BV production and pathogenesis suggests that the observed effects of the Ac23 knockout are not the result of decreased BV production in vivo and did not result from an obvious delay in the systemic spread of viral infection. Thus, the point at which Ac23 exerts its effect in the cell is not clear and requires further study. A recent report that the OpMNPV F protein (Op21) is more tightly associated with the nucleocapsid than it is with a membrane fraction containing GP64 (27) is intriguing and further supports the contention that the F protein homolog plays a distinctively different role from that of GP64.
In conclusion, we used a new bacmid-based knockout system to delete the AcMNPV F homolog gene (Ac23) and to examine the function of Ac23 in the context of infected insect cells in culture and in orally infected insect larvae. We show that Ac23 is not essential for viral replication or pathogenesis but plays an important role in accelerating mortality in the host insect and thus represents a newly identified pathogenicity factor in the Baculoviridae.
Acknowledgments
We acknowledge Pat Hughes and Li Guoxun for help and advice with experimental methods in animal experiments, and we thank Yoshifumi Hashimoto for reviewing the manuscript. We also thank Just Vlak, Douwe Zuidema, and Marcel Westenberg for discussions on F protein function and analysis.
This work was supported by NIH grant AI31130 and by a postdoctoral fellowship from the Boyce Thompson Institute to O.Y.L.
REFERENCES
- 1.Ayres, M. D., S. C. Howard, J. Kuzio, M. Lopez-Ferber, and R. D. Possee. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586-605. [DOI] [PubMed] [Google Scholar]
- 2.Blissard, G., B. Black, N. Crook, B. A. Keddie, R. Possee, G. Rohrmann, D. Theilmann, and L. E. Volkman. 2000. Baculoviridae: taxonomic structure and properties of the family, p. 195-202. In M. H. V. van Regenmortel et al. (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee for the Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 3.Blissard, G. W., and J. R. Wenz. 1992. Baculovirus GP64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 66:6829-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhard, E. K., L. N. W. Kam-Morgan, J. O. Washburn, and L. E. Volkman. 1994. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 91:3224-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flipsen, J. T. M., R. M. W. Mans, A. W. F. Kleefsman, D. Knebelmorsdorf, and J. M. Vlak. 1995. Deletion of the baculovirus ecdysteroid UDP-glucosyltransferase gene induces early degeneration of Malpighian tubules in infected insects. J. Virol. 69:4529-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flipsen, J. T. M., J. W. M. Martens, M. M. Van-Oers, J. M. Vlak, and J. W. M. Van-Lent. 1995. Passage of Autographa californica nuclear polyhedrosis virus through the midgut epithelium of Spodoptera exigua larvae. Virology 208:328-335. [DOI] [PubMed] [Google Scholar]
- 8.Hefferon, K., A. Oomens, S. Monsma, C. Finnerty, and G. Blissard. 1999. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology 258:455-468. [DOI] [PubMed] [Google Scholar]
- 9.Herniou, E. A., T. Luque, X. Chen, J. M. Vlak, D. Winstanley, J. S. Cory, and D. R. O'Reilly. 2001. Use of whole-genome sequence data to infer baculovirus phylogeny. J. Virol. 75:8117-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hink, W. F. 1970. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature 226:466-467. [DOI] [PubMed] [Google Scholar]
- 11.Hohmann, A. W., and P. Faulkner. 1983. Monoclonal antibodies to baculovirus structural proteins: determination of specificities by Western blot analysis. Virology 125:432-444. [DOI] [PubMed] [Google Scholar]
- 12.Hu, Z. H., B. M. Arif, J. S. Sun, X. W. Chen, D. Zuidema, R. W. Goldbach, and J. M. Vlak. 1998. Genetic organization of the HindIII-I region of the single-nucleocapsid nucleopolyhedrovirus of Buzura suppressaria. Virus Res. 55:71-82. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, P. R., and H. A. Wood. 1981. A synchronous peroral technique for the bioassay of insect viruses. J. Invertebr. Pathol. 37:154-159. [Google Scholar]
- 14.IJkel, W. F. J., E. A. van Strien, J. G. Heldens, R. Broer, D. Zuidema, R. W. Goldbach, and J. M. Vlak. 1999. Sequence and organization of the Spodoptera exigua multicapsid nucleopolyhedrovirus genome. J. Gen. Virol. 80:3289-3304. [DOI] [PubMed] [Google Scholar]
- 15.IJkel, W. F. J., M. Westenberg, R. W. Goldbach, G. W. Blissard, J. M. Vlak, and D. Zuidema. 2000. A novel baculovirus envelope fusion protein with a proprotein convertase cleavage site. Virology 275:30-41. [DOI] [PubMed] [Google Scholar]
- 16.Kuzio, J., M. N. Pearson, S. H. Harwood, C. J. Funk, J. T. Evans, J. M. Slavicek, and G. F. Rohrmann. 1999. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology 253:17-34. [DOI] [PubMed] [Google Scholar]
- 17.Luckow, V. A., S. C. Lee, G. F. Barry, and P. O. Olins. 1993. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 67:4566-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lung, O., M. Westenberg, J. M. Vlak, D. Zuidema, and G. W. Blissard. 2002. Pseudotyping Autographa californica multicapsid Nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J. Virol. 76:5729-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik, H. S., S. Henikoff, and T. H. Eickbush. 2000. Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Res. 10:1307-1318. [DOI] [PubMed] [Google Scholar]
- 20.Monsma, S. A., and G. W. Blissard. 1995. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 envelope fusion protein. J. Virol. 69:2583-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monsma, S. A., A. G. P. Oomens, and G. W. Blissard. 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J. Virol. 70:4607-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 23.Oomens, A. G. P., and G. W. Blissard. 1999. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology 254:297-314. [DOI] [PubMed] [Google Scholar]
- 24.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors: a laboratory manual. W. H. Freeman & Co., New York, N.Y.
- 25.Pearson, M. N., C. Groten, and G. F. Rohrmann. 2000. Identification of the Lymantria dispar nucleopolyhedrovirus envelope fusion protein provides evidence for a phylogenetic division of the Baculoviridae. J. Virol. 74:6126-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, M. N., and G. F. Rohrmann. 2002. Transfer, incorporation, and substitution of envelope fusion proteins among members of the Baculoviridae, Orthomyxoviridae, and Metaviridae (insect retrovirus) families. J. Virol. 76:5301-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson, M. N., R. L. Russell, and G. F. Rohrmann. 2001. Characterization of a baculovirus-encoded protein that is associated with infected-cell membranes and budded virions. Virology 291:22-31. [DOI] [PubMed] [Google Scholar]
- 28.Rohrmann, G. F., and P. A. Karplus. 2001. Relatedness of baculovirus and gypsy retrotransposon envelope proteins. BMC E vol. Biol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Beek, N. A. M., and P. R. Hughes. 1998. The response time of insect larvae infected with recombinant baculoviruses. J. Invertebr. Pathol. 72:338-347. [DOI] [PubMed] [Google Scholar]
- 31.Volkman, L. E., P. A. Goldsmith, R. T. Hess, and P. Faulkner. 1984. Neutralization of budded Autographa californica NPV by a monoclonal antibody: identification of the target antigen. Virology 133:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westenberg, M., H. Wang, W. F. IJkel, R. W. Goldbach, J. M. Vlak, and D. Zuidema. 2002. Furin is involved in baculovirus envelope fusion protein activation. J. Virol. 76:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanotto, P. M., B. D. Kessing, and J. E. Maruniak. 1993. Phylogenetic interrelationships among baculoviruses: evolutionary rates and host associations. J. Invertebr. Pathol. 62:147-164. [DOI] [PubMed] [Google Scholar]